Effects of Spaghetti Differing in Soluble Fiber and Protein Content on Glycemic Responses in Humans: A Randomized Clinical Trial in Healthy Subjects
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Study Design
2.3. Test Meals
2.4. Blood Glucose and Salivary Insulin Concentrations
2.5. Blood Pressure Measurements and Dietary Intake Analysis
2.6. Statistical Analysis
3. Results
3.1. Subjects’ Characteristics
3.2. Glycemic Index (GI) of Three Spaghetti Types
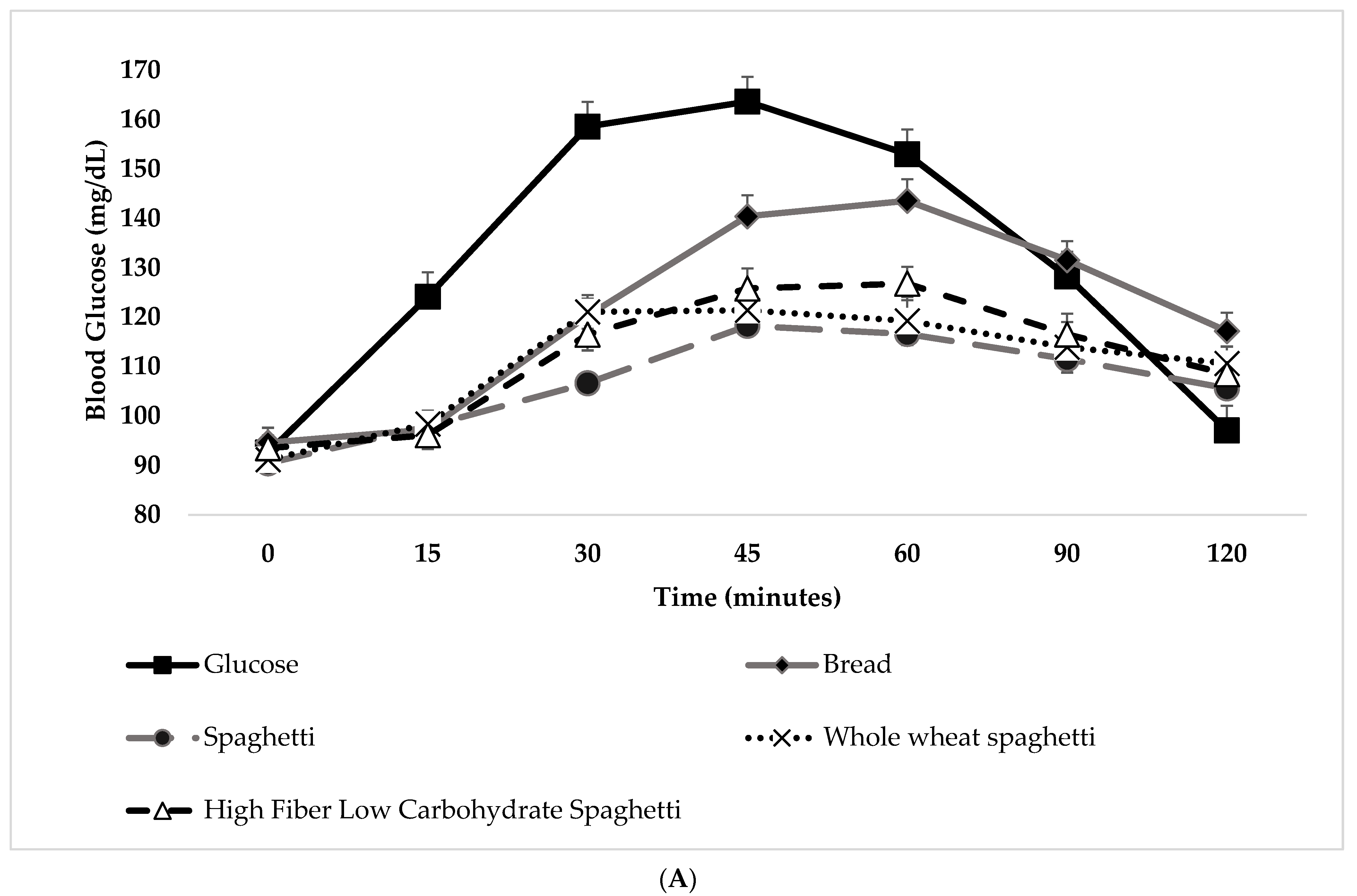
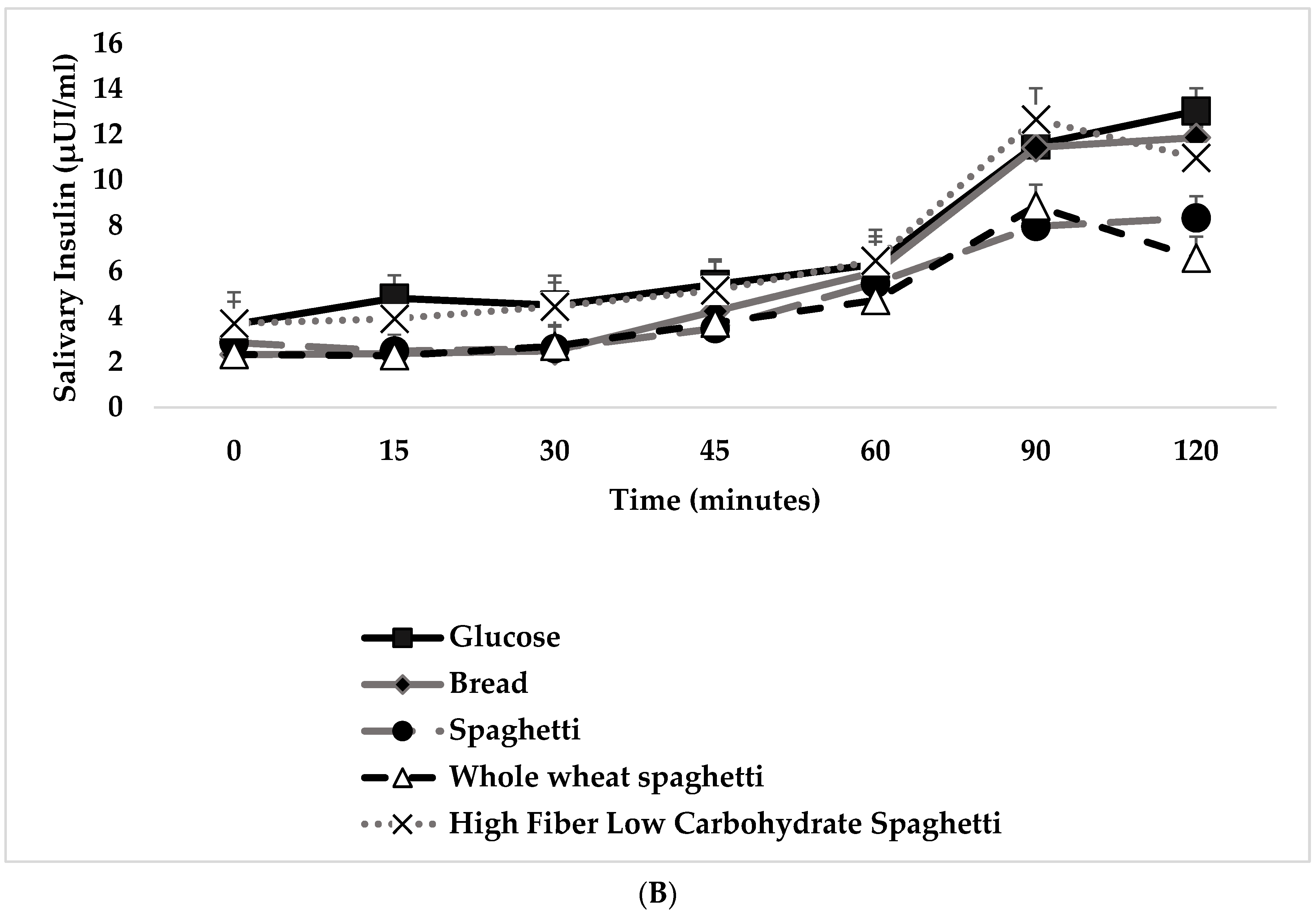
3.3. Blood Glucose and Salivary Insulin Concentrations
3.4. Blood Pressure and Subjective Appetite
4. Discussion
4.1. Glycemic Index (GI) and Glycemic Responses: Fiber and Protein Implications
4.1.1. Blood and Salivary Insulin Responses
4.1.2. Blood Pressure
4.2. Study Limitations and Advantages
4.3. Practical Applications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ISO 26642; Food Products—Determination of the Glycaemic Index (GI) and Recommendation for Food Classification. International Standards Organization: Geneva, Switzerland, 2010.
- FAO and WHO. Carbohydrates in human nutrition. Report of a Joint FAO/WHO Expert Consultation. FAO Food Nutr. Pap. 1998, 66, 1–140. [Google Scholar]
- Augustin, L.S.; Kendall, C.W.; Jenkins, D.J.; Willett, W.C.; Astrup, A.; Barclay, A.W.; Bjorck, I.; Brand-Miller, J.C.; Brighenti, F.; Buyken, A.E.; et al. Glycemic index, glycemic load and glycemic response: An International Scientific Consensus Summit from the International Carbohydrate Quality Consortium (ICQC). Nutr. Metab. Cardiovasc. Dis. 2015, 25, 795–815. [Google Scholar] [CrossRef] [PubMed]
- Brouns, F.; Bjorck, I.; Frayn, K.N.; Gibbs, A.L.; Lang, V.; Slama, G.; Wolever, T.M. Glycaemic index methodology. Nutr. Res. Rev 2005, 18, 145–171. [Google Scholar] [CrossRef]
- Wolever, T.M.; Jenkins, D.J.; Jenkins, A.L.; Josse, R.G. The glycemic index: Methodology and clinical implications. Am. J. Clin. Nutr. 1991, 54, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Fardet, A.; Leenhardt, F.; Lioger, D.; Scalbert, A.; Remesy, C. Parameters controlling the glycaemic response to breads. Nutr. Res. Rev. 2006, 19, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A.; Esposito, K.; Piconi, L.; Ihnat, M.A.; Thorpe, J.E.; Testa, R.; Boemi, M.; Giugliano, D. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes 2008, 57, 1349–1354. [Google Scholar] [CrossRef]
- Monnier, L.; Colette, C. Target for glycemic control: Concentrating on glucose. Diabetes Care 2009, 32 (Suppl. 2), S199–S204. [Google Scholar] [CrossRef]
- Bao, J.; Atkinson, F.; Petocz, P.; Willett, W.C.; Brand-Miller, J.C. Prediction of postprandial glycemia and insulinemia in lean, young, healthy adults: Glycemic load compared with carbohydrate content alone. Am. J. Clin. Nutr. 2011, 93, 984–996. [Google Scholar] [CrossRef]
- Barclay, A.W.; Petocz, P.; McMillan-Price, J.; Flood, V.M.; Prvan, T.; Mitchell, P.; Brand-Miller, J.C. Glycemic index, glycemic load, and chronic disease risk--a meta-analysis of observational studies. Am. J. Clin. Nutr. 2008, 87, 627–637. [Google Scholar] [CrossRef]
- Greenwood, D.C.; Threapleton, D.E.; Evans, C.E.; Cleghorn, C.L.; Nykjaer, C.; Woodhead, C.; Burley, V.J. Glycemic index, glycemic load, carbohydrates, and type 2 diabetes: Systematic review and dose-response meta-analysis of prospective studies. Diabetes Care 2013, 36, 4166–4171. [Google Scholar] [CrossRef]
- FAO and WHO. Carbohydrate and nutrition. Nurs. Stand. 1998, 12, 32–33. [Google Scholar]
- Evert, A.B.; Boucher, J.L.; Cypress, M.; Dunbar, S.A.; Franz, M.J.; Mayer-Davis, E.J.; Neumiller, J.J.; Nwankwo, R.; Verdi, C.L.; Urbanski, P.; et al. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care 2013, 36, 3821–3842. [Google Scholar] [CrossRef] [PubMed]
- Chiavaroli, L.; Lee, D.; Ahmed, A.; Cheung, A.; Khan, T.A.; Mejia, S.B.; Mirrahimi, A.; Jenkins, D.J.A.; Livesey, G.; Wolever, T.M.S.; et al. Effect of low glycaemic index or load dietary patterns on glycaemic control and cardiometabolic risk factors in diabetes: Systematic review and meta-analysis of randomised controlled trials. BMJ 2021, 374, n1651. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.; Elliott, E.J. Low glycaemic index, or low glycaemic load, diets for diabetes mellitus. Cochrane Database Syst. Rev. 2009, 1. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, M.L.; Dunbar, S.A.; Jaacks, L.M.; Karmally, W.; Mayer-Davis, E.J.; Wylie-Rosett, J.; Yancy, W.S., Jr. Macronutrients, food groups, and eating patterns in the management of diabetes: A systematic review of the literature, 2010. Diabetes Care 2012, 35, 434–445. [Google Scholar] [CrossRef]
- de la Hera, E.; Rosell, C.M.; Gomez, M. Effect of water content and flour particle size on gluten-free bread quality and digestibility. Food Chem. 2014, 151, 526–531. [Google Scholar] [CrossRef]
- Scazzina, F.; Dall’Asta, M.; Casiraghi, M.C.; Sieri, S.; Del Rio, D.; Pellegrini, N.; Brighenti, F. Glycemic index and glycemic load of commercial Italian foods. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 419–429. [Google Scholar] [CrossRef]
- Jenkins, A.L.; Jenkins, D.J.; Zdravkovic, U.; Wursch, P.; Vuksan, V. Depression of the glycemic index by high levels of beta-glucan fiber in two functional foods tested in type 2 diabetes. Eur. J. Clin. Nutr. 2002, 56, 622–628. [Google Scholar] [CrossRef]
- Ray, K.S.; Singhania, P.R. Glycemic and insulinemic responses to carbohydrate rich whole foods. J. Food Sci. Technol. 2014, 51, 347–352. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Orfanakos, N.; Farajian, P.; Kapetanakou, A.E.; Makariti, I.P.; Grivokostopoulos, N.; Ha, M.A.; Skandamis, P.N. Short-term effects of a low glycemic index carob-containing snack on energy intake, satiety, and glycemic response in normal-weight, healthy adults: Results from two randomized trials. Nutrition 2017, 42, 12–19. [Google Scholar] [CrossRef]
- Gourdomichali, T.; Papakonstantinou, E. Short-term effects of six Greek honey varieties on glycemic response: A randomized clinical trial in healthy subjects. Eur. J. Clin. Nutr. 2018, 72, 1709–1716. [Google Scholar] [CrossRef] [PubMed]
- Papakonstantinou, E.; Chaloulos, P.; Papalexi, A.; Mandala, I. Effects of bran size and carob seed flour of optimized bread formulas on glycemic responses in humans: A randomized clinical trial. J. Funct. Foods 2018, 46, 345–355. [Google Scholar]
- Di Pede, G.; Dodi, R.; Scarpa, C.; Brighenti, F.; Dall’Asta, M.; Scazzina, F. Glycemic Index Values of Pasta Products: An Overview. Foods 2021, 10, 2541. [Google Scholar] [CrossRef] [PubMed]
- Wolever, T.M.; Vorster, H.H.; Bjorck, I.; Brand-Miller, J.; Brighenti, F.; Mann, J.I.; Ramdath, D.D.; Granfeldt, Y.; Holt, S.; Perry, T.L.; et al. Determination of the glycaemic index of foods: Interlaboratory study. Eur. J. Clin. Nutr. 2003, 57, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Pandolfo, A.; Messina, B.; Russo, G. Evaluation of Glycemic Index of Six Different Samples of Commercial and Experimental Pasta Differing in Wheat Varieties and Production Processes. Foods 2021, 10, 2221. [Google Scholar] [CrossRef]
- Urbaniak, G.C.; Plous, S. Research Randomizer (version 4.0) [Computer Software]. Available online: http://www.randomizer.org (accessed on 1 March 2021).
- Kaplan, E.A. The effect of durum wheat genotypes on cooking quality of pasta. Eur. J. Food Res. Technol. 2022, 248, 815–824. [Google Scholar] [CrossRef]
- Flint, A.; Raben, A.; Blundell, J.E.; Astrup, A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 38–48. [Google Scholar] [CrossRef]
- Myette-Cote, E.; Baba, K.; Brar, R.; Little, J.P. Detection of Salivary Insulin Following Low versus High Carbohydrate Meals in Humans. Nutrients 2017, 9, 1204. [Google Scholar] [CrossRef]
- Schulze, M.B.; Liu, S.; Rimm, E.B.; Manson, J.E.; Willett, W.C.; Hu, F.B. Glycemic index, glycemic load, and dietary fiber intake and incidence of type 2 diabetes in younger and middle-aged women. Am. J. Clin. Nutr. 2004, 80, 348–356. [Google Scholar] [CrossRef]
- Karl, J.P.; Meydani, M.; Barnett, J.B.; Vanegas, S.M.; Goldin, B.; Kane, A.; Rasmussen, H.; Saltzman, E.; Vangay, P.; Knights, D.; et al. Substituting whole grains for refined grains in a 6-wk randomized trial favorably affects energy-balance metrics in healthy men and postmenopausal women. Am. J. Clin. Nutr. 2017, 105, 589–599. [Google Scholar] [CrossRef]
- Wu, H.; Flint, A.J.; Qi, Q.; van Dam, R.M.; Sampson, L.A.; Rimm, E.B.; Holmes, M.D.; Willett, W.C.; Hu, F.B.; Sun, Q. Association between dietary whole grain intake and risk of mortality: Two large prospective studies in US men and women. JAMA Intern. Med. 2015, 175, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, D.R., Jr.; Andersen, L.F.; Blomhoff, R. Whole-grain consumption is associated with a reduced risk of noncardiovascular, noncancer death attributed to inflammatory diseases in the Iowa Women’s Health Study. Am. J. Clin. Nutr. 2007, 85, 1606–1614. [Google Scholar] [CrossRef] [PubMed]
- Hon, H.W.H.; Wong, T.H.T.; Tse, I.M.Y.; Louie, J.C.Y. The effect of a low glycaemic index diet on reducing day-long glycaemia in healthy young adults: A randomized crossover trial. Diabetes Obes. Metab. 2020, 22, 2398–2407. [Google Scholar] [CrossRef] [PubMed]
- Papakonstantinou, E.; Oikonomou, C.; Nychas, G.; Dimitriadis, G.D. Effects of Diet, Lifestyle, Chrononutrition and Alternative Dietary Interventions on Postprandial Glycemia and Insulin Resistance. Nutrients 2022, 14, 823. [Google Scholar] [CrossRef]
- Brynes, A.E.; Adamson, J.; Dornhorst, A.; Frost, G.S. The beneficial effect of a diet with low glycaemic index on 24 h glucose profiles in healthy young people as assessed by continuous glucose monitoring. Br. J. Nutr. 2005, 93, 179–182. [Google Scholar] [CrossRef]
- Vlachos, D.; Malisova, S.; Lindberg, F.A.; Karaniki, G. Glycemic Index (GI) or Glycemic Load (GL) and Dietary Interventions for Optimizing Postprandial Hyperglycemia in Patients with T2 Diabetes: A Review. Nutrients 2020, 12, 561. [Google Scholar] [CrossRef]
- Kim, H.; Stote, K.S.; Behall, K.M.; Spears, K.; Vinyard, B.; Conway, J.M. Glucose and insulin responses to whole grain breakfasts varying in soluble fiber, beta-glucan: A dose response study in obese women with increased risk for insulin resistance. Eur. J. Nutr. 2009, 48, 170–175. [Google Scholar] [CrossRef]
- Ibrugger, S.; Vigsnaes, L.K.; Blennow, A.; Skuflic, D.; Raben, A.; Lauritzen, L.; Kristensen, M. Second meal effect on appetite and fermentation of wholegrain rye foods. Appetite 2014, 80, 248–256. [Google Scholar] [CrossRef]
- Nilsson, A.C.; Ostman, E.M.; Granfeldt, Y.; Bjorck, I.M. Effect of cereal test breakfasts differing in glycemic index and content of indigestible carbohydrates on daylong glucose tolerance in healthy subjects. Am. J. Clin. Nutr. 2008, 87, 645–654. [Google Scholar] [CrossRef]
- Nilsson, A.C.; Ostman, E.M.; Holst, J.J.; Bjorck, I.M. Including indigestible carbohydrates in the evening meal of healthy subjects improves glucose tolerance, lowers inflammatory markers, and increases satiety after a subsequent standardized breakfast. J. Nutr. 2008, 138, 732–739. [Google Scholar] [CrossRef]
- Rosen, L.A.; Ostman, E.M.; Bjorck, I.M. Postprandial glycemia, insulinemia, and satiety responses in healthy subjects after whole grain rye bread made from different rye varieties. 2. J. Agric. Food Chem. 2011, 59, 12149–12154. [Google Scholar] [CrossRef] [PubMed]
- ADA. American Diabetes Association. 4. Lifestyle Management. Diabetes Care 2017, 40, S33–S43. [Google Scholar] [CrossRef] [PubMed]
- Wolever, T.M.; Jenkins, D.J.; Kalmusky, J.; Giordano, C.; Giudici, S.; Jenkins, A.L.; Thompson, L.U.; Wong, G.S.; Josse, R.G. Glycemic response to pasta: Effect of surface area, degree of cooking, and protein enrichment. Diabetes Care 1986, 9, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Scazzina, F.; Siebenhandl-Ehn, S.; Pellegrini, N. The effect of dietary fibre on reducing the glycaemic index of bread. Br. J. Nutr. 2013, 109, 1163–1174. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Wolever, T.M.; Jenkins, A.L.; Lee, R.; Wong, G.S.; Josse, R. Glycemic response to wheat products: Reduced response to pasta but no effect of fiber. Diabetes Care 1983, 6, 155–159. [Google Scholar] [CrossRef]
- Zavitsanou, S.; Massa, J.; Deshpande, S.; Pinsker, J.E.; Church, M.M.; Andre, C.; Doyle Iii, F.J.; Michelson, A.; Creason, J.; Dassau, E.; et al. The Effect of Two Types of Pasta Versus White Rice on Postprandial Blood Glucose Levels in Adults with Type 1 Diabetes: A Randomized Crossover Trial. Diabetes Technol. Ther. 2019, 21, 485–492. [Google Scholar] [CrossRef]
- Camps, S.G.; Lim, J.; Koh, M.X.N.; Henry, C.J. The Glycaemic and Insulinaemic Response of Pasta in Chinese and Indians Compared to Asian Carbohydrate Staples: Taking Spaghetti Back to Asia. Nutrients 2021, 13, 451. [Google Scholar] [CrossRef]
- Atkinson, F.S.; Brand-Miller, J.C.; Foster-Powell, K.; Buyken, A.E.; Goletzke, J. International tables of glycemic index and glycemic load values 2021: A systematic review. Am. J. Clin. Nutr. 2021. [Google Scholar] [CrossRef]
- Atkinson, F.S.; Foster-Powell, K.; Brand-Miller, J.C. International tables of glycemic index and glycemic load values: 2008. Diabetes Care 2008, 31, 2281–2283. [Google Scholar] [CrossRef]
- Wolever, T.M. Relationship between dietary fiber content and composition in foods and the glycemic index. Am. J. Clin. Nutr. 1990, 51, 72–75. [Google Scholar] [CrossRef]
- Bjorck, I.; Elmstahl, H.L. The glycaemic index: Importance of dietary fibre and other food properties. Proc. Nutr. Soc. 2003, 62, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, D.S.; Hu, F.B.; Tappy, L.; Brand-Miller, J. Dietary carbohydrates: Role of quality and quantity in chronic disease. BMJ 2018, 361, k2340. [Google Scholar] [CrossRef]
- Tosh, S.M.; Chu, Y. Systematic review of the effect of processing of whole-grain oat cereals on glycaemic response. Br. J. Nutr. 2015, 114, 1256–1262. [Google Scholar] [CrossRef]
- Reynolds, A.N.; Mann, J.; Elbalshy, M.; Mete, E.; Robinson, C.; Oey, I.; Silcock, P.; Downes, N.; Perry, T.; Te Morenga, L. Wholegrain Particle Size Influences Postprandial Glycemia in Type 2 Diabetes: A Randomized Crossover Study Comparing Four Wholegrain Breads. Diabetes Care 2020, 43, 476–479. [Google Scholar] [CrossRef] [PubMed]
- Blasel, H.M.; Hoffman, P.C.; Shaver, R.D. Degree of starch access: An enzymatic method to determine starch degradation potential of corn grain and corn silage. Anim. Feed. Sci. Technol. 2006, 128, 96–107. [Google Scholar] [CrossRef]
- Parada, J.; Aguilera, J.M. Review: Starch matrices and the glycemic response. Food Sci. Technol. Int. 2011, 17, 187–204. [Google Scholar] [CrossRef]
- Holt, S.H.; Miller, J.B. Particle size, satiety and the glycaemic response. Eur. J. Clin. Nutr. 1994, 48, 496–502. [Google Scholar] [PubMed]
- Jenkins, D.J.; Wolever, T.M.; Buckley, G.; Lam, K.Y.; Giudici, S.; Kalmusky, J.; Jenkins, A.L.; Patten, R.L.; Bird, J.; Wong, G.S.; et al. Low-glycemic-index starchy foods in the diabetic diet. Am. J. Clin. Nutr. 1988, 48, 248–254. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Wolever, T.M.; Jenkins, A.L. Starchy foods and glycemic index. Diabetes Care 1988, 11, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Gopirajah, R.; Raichurkar, K.P.; Wadhwa, R.; Anandharamakrishnan, C. The glycemic response to fibre rich foods and their relationship with gastric emptying and motor functions: An MRI study. Food Funct. 2016, 7, 3964–3972. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Wolever, T.M.; Collier, G.R.; Ocana, A.; Rao, A.V.; Buckley, G.; Lam, Y.; Mayer, A.; Thompson, L.U. Metabolic effects of a low-glycemic-index diet. Am. J. Clin. Nutr. 1987, 46, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Nesti, L.; Mengozzi, A.; Trico, D. Impact of Nutrient Type and Sequence on Glucose Tolerance: Physiological Insights and Therapeutic Implications. Front. Endocrinol. 2019, 10, 144. [Google Scholar] [CrossRef] [PubMed]
- Trico, D.; Natali, A. Modulation of postprandial glycemic responses by noncarbohydrate nutrients provides novel approaches to the prevention and treatment of type 2 diabetes. Am. J. Clin. Nutr. 2017, 106, 701–702. [Google Scholar] [CrossRef][Green Version]
- Meng, H.; Matthan, N.R.; Ausman, L.M.; Lichtenstein, A.H. Effect of macronutrients and fiber on postprandial glycemic responses and meal glycemic index and glycemic load value determinations. Am. J. Clin. Nutr. 2017, 105, 842–853. [Google Scholar] [CrossRef] [PubMed]
- Turco, I.; Bacchetti, T.; Morresi, C.; Padalino, L.; Ferretti, G. Polyphenols and the glycaemic index of legume pasta. Food Funct. 2019, 10, 5931–5938. [Google Scholar] [CrossRef]
- Goni, I.; Valentil-Gamazo, C. Chickpea flour ingredient slows glycemic response to pasta in healthy volunteers. Food Chem. 2003, 81, 511–515. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Triantafillidou, D.; Panagiotakos, D.B.; Iraklianou, S.; Berdanier, C.D.; Zampelas, A. A high protein low fat meal does not influence glucose and insulin responses in obese individuals with or without type 2 diabetes. J. Hum. Nutr. Diet 2010, 23, 183–189. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Triantafillidou, D.; Panagiotakos, D.B.; Koutsovasilis, A.; Saliaris, M.; Manolis, A.; Melidonis, A.; Zampelas, A. A high-protein low-fat diet is more effective in improving blood pressure and triglycerides in calorie-restricted obese individuals with newly diagnosed type 2 diabetes. Eur. J. Clin. Nutr. 2010, 64, 595–602. [Google Scholar] [CrossRef]
- Dong, J.Y.; Zhang, Z.L.; Wang, P.Y.; Qin, L.Q. Effects of high-protein diets on body weight, glycaemic control, blood lipids and blood pressure in type 2 diabetes: Meta-analysis of randomised controlled trials. Br. J. Nutr. 2013, 110, 781–789. [Google Scholar] [CrossRef]
- Scazzina, F.; Del Rio, D.; Pellegrini, N.; Brighenti, F. Sourdough bread: Starch digestibility and postprandial glycemic response. J. Cereal Sci. 2009, 49, 419–421. [Google Scholar] [CrossRef]
- Desai, G.S.; Mathews, S.T. Saliva as a non-invasive diagnostic tool for inflammation and insulin-resistance. World. J. Diabetes 2014, 5, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Fabre, B.; Maccallini, G.; Oneto, A.; Gonzalez, D.; Hirschler, V.; Aranda, C.; Berg, G. Measurement of fasting salivary insulin and its relationship with serum insulin in children. Endocr. Connect. 2012, 1, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Pasic, J.; Pickup, J.C. Salivary insulin in normal and type I diabetic subjects. Diabetes Care 1988, 11, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Eelderink, C.; Noort, M.W.J.; Sozer, N.; Koehorst, M.; Holst, J.J.; Deacon, C.F.; Rehfeld, J.F.; Poutanen, K.; Vonk, R.J.; Oudhuis, L.; et al. Difference in postprandial GLP-1 response despite similar glucose kinetics after consumption of wheat breads with different particle size in healthy men. Eur. J. Nutr. 2017, 56, 1063–1076. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.; Wesson, V.; Wolever, T.M.; Jenkins, A.L.; Kalmusky, J.; Guidici, S.; Csima, A.; Josse, R.G.; Wong, G.S. Wholemeal versus wholegrain breads: Proportion of whole or cracked grain and the glycaemic response. BMJ 1988, 297, 958–960. [Google Scholar] [CrossRef]

| Semolina Spaghetti No 7 | Wholegrain Spaghetti No 7 | High soluble Fiber Low Carbohydrate Spaghetti No 7 | |
|---|---|---|---|
| Energy (kcal) | 1500 kJ/354 kcal | 1502 kJ/355 kcal | 1398 kJ/333 kcal |
| Fat (gr) Saturated fat (gr) | 1.5 0.3 | 2.1 0.4 | 4.6 0.8 |
| Carbohydrates (gr) Sugar (gr) Polyols (gr) | 72 3.8 11 | 67.6 2.6 12.8 | 47.4 2.2 5.4 |
| Dietary fiber (gr) | 1.8 | 7.0 | 21.1 |
| Protein (gr) | 12 | 12.8 | 14.9 |
| Salt (gr) | 0.03 | 0.15 | 0.18 |
| Spaghetti Type | Cooking Time * (min) | Protein Content (%DM) | Ash Content (%DM) | Available Carbohydrates (%DM) | Total Dietary Fibers (%DM) | |
|---|---|---|---|---|---|---|
| Spaghetti | Raw | 10.16 ± 0.15 a** | 0.93 ± 0.04 a | 84.99 ± 1.65 a | 1.82 ± 0.08 a | |
| Cooked | 8.0 | 7.20 ± 0.11 b | 0.66 ± 0.02 b | 84.04 ± 1.29 a | 1.79 ± 0.06 a | |
| 8.5 | 6.98 ± 0.13 c | 0.62 ± 0.02 b,c | 83.01 ± 1.56 a | 1.77 ± 0.07 a | ||
| 9.0 | 6.63 ± 0.12 d | 0.59 ± 0.03 c | 81.74 ± 1.83 a | 1.79 ± 0.04 a | ||
| Wholegrain Spaghetti | Raw | 14.97 ± 0.13 a | 1.65 ± 0.05 a | 75.94 ± 1.15 a | 7.04 ± 0.06 a | |
| Cooked | 9.0 | 13.43 ± 0.10 b | 1.24 ± 0.01 b | 75.43 ± 1.53 a | 7.01 ± 0.05 a | |
| 9.5 | 13.26 ± 0.07 b | 1.22 ± 0.01 b,c | 74.99 ± 1.66 a | 6.96 ± 0.07 a | ||
| 10.0 | 12.79 ± 0.08 c | 1.21 ± 0.01 c | 73.75 ± 1.27 a | 6.99 ± 0.05 a | ||
| High soluble Fiber low Carbohydrate Spaghetti | Raw | 12.39 ± 0.09 a | 3.22 ± 0.03 a | 55.16 ± 1.62 a | 21.13 ± 0.09 a | |
| Cooked | 8.0 | 10.57 ± 0.12 b | 3.05 ± 0.02 b | 55.04 ± 1.48 a | 21.05 ± 0.06 a | |
| 8.5 | 10.04 ± 0.10 c | 3.03 ± 0.01 b,c | 54.78 ± 1.55 a | 21.08 ± 0.06 a | ||
| 9.0 | 9.34 ± 0.10 d | 2.82 ± 0.03 c | 52.04 ± 1.59 a | 20.89 ± 0.05 a |
| Characteristics | Total |
|---|---|
| N | 14 (4 men, 10 women) |
| Age (years) | 25.21 ± 0.91 |
| Weight (kg) | 64.51 ± 4.44 |
| Height (cm) | 167.43 ± 0.10 |
| Body mass index (BMI; kg/m2) | 22.67 ± 0.89 |
| Basal metabolic rate (BMR, kcal) | 1534.46 ± 143.92 |
| Body fat (kg) | 14.78 ± 1.62 |
| Muscle mass (kg) | 27.16 ± 2.28 |
| Waist circumference (cm) | 77.54 ± 2.98 |
| Hip circumference (cm) | 105.89 ± 7.36 |
| Dietary intake (from 24-h recall) | |
| Protein (gr) | 67.0 ± 7.662 |
| Carbohydrate (gr) | 206.27 ± 21.28 |
| Fat (gr) | 65.17 ± 7.51 |
| Saturated fat (gr) | 21.58 ± 2.71 |
| Total cholesterol(gr) | 218.74 ± 26.99 |
| Fiber (gr) | 18.06 ± 1.96 |
| Sodium (gr) | 2648.74 ± 509.83 |
| Energy intake (kcal) | 1668.85 ± 165.97 |
| Food (Serving Size Containing 50 g Available Carbohydrates) | iAUC (mmol 120 min l−1) | GI (Glucose as Reference Food) | GI (WB as Reference Food) | GL (Glucose as Reference Food) | GL (WB as Reference Food) | Glucose Peak Value (mg/dL) |
|---|---|---|---|---|---|---|
| Glucose | 4478 ± 228 a | 100 a | _ | _ | _ | 79.63 ± 4.23 a |
| WB | 3415 ± 228 b | 73.55 ± 5.47 b | _ | 36.77 ± 2.74 b | _ | 55.79 ± 3.53 b |
| S (163.67 g) | 2144 ± 324 c | 32.97 ± 4.29 c | 46.26 ± 5.24 | 17.48 ± 2.28 c | 24.52 ± 2.78 | 32.63 ± 4.23 c |
| WS (186.26 g) | 2547 ± 324 b,c | 38.31 ± 3.77 c | 48.84 ± 4.81 | 18.00 ± 1.77 c | 22.95 ± 2.26 | 37.44 ± 4.67 b |
| HFLowCS (223.06 g) | 2567 ± 324 b,c | 40.55 ± 4.37 c | 47.33 ± 4.35 | 15.00 ± 1.62 c | 17.51 ± 1.61 | 39.57 ± 3.95 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papakonstantinou, E.; Xaidara, M.; Siopi, V.; Giannoglou, M.; Katsaros, G.; Theodorou, G.; Maratou, E.; Poulia, K.-A.; Dimitriadis, G.D.; Skandamis, P.N. Effects of Spaghetti Differing in Soluble Fiber and Protein Content on Glycemic Responses in Humans: A Randomized Clinical Trial in Healthy Subjects. Int. J. Environ. Res. Public Health 2022, 19, 3001. https://doi.org/10.3390/ijerph19053001
Papakonstantinou E, Xaidara M, Siopi V, Giannoglou M, Katsaros G, Theodorou G, Maratou E, Poulia K-A, Dimitriadis GD, Skandamis PN. Effects of Spaghetti Differing in Soluble Fiber and Protein Content on Glycemic Responses in Humans: A Randomized Clinical Trial in Healthy Subjects. International Journal of Environmental Research and Public Health. 2022; 19(5):3001. https://doi.org/10.3390/ijerph19053001
Chicago/Turabian StylePapakonstantinou, Emilia, Marina Xaidara, Vassiliki Siopi, Marianna Giannoglou, George Katsaros, Georgios Theodorou, Eirini Maratou, Kalliopi-Anna Poulia, George D. Dimitriadis, and Panagiotis N. Skandamis. 2022. "Effects of Spaghetti Differing in Soluble Fiber and Protein Content on Glycemic Responses in Humans: A Randomized Clinical Trial in Healthy Subjects" International Journal of Environmental Research and Public Health 19, no. 5: 3001. https://doi.org/10.3390/ijerph19053001
APA StylePapakonstantinou, E., Xaidara, M., Siopi, V., Giannoglou, M., Katsaros, G., Theodorou, G., Maratou, E., Poulia, K.-A., Dimitriadis, G. D., & Skandamis, P. N. (2022). Effects of Spaghetti Differing in Soluble Fiber and Protein Content on Glycemic Responses in Humans: A Randomized Clinical Trial in Healthy Subjects. International Journal of Environmental Research and Public Health, 19(5), 3001. https://doi.org/10.3390/ijerph19053001









