Effect of Treadmill Training Interventions on Spatiotemporal Gait Parameters in Older Adults with Neurological Disorders: Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Selection Criteria
2.2. Search Strategy
2.3. Data Extraction
2.4. Quantitative Data Synthesis
2.5. Study Quality Assessment
3. Results
3.1. Study Selection
3.2. Basic Characteristics of Selected Studies
3.3. Gait Outcomes
3.4. Meta-Analysis
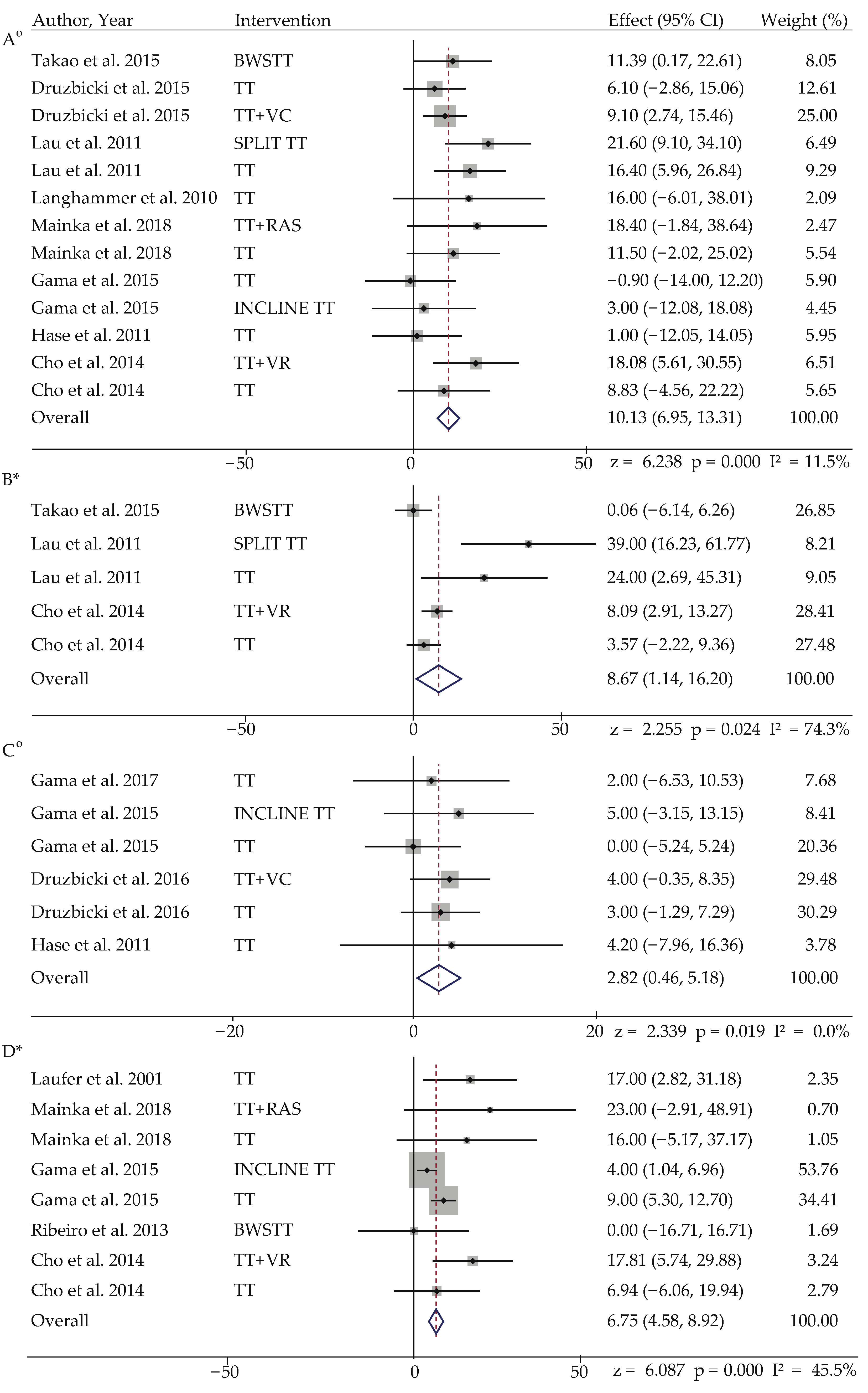
3.4.1. Meta-Analysis in Stroke
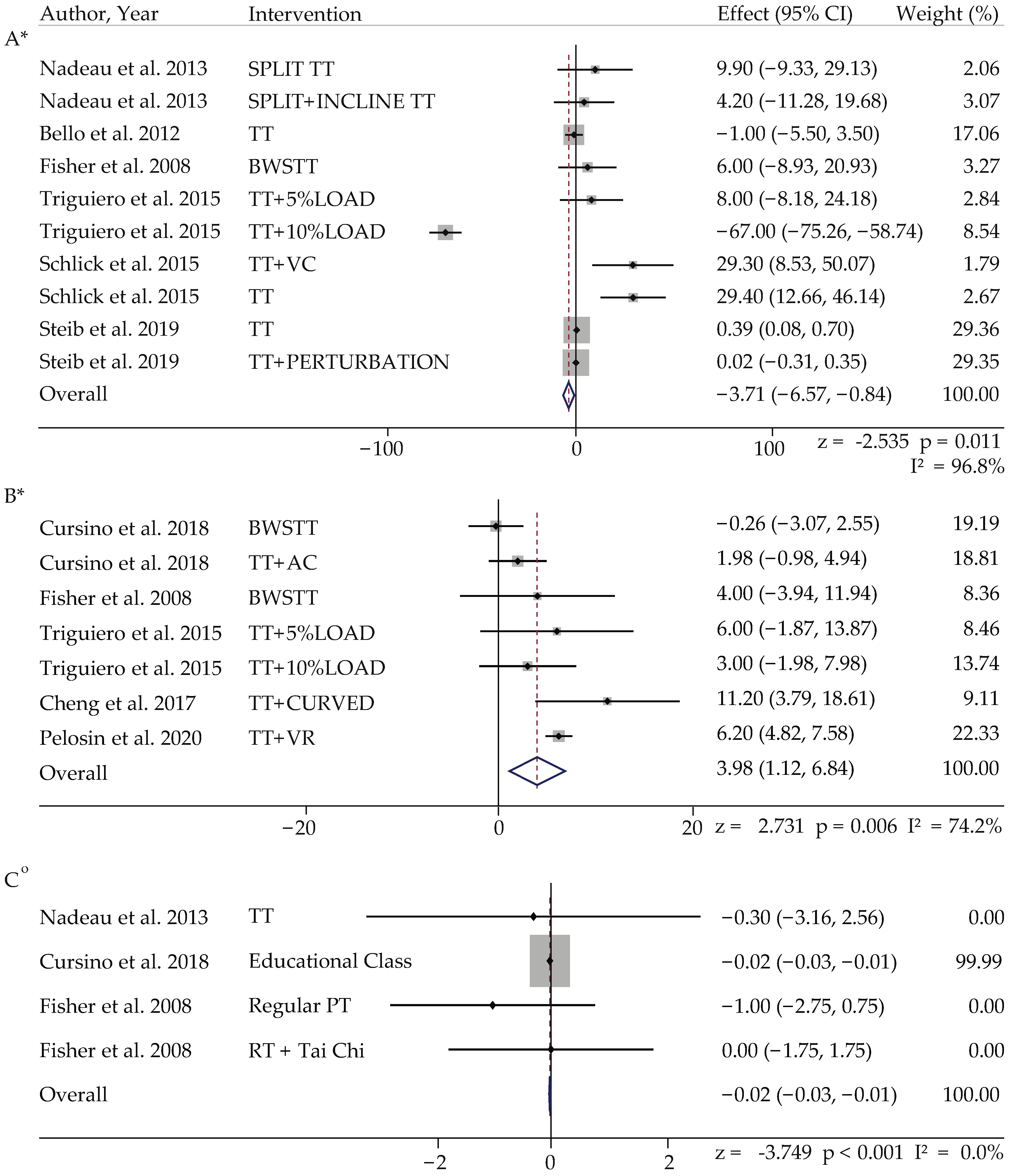
3.4.2. Meta-Analysis in PD
3.4.3. Meta-Analysis in Active Control of PD Population
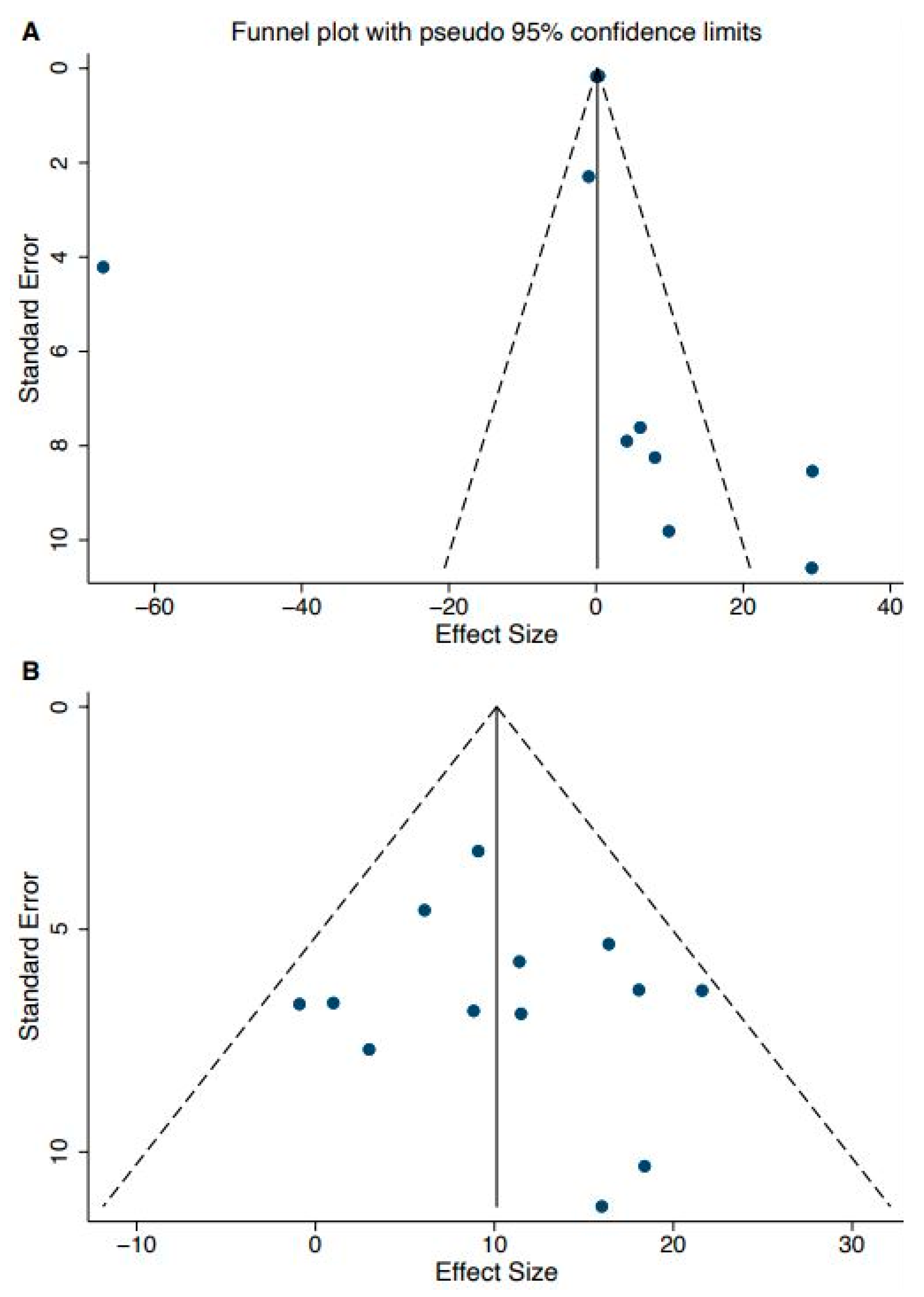
3.5. Publication Bias
3.6. Study Quality Assessment
4. Discussion
4.1. Pure Treadmill Training
4.2. Treadmill Training with an Incline or Speed-Dependent Treadmill Training
4.3. Treadmill Training with Sensory Feedback
4.4. Treadmill Training with Bodyweight Support
4.5. Other Treadmill training: Curved TT and Perturbation TT
4.6. Biomechanical and Physiological Mechanisms behind Impact of TT in Adults with PD and Stroke
4.7. Adverse Effects of Treadmill Training
4.8. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Search Algorithm for Each Database
| Database | Key Terms, Algorithm, and Number of Articles Extracted |
|---|---|
| PubMed/Medline (611) | ((walk OR ambulatory OR mobility OR gait) AND (variability OR complexity OR unsteadiness or inconsist* OR stability OR equilibrium OR dynamics OR balance OR ataxia) AND (“neurological disorder” OR “neurological pathology” OR “multiple sclerosis” OR “Parkinson’s disease” OR “Huntington’s disease” OR ALS OR “cerebellar ataxia” OR Alzheimer OR stroke) AND (intervention OR therapy OR treatment OR “best practices”) AND (older adults OR elderly OR aged OR elder OR older OR senior OR geriatric) NOT (musculoskeletal OR posture OR postural OR animal OR robot OR amputee OR trunk OR knee OR Hip OR freezing of gait OR spasticity OR heart OR blood OR cardiac)) Refined by: LANGUAGES: (ENGLISH) Refined by: AGES: (Middle Aged: 45–64; Aged: 65+ years) Refined by: PUBLICATION DATES: (From 1 January 2000 to 31 December 2021) Total articles extracted: 611 |
| CINAHL (125) | ((walk OR ambulatory OR mobility OR gait) AND (variability OR complexity OR unsteadiness or inconsist* OR stability OR equilibrium OR dynamics OR balance OR ataxia) AND (“neurological disorder” OR “neurological pathology” OR “multiple sclerosis” OR “Parkinson’s disease” OR “Huntington’s disease” OR ALS OR “cerebellar ataxia” OR Alzheimer) AND (intervention OR therapy OR treatment OR “best practices”) AND (older adults OR elderly OR aged OR elder OR older OR senior OR geriatric) NOT (posture OR postural OR animal OR robot OR amputee OR trunk OR knee OR Hip OR freezing of gait OR spasticity OR heart OR blood OR cardiac)) Total articles extracted: 125 |
| Scopus (732) | (walk OR ambulatory OR mobility OR gait OR locomot*) AND (variability OR complexity OR unsteadiness OR inconsist* OR stability OR equilibrium OR dynamics OR balance OR ataxia) AND ((“neurological disorder” OR “neurological pathology” OR “multiple sclerosis” OR “Parkinson’s disease” OR “Huntington’s disease” OR als OR “cerebellar ataxia” OR alzheimer)) AND ((intervention OR therapy OR treatment OR “best practices”)) AND ((older AND adults OR elderly OR aged OR elder OR older OR senior OR geriatric)) AND NOT ((posture OR postural OR animal OR robot OR amputee OR trunk OR knee OR hip OR freezing AND of AND gait OR spasticity OR heart OR blood OR cardiac OR depression)) AND (LIMIT-TO (LANGUAGE, “English”)) AND (LIMIT-TO (EXACTKEYWORD, “Gait Disorder”) OR LIMIT-TO (EXACTKEYWORD, “Elderly”) OR LIMIT-TO (EXACTKEYWORD, “Gait”)) AND (LIMIT- TO (PUBYEAR, 2021) OR LIMIT-TO (PUBYEAR, 2020) OR LIMIT- TO (PUBYEAR, 2019) OR LIMIT-TO (PUBYEAR, 2018) OR LIMIT- TO (PUBYEAR, 2017) OR LIMIT-TO (PUBYEAR, 2016) OR LIMIT- TO (PUBYEAR, 2015) OR LIMIT-TO (PUBYEAR, 2014) OR LIMIT- TO (PUBYEAR, 2013) OR LIMIT-TO (PUBYEAR, 2012) OR LIMIT- TO (PUBYEAR, 2011) OR LIMIT-TO (PUBYEAR, 2010) OR LIMIT- TO (PUBYEAR, 2009) OR LIMIT-TO (PUBYEAR, 2008) OR LIMIT- TO (PUBYEAR, 2007) OR LIMIT-TO (PUBYEAR, 2006) OR LIMIT- TO (PUBYEAR, 2005) OR LIMIT-TO (PUBYEAR, 2004) OR LIMIT- TO (PUBYEAR, 2003) OR LIMIT-TO (PUBYEAR, 2002) OR LIMIT- TO (PUBYEAR, 2001) OR LIMIT-TO (PUBYEAR, 2000)) AND (LIMIT- TO (PUBSTAGE, “final”)) AND (LIMIT-TO (DOCTYPE, “ar”)) Total articles extracted: 732 |
| Web of Science (314) | (TS = (walk OR locomot* OR ambulatory OR mobility OR gait) AND TS = (variability OR complexity OR unsteadiness or inconsist* OR stability OR equilibrium OR dynamics OR balance OR ataxia) AND TS = (“neurological disorder” OR “neurological pathology” OR “multiple sclerosis” OR “Parkinson’s disease” OR “Huntington’s disease” OR ALS OR “cerebellar ataxia” OR Alzheimer) AND TS = (intervention OR therapy OR treatment OR “best practices”) AND TS = (older adults OR elderly OR aged OR elder OR older OR senior OR geriatric) NOT TS = (posture OR postural OR dual task OR animal OR robot OR amputee OR trunk OR knee OR Hip OR freezing of gait OR spasticity OR heart OR blood OR cardiac OR depression)) AND LANGUAGE: (English) AND DOCUMENT TYPES: (Article) Timespan: 2000–2021. Indexes: SCI-EXPANDED. Total articles extracted: 314 |
Appendix B
| Study ID | Outcome Measure | Stroke Control | Stroke Intervention | ||
| Pre | Post | Pre | Post | ||
| 1 | Stride length | 0.72 ± 0.17 | 0.79 ± 0.18 | 0.82 ± 0.25 (VF) | 0.86 ± 0.28 (VF) |
| 0.60 ± 0.19 (AF) | 0.70 ± 0.23 (AF) | ||||
| Cadence | 157.0 ± 26.1 | 162.5 ± 29.4 | 162.5 ± 29.4 (VF) | 159.0 ± 23.3 (VF) | |
| 156.2 ± 29.8 (AF) | 161.5 ± 37.2 (AF) | ||||
| 2 | Step length | 38.31 ± 8.31 | 41.88 ± 7.86 | 40.1 ± 6.47 | 48.19 ± 7.92 |
| Stride length | 74.96 ± 18.31 | 81.90 ± 18.01 | 76.29 ± 14.99 | 94.10 ± 18.54 | |
| Cadence | 75.93 ± 18.13 | 84.76 ± 19.26 | 81.56 ± 19.88 | 99.64 ± 14.55 | |
| SL support % | 25.18 ± 5.86 | 28.09 ± 5.95 | 27.63 ± 5.27 | 33.14 ± 4.33 | |
| 3 | Cadence | 67.5 ± 15.5 | 73.6 ± 16.8 | 68.9 ± 10.6 | 78 ± 12.3 |
| 4 | Step length NP | 33 ± 7 | 32 ± 7 | 31 ± 7 | 29 ± 6 |
| Step length P | 25 ± 6 | 28 ± 6 | 24 ± 7 | 42.7 ± 14.5 | |
| 5 | Step length | 30 ± 9 | 33 ± 11 | 38 ± 10 | 41 ± 9 |
| Stride length | 67 ± 4 | 71 ± 4 | 76 ± 5 | 85 ± 5 | |
| Cadence | 70.1 ± 19.54 | 69.2 ± 15.61 | 67.2 ± 20.21 | 70.2 ± 20.51 | |
| 6 | Step length NP | 37 ± 13 | 39 ± 14 | 35 ± 11 | 39 ± 14 |
| Step length P | 39 ± 14 | 46 ± 11 | 40 ± 11 | 42 ± 12 | |
| SL support % NP | 36.0 | 37.6 ± 5.9 | 36.1 ± 4.6 | 36 ± 4.9 | |
| SL support % P | 26.0 ± 6.33 | 28.4 ± 4.0 | 25.7 ± 8.1 | 26.2 ± 7.6 | |
| 7 | Step length NP | 36.6 ± 15.8 | 38.8 ± 13.5 | 43.5 ± 10.3 | 45.3 ± 8.5 |
| Step length P | 38.5± 14.6 | 37 ± 8.1 | 38.5 ± 14.6 | 42.7 ± 14.5 | |
| Cadence | 93.1 ± 13 | 97.6 ± 15.1 | 90.7 ± 16.2 | 91.7 ± 15 | |
| SL support % NP | 30.5 ± 3.5 | 33.6 ± 3.6 | 31.1 ± 3.6 | 30.6 ± 5.1 | |
| SL support % P | 23 ± 6.9 | 26.9 ± 5 | 25.8 ± 7 | 25.5 ± 9.3 | |
| 8 | SL support % | 20 ± 6.5 | 22 ± 7 | 20 ± 5.6 | 20 ± 5.6 |
| 9 | Step length (right) | 97 ± 40 | 110 ± 40 | 110 ± 20 | 110 ± 30 |
| Step length (left) | 97 ± 40 | 92 ± 30 | 100 ± 20 | 110 ± 40 | |
| Step width | 11.3 ± 5.6 | 12.3 ± 5.3 | 7.2 ± 5.2 | 7.9 ± 5.3 | |
| Cadence | 99.3 ± 30.1 | 108.1 ± 35.1 | 81.6 ± 45.3 | 97.6 ± 24.2 | |
| 10 | Step length | 68 ± 24 | 92 ± 31 | 65 ± 23 | 104 ± 35 |
| Cadence | 39.5 ± 15 | 55.9 ± 12 | 36.9 ± 16.6 | 58.5 ± 15.9 | |
| 11 | Step length | 40 ± 16 | 47 ± 18 | 36 ± 14 | 53 ± 22 |
| 99 ± 12 (HOA) | |||||
| 12 | Step width | NR | 16.32 ± 3.2 | NR | 16.5 ± 2.9 |
| Stride length | NR | 75.6 ± 17.3 | NR | 70.9 ± 19.6 | |
| 13 | Step length | 96 ± 26 | 112 ± 29 | 99 ± 31 | 122 ± 31 |
| Cadence | 91.2 ± 19.6 | 102.7 ± 15.3 | 96.6 ± 25 | 115 ± 23.4 | |
| 14 | SL support % | NR | 8.8 ± 3.9 | NR | 2.8 ± 4.2 |
| 15 | Stride length | 70 ± 20 | 70 ± 10 | 80 ± 20 | 80 ± 20 |
| DL support (s) | 0.6 ± 0.2 | 0.6 ± 0.2 | 0.5 ± 0.2 | 0.5 ± 0.2 | |
| 16 | Step length NP | NR | 34.62 ± 8.30 | NR | 36.29 ± 10.45 (VF) |
| NR | 36.09 ± 10.15 (RAC) | ||||
| Step length P | NR | 31.50 ± 7.94 | NR | 33.23 ± 9.74 (VF) | |
| NR | 34.68 ± 9.35 (RAC) | ||||
| 17 | Step length NP (no SD) | 36 | 36 | 37 | 37 |
| Step length P (no SD) | 37 | 37 | 37 | 43 | |
| 18 | Step length | 50 ± 11 | 0.01 ± 0.04 | 50 ± 11 | 0.06 ± 0.06 * |
| Cadence | 94.4 ± 22.8 | 0.93 ± 6.10 | 108 ± 30.6 | 11.39 ± 18.10 * | |
| 19 | DL support (s) | 0.75 ± 0.53 | 0.60 ± 0.36 | 0.64 ± 0.38 | 0.51 ± 0.22 |
| Study ID | Outcome Measure | PD Control | PD Intervention | ||
| Pre | Post | Pre | Post | ||
| 20 | Stride length | 136 ± 5 | 135 ± 7 | 129 ± 3 | 128 ± 7 |
| Cadence | 118.8 ± 4.34 | 118.8 ± 4.7 | 117.6 ± 3.62 | 117.6 ± 4.34 | |
| 21 | Cadence | 95.7 ± 12.7 | 96.2 ± 13.4 | 91.6 ± 10.0 | 82.7 ± 10.8 |
| Step length | 38.0 ± 5.7 | 39.0 ± 7.6 | 38.3 ± 7.7 | 49.5 ± 10.6 | |
| 22 | Step length | 59.2 ± 5.24 | 64.68 ± 5.88 | 65.83 ± 2.5 (PBWS) | 65.57 ± 2.85 (PBWS) |
| 66.69 ± 2.73 (GAS) | 68.67 ± 2.91 (GAS) | ||||
| Step width | 0.11 ± 0.01 | 0.09 ± 0.01 | 0.06 ± 0.01 (PBWS) | 0.08 ± 0.01 (PBWS) | |
| 0.08 ± 0.01 (GAS) | 0.07 ± 0.01 (GAS) | ||||
| 23 | Step length | 71 ± 8 | 71 ± 11 | 73 ± 10 (HI) | 77 ± 8 (HI) |
| 68 ± 11 (LI) | 72 ± 7 (LI) | ||||
| Step width | 12 ± 2 | 11 ± 2 | 11 ± 2 (HI) | 11 ± 2 (HI) | |
| 10 ± 2 (LI) | 10 ± 2 (LI) | ||||
| Stride length | 137 ± 23 | 141 ± 23 | 148 ± 18 (HI) | 154 ± 16 (HI) | |
| 143 ± 15 (LI) | 144 ± 14 (LI) | ||||
| Cadence | 120.33 ± 9.26 | 121.09 ± 8.6 | 120.66 ± 10.4 (HI) | 120.85 ± 8.5 (HI) | |
| 120.57 ± 11.6 (LI) | 118.94 ± 10.2 m(LI) | ||||
| DL support % | 24.04 ± 6.17 | 21.22 ± 4.03 | 21.2 ± 3.35 (HI) | 19.68 ± 2.58 (HI) | |
| 19.53 ± 4.49 (LI) | 19.87 ± 3.58 (LI) | ||||
| 24 | Stride cycle (cycle/s) | 0.6 ± 0.1 | 0.7 ± 0.1 | 0.6 ± 0.2 | 0.8 ± 0.2 |
| 25 ** | Stride length | 4.25 | 4.04 | 4.44 | 3.8 |
| Cadence | 3.15 | 3.28 | 3.59 | 2.97 | |
| DL support % | 5.10 | 4.81 | 5.07 | 4.76 | |
| 26 | Cadence (steps/10 m) | 22.8 ± 2.2 | 22.7 ± 2.0 | 23.4 ± 2.3 | 20 ± 2.1 |
| 27 | Step width | 8.1 ± 3.8 | 7.8 ± 3.0 | 8.8 ± 3 (STT) | 8.7 ± 2.8 (STT) |
| 8.6 ± 3.9 (MTT) | 8.2 ± 3.9 (MTT) | ||||
| Stride length | 133 ± 19.4 | 135 ± 20.8 | 111.9 ± 25.9 (STT) | 121.8 ± 22 (STT) | |
| 136.4 ± 17.6 (MTT) | 140.6 ± 19.4 (MTT) | ||||
| Cadence | 113.7 ± 4.6 | 115.1 ± 4.8 | 109.1 ± 13.7 (STT) | 110.2 ± 16 (STT) | |
| 108.2 ± 8.7 (MTT) | 111.2 ± 6.2 (MTT) | ||||
| SL support % | 37.9 ± 1.8 | 38.2 ± 1.8 | 37.4 ± 3.1 (STT) | 38.4 ± 2.4 (STT) | |
| 37.5 ± 1.3 (MTT) | 38.2 ± 1.2 (MTT) | ||||
| DL support % | 24.4 ± 3.5 | 23.8 ± 3.4 | 25.5 ± 6.2 (STT) | 23.4 ± 4.6 (STT) | |
| 25.1 ± 2.7 (MTT) | 23.7 ± 2.5 (MTT) | ||||
| 28 | Stride length (right) | 60.2 ± 13.3 | 60.4 ± 10.0 | 66.5 ± 13.7 | 71.1 ± 14.4 |
| Stride length (left) | 61.0 ± 15.4 | 60.8 ± 10.9 | 68.7 ± 14.9 | 72.9 ± 17.0 | |
| Cadence | 117.7 ± 13.0 | 124.3 ± 15.1 | 112.8 ± 7.2 | 120.3 ± 8.2 | |
| 29 | Stride length | 75.1 ± 18.2 | 104.5 ± 21.7 | 61.1 ± 29.6 | 90.4 ± 21.7 |
| Cadence | 107.4 ± 21.8 | 110 ± 13.2 | 99.9 ± 28.1 | 95.4 ± 10.9 | |
| 30 * | Step length | 0 | 1.15 | 0 | −1.85 |
| DL support % | 0 | 0.10 | 0 | 0.68 | |
| 31 | Step length | 56 ± 4 | 57 ± 6 | 45 ± 9 (5% load) | 51 ± 8 (5% load) |
| 58 ± 3 (10% load) | 61 ± 7 (10% load) | ||||
| Stride length | 111 ± 11 | 115 ± 9 | 93 ± 18 (5% load) | 101 ± 17 (5% load) | |
| 191 ± 4 (10% load) | 124 ± 12 (10% load) | ||||
| 32 | Step length | ||||
References
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.; Sung, J.; An, R.; Hernandez, M.E.; Sosnoff, J. Gait variability in people with neurological disorders: A systematic review and meta-analysis. Hum. Mov. Sci. 2016, 47, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Warburton, D.E.R.; Nicol, C.W.; Bredin, S.S.D. Health benefits of physical activity: The evidence. Can. Med. Assoc. J. 2006, 174, 801–809. [Google Scholar] [CrossRef] [Green Version]
- Eng, J.J.; Tang, P.-F. Gait training strategies to optimize walking ability in people with stroke: A synthesis of the evidence. Expert Rev. Neurother. 2007, 7, 1417–1436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gelaw, A.Y.; Janakiraman, B.; Teshome, A.; Ravichandran, H. Effectiveness of treadmill assisted gait training in stroke survivors: A systematic review and meta-analysis. Glob. Epidemiol. 2019, 1, 100012. [Google Scholar] [CrossRef]
- Latham, N.K.; Jette, D.U.; Slavin, M.; Richards, L.G.; Procino, A.; Smout, R.J.; Horn, S.D. Physical Therapy During Stroke Rehabilitation for People with Different Walking Abilities. Arch. Phys. Med. Rehabil. 2005, 86, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Peurala, S.; Karttunen, A.; Sjogren, T.; Paltamaa, J.; Heinonen, A. Evidence for the effectiveness of walking training on walking and self-care after stroke: A systematic review and meta-analysis of randomized controlled trials. J. Rehabil. Med. 2014, 46, 387–399. [Google Scholar] [CrossRef] [Green Version]
- Hausdorff, J.M. Gait dynamics in Parkinson’s disease: Common and distinct behavior among stride length, gait variability, and fractal-like scaling. Chaos Interdiscip. J. Nonlinear Sci. 2009, 19, 026113. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.M.; Kim, D.H.; Yang, Y.; Ha, S.W.; Han, J.H. Gait Patterns in Parkinson’s Disease with or without Cognitive Impairment. Dement. Neurocogn. Disord. 2018, 17, 57–65. [Google Scholar] [CrossRef]
- Polese, J.C.; Ada, L.; Dean, C.; Nascimento, L.R.; Teixeira-Salmela, L.F. Treadmill training is effective for ambulatory adults with stroke: A systematic review. J. Physiother. 2013, 59, 73–80. [Google Scholar] [CrossRef] [Green Version]
- Meder, K.G.; LoJacono, C.T.; Rhea, C.K. A Systematic Review of Non-Pharmacological Interventions to Improve Gait Asymmetries in Neurological Populations. Symmetry 2022, 14, 281. [Google Scholar] [CrossRef]
- Luo, L.; Zhu, S.; Shi, L.; Wang, P.; Li, M.; Yuan, S. High Intensity Exercise for Walking Competency in Individuals with Stroke: A Systematic Review and Meta-Analysis. J. Stroke Cerebrovasc. Dis. 2019, 28, 104414. [Google Scholar] [CrossRef]
- NIH Quality Assessment Tool. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 11 February 2020).
- Brasileiro, A.; Gama, G.; Trigueiro, L.; Ribeiro, T.; Silva, E.; Galvão, E.; Lindquist, A. Influence of visual and auditory biofeedback on partial body weight support treadmill training of individuals with chronic hemiparesis: A randomized controlled clinical trial. Eur. J. Phys. Rehabil. Med. 2015, 51, 49–58. [Google Scholar]
- Cho, K.H.; Lee, W.H. Effect of treadmill training based real-world video recording on balance and gait in chronic stroke patients: A randomized controlled trial. Gait Posture 2014, 39, 523–528. [Google Scholar] [CrossRef]
- Drużbicki, M.; Guzik, A.; Przysada, G.; Kwolek, A.; Brzozowska-Magoń, A. Efficacy of gait training using a treadmill with and without visual biofeedback in patients after stroke: A randomized study. J. Rehabil. Med. 2015, 47, 419–425. [Google Scholar] [CrossRef] [Green Version]
- Drużbicki, M.; Guzik, A.; Przysada, G.; Kwolek, A.; Brzozowska-Magon, A.; Sobolewski, M. Changes in Gait Symmetry After Training on a Treadmill with Biofeedback in Chronic Stroke Patients: A 6-Month Follow-Up from a Randomized Controlled Trial. Med. Sci. Monit. 2016, 22, 4859–4868. [Google Scholar] [CrossRef] [Green Version]
- Gama, G.L.; de Lucena Trigueiro, L.C.; Simão, C.R.; de Sousa, A.V.C.; de Souza e Silva, E.M.G.; Galvão, E.R.V.P.; Rodrigues Lindquist, A.R. Effects of Treadmill Inclination on Hemiparetic Gait. Am. J. Phys. Med. Rehabil. 2015, 94, 718–727. [Google Scholar] [CrossRef]
- Gama, G.L.; Celestino, M.; Barela, J.A.; Forrester, L.; Whitall, J.; Barela, A. Effects of Gait Training with Body Weight Support on a Treadmill Versus Overground in Individuals with Stroke. Arch. Phys. Med. Rehabil. 2017, 98, 738–745. [Google Scholar] [CrossRef] [Green Version]
- Hase, K.; Suzuki, E.; Matsumoto, M.; Fujiwara, T.; Liu, M. Effects of Therapeutic Gait Training Using a Prosthesis and a Treadmill for Ambulatory Patients with Hemiparesis. Arch. Phys. Med. Rehabil. 2011, 92, 1961–1966. [Google Scholar] [CrossRef]
- Hornby, T.G.; Campbell, D.D.; Kahn, J.H.; DeMott, T.; Moore, J.L.; Roth, H.R. Enhanced Gait-Related Improvements After Therapist- Versus Robotic-Assisted Locomotor Training in Subjects with Chronic Stroke. Stroke 2008, 39, 1786–1792. [Google Scholar] [CrossRef]
- Langhammer, B.; Stanghelle, J.K. Exercise on a treadmill or walking outdoors? A randomized controlled trial comparing effectiveness of two walking exercise programmes late after stroke. Clin. Rehabil. 2010, 24, 46–54. [Google Scholar] [CrossRef]
- Lau, K.W.K.; Mak, K.Y.M. Speed-dependent treadmill training is effective to improve gait and balance performance in patients with sub-acute stroke. J. Rehabil. Med. 2011, 43, 709–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laufer, Y.; Dickstein, R.; Chefez, Y.; Marcovitz, E. The effect of treadmill training on the ambulation of stroke survivors in the early stages of rehabilitation: A randomized study. J. Rehabil. Res. Dev. 2001, 38, 69–78. [Google Scholar] [PubMed]
- Lura, D.J.; Venglar, M.C.; Van Duijn, A.J.; Csavina, K.R. Body weight supported treadmill vs. overground gait training for acute stroke gait rehabilitation. Int. J. Rehabil. Res. 2019, 42, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Mainka, S.; Wissel, J.; Völler, H.; Evers, S. The Use of Rhythmic Auditory Stimulation to Optimize Treadmill Training for Stroke Patients: A Randomized Controlled Trial. Front. Neurol. 2018, 9, 755. [Google Scholar] [CrossRef] [PubMed]
- McCain, K.J.; Pollo, F.E.; Baum, B.; Coleman, S.C.; Baker, S.; Smith, P.S. Locomotor Treadmill Training with Partial Body-Weight Support Before Overground Gait in Adults with Acute Stroke: A Pilot Study. Arch. Phys. Med. Rehabil. 2008, 89, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, T.; Britto, H.; Oliveira, D.; Silva, E.; Galvão, E.; Lindquist, A. Effects of treadmill training with partial body weight support and the proprioceptive neuromuscular facilitation method on hemiparetic gait: A randomized controlled study. Eur. J. Phys. Rehabil. Med. 2013, 49, 451–461. [Google Scholar]
- Ribeiro, T.S.; Silva, E.M.; Silva, I.A.; Costa, M.F.; Cavalcanti, F.A.; Lindquist, A.R. Effects of treadmill training with load addition on non-paretic lower limb on gait parameters after stroke: A randomized controlled clinical trial. Gait Posture 2017, 54, 229–235. [Google Scholar] [CrossRef]
- Shin, J.; Chung, Y. Influence of visual feedback and rhythmic auditory cue on walking of chronic stroke patient induced by treadmill walking in real-time basis. NeuroRehabilitation 2017, 41, 445–452. [Google Scholar] [CrossRef]
- Takao, T.; Tanaka, N.; Iizuka, N.; Saitou, H.; Tamaoka, A.; Yanagi, H. Improvement of gait ability with a short-term intensive gait rehabilitation program using body weight support treadmill training in community dwelling chronic poststroke survivors. J. Phys. Ther. Sci. 2015, 27, 159–163. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, T.S.; Gomes de Souza e Silva, E.A.; Regalado, I.C.R.; Silva, S.T.da.P.; de Oliviera Sousa, C.; Ribeiro Bezerra de Figueiredo, K.M.O.; Lindquist Rodrigues, A.R. Effects of Load Addition during Gait Training on Weight-Bearing and Temporal Asymmetry after Stroke: A Randomized Clinical Trial. Am. J. Phys. Med. Rehabil. 2020, 99, 250–256. [Google Scholar] [CrossRef]
- Bello, O.; Sanchez, J.A.; Lopez-Alonso, V.; Márquez, G.; Morenilla-Burló, L.; Castro, X.; Giráldez-García, M.A.; Santos-García, D.; del Olmo, M.F. The effects of treadmill or overground walking training program on gait in Parkinson’s disease. Gait Posture 2013, 38, 590–595. [Google Scholar] [CrossRef] [Green Version]
- Cheng, F.-Y.; Yang, Y.-R.; Wu, Y.-R.; Cheng, S.-J.; Wang, R.-Y. Effects of curved-walking training on curved-walking performance and freezing of gait in individuals with Parkinson’s disease: A randomized controlled trial. Park. Relat. Disord. 2017, 43, 20–26. [Google Scholar] [CrossRef]
- Cursino, M.P.; Raquel, D.F.; Hallal, C.Z.; Faganello-Navega, F.R. Kinematic variables of gait and quality of life in Parkinsonians after different treadmill trainings: A randomized control trial. Motricidade 2018, 14, 29–39. [Google Scholar] [CrossRef] [Green Version]
- Fisher, B.E.; Wu, A.D.; Salem, G.J.; Song, J.; Janice Lin, C.-H.; Yip, J.; Cen, S.; Gordon, J.; Jakowec, M.; Petzinger, G. The Effect of Exercise Training in Improving Motor Performance and Corticomotor Excitability in People with Early Parkinson’s Disease. Arch. Phys. Med. Rehabil. 2008, 89, 1221–1229. [Google Scholar] [CrossRef] [Green Version]
- Frazzitta, G.; Maestri, R.; Uccellini, D.; Bertotti, G.; Abelli, P. Rehabilitation treatment of gait in patients with Parkinson’s disease with freezing: A comparison between two physical therapy protocols using visual and auditory cues with or without treadmill training. Mov. Disord. 2009, 24, 1139–1143. [Google Scholar] [CrossRef]
- Klamroth, S.; Steib, S.; Gaßner, H.; Goßler, J.; Winkler, J.; Eskofier, B.; Klucken, J.; Pfeifer, K. Immediate effects of perturbation treadmill training on gait and postural control in patients with Parkinson’s disease. Gait Posture 2016, 50, 102–108. [Google Scholar] [CrossRef]
- Miyai, I.; Fujimoto, Y.; Yamamoto, H.; Ueda, Y.; Saito, T.; Nozaki, S.; Kang, J. Long-term effect of body weight–supported treadmill training in Parkinson’s disease: A randomized controlled trial. Arch. Phys. Med. Rehabil. 2002, 83, 1370–1373. [Google Scholar] [CrossRef]
- Nadeau, A.; Pourcher, E.; Corbeil, P. Effects of 24 wk of Treadmill Training on Gait Performance in Parkinson’s Disease. Med. Sci. Sports Exerc. 2014, 46, 645–655. [Google Scholar] [CrossRef]
- Protas, E.J.; Mitchell, K.; Williams, A.; Qureshy, H.; Caroline, K.; Lai, E.C. Gait and step training to reduce falls in Parkinson’s disease. NeuroRehabilitation 2005, 20, 183–190. [Google Scholar] [CrossRef] [Green Version]
- Schlick, C.; Ernst, A.; Bötzel, K.; Plate, A.; Pelykh, O.; Ilmberger, J. Visual cues combined with treadmill training to improve gait performance in Parkinson’s disease: A pilot randomized controlled trial. Clin. Rehabil. 2016, 30, 463–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steib, S.; Klamroth, S.; Gaßner, H.; Pasluosta, C.; Eskofier, B.; Winkler, J.; Klucken, J.; Pfeifer, K. Exploring gait adaptations to perturbed and conventional treadmill training in Parkinson’s disease: Time-course, sustainability, and transfer. Hum. Mov. Sci. 2019, 64, 123–132. [Google Scholar] [CrossRef] [PubMed]
- de Lucena Trigueiro, L.C.; Lopes Gama, G.; Simão, C.R.; de Sousa, A.V.C.; de Oliviera Godeiro Júnior, C.; Rodrigues Lindquist, A.R. Effects of Treadmill Training with Load on Gait in Parkinson Disease. Am. J. Phys. Med. Rehabil. 2015, 94, 830–837. [Google Scholar] [CrossRef] [PubMed]
- Pelosin, E.; Cerulli, C.; Ogliastro, C.; LaGravinese, G.; Mori, L.; Bonassi, G.; Mirelman, A.; Hausdorff, J.M.; Abbruzzese, G.; Marchese, R.; et al. A multimodal training modulates short-afferent inhibition and improves complex walking in a cohort of faller older adults with an increased prevalence of Parkinson’s disease. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.G.; Dennett, A.M.; Snowdon, D.A. Treadmill training may be an effective form of task-specific training for improving mobility in people with Parkinson’s disease and multiple sclerosis: A systematic review and meta-analysis. Physiotherapy 2019, 105, 174–186. [Google Scholar] [CrossRef]
- Baram, Y. Virtual Sensory Feedback for Gait Improvement in Neurological Patients. Front. Neurol. 2013, 4, 138. [Google Scholar] [CrossRef] [Green Version]
- Luna, N.M.S.; Brech, G.C.; Canonica, A.; Ernandes, R.D.C.; Bocalini, D.S.; Greve, J.M.D.; Alonso, A.C. Effects of treadmill training on gait of elders with Parkinson’s disease: A literature review. Einstein 2020, 18, eRW5233. [Google Scholar] [CrossRef]
- Apte, S.; Plooij, M.; Vallery, H. Influence of body weight unloading on human gait characteristics: A systematic review. J. Neuroeng. Rehabil. 2018, 15, 53. [Google Scholar] [CrossRef] [Green Version]
- Lamontagne, A.; Fung, J.; McFadyen, B.J.; Faubert, J. Modulation of walking speed by changing optic flow in persons with stroke. J. Neuroeng. Rehabil. 2007, 4, 22. [Google Scholar] [CrossRef] [Green Version]
- Barbieri, A.F.; Vitório, R. Locomotion and Posture in Older Adults; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Mahoney, J.R.; Verghese, J. Visual-Somatosensory Integration and Quantitative Gait Performance in Aging. Front. Aging Neurosci. 2018, 10, 377. [Google Scholar] [CrossRef]
- Mahoney, J.R.; Cotton, K.; Verghese, J. Multisensory Integration Predicts Balance and Falls in Older Adults. J. Gerontol. A 2019, 74, 1429–1435. [Google Scholar] [CrossRef] [PubMed]
- Arfa-Fatollahkhani, P.; Safar Cherati, A.; Habibi, S.A.H.; Shahidi, G.A.; Sohrabi, A.; Zamani, B. Effects of treadmill training on the balance, functional capacity and quality of life in Parkinson’s disease: A randomized clinical trial. J. Complement. Integr. Med. 2019, 17, 20180245. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-del-Olmo, M.A.; Sanchez, J.A.; Bello, O.; Lopez-Alonso, V.; Marquez, G.; Morenilla, L.; Castro, X.; Giraldez, M.; Santos-Garcia, D. Treadmill training improves overground walking economy in Parkinson’s disease: A randomized, controlled pilot study. Front. Neurol. 2014, 5, 191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, F.-Y.; Yang, Y.-R.; Chen, L.-M.; Wu, Y.-R.; Cheng, S.-J.; Wang, R.-Y. Positive Effects of Specific Exercise and Novel Turning-based Treadmill Training on Turning Performance in Individuals with Parkinson’s disease: A Randomized Controlled Trial. Sci. Rep. 2016, 6, 33242. [Google Scholar] [CrossRef] [Green Version]
- Małczyńska-Sims, P.; Chalimoniuk, M.; Sułek, A. The Effect of Endurance Training on Brain-Derived Neurotrophic Factor and Inflammatory Markers in Healthy People and Parkinson’s Disease. A Narrative Review. Front. Physiol. 2020, 11, 578981. [Google Scholar] [CrossRef]
- Xiao, X.; Huang, D.; O’Young, B. Gait improvement after treadmill training in ischemic stroke survivors: A critical review of functional MRI studies. Neural Regen. Res. 2012, 7, 2457–2464. [Google Scholar] [CrossRef]
- Macko, R.F.; Smith, G.V.; Dobrovolny, C.; Sorkin, J.D.; Goldberg, A.P.; Silver, K.H. Treadmill training improves fitness reserve in chronic stroke patients. Arch. Phys. Med. Rehabil. 2001, 82, 879–884. [Google Scholar] [CrossRef] [Green Version]
- Acheampong, I.K.; Moses, M.O.; Baffour-Awuah, B.; Essaw, E.; Mensah, W.; Afrifa, D.; Owusu, L. Effectiveness of combined and conventional exercise trainings on the biochemical responses of stroke patients. J. Exerc. Rehabil. 2018, 14, 473–480. [Google Scholar] [CrossRef] [Green Version]
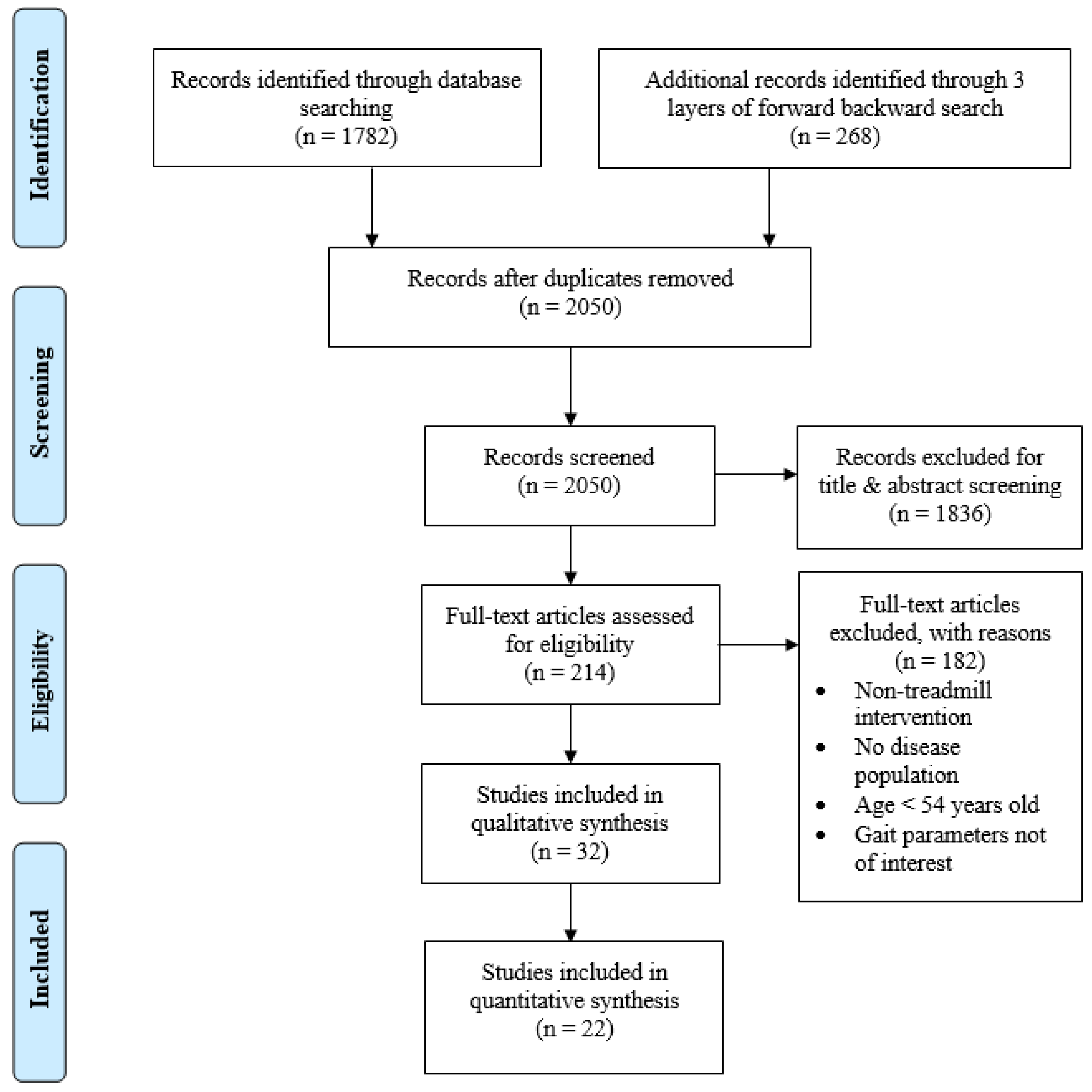
| Study ID | Author (Year) | Age (Mean ± SD (y)) | Sample Size | |||
|---|---|---|---|---|---|---|
| Stroke | ||||||
| Int | Control | Int | Control | Type | ||
| 1 | Brasileiro et al. (2015) [14] | 52.4 ± 5.9 (Exp 1) 58.8 ± 7.9 (Exp 2) | 57.9 ± 4.9 | 10 10 | 10 | C, H and I |
| 2 | Cho et al. (2014) † [15] | 65.86 ± 5.73 | 63.53 ± 5.54 | 30 | 15 | C |
| 3 | Druzbicki et al. (2015) † [16] | 59.8 ± 11.7 | 61.9 ± 11.4 | 25 | 25 | C, I |
| 4 | Druzbicki et al. (2016) [17] | 61.9 ± 11.4 | 59.8 ± 11.7 | 15 | 15 | NR |
| 5 | Gama et al. (2015) † [18] | 52.92 ± 9.51 | 57.64 ± 8.15 | 14 | 14 | C, H and I |
| 6 | Gama et al. (2017) † [19] | 58.7 ± 8.4 | 57.7 ± 10.1 | 14 | 14 | C, H and I |
| 7 | Hase et al. (2011) † [20] | 62.3 ± 9.2 | 60.1 ± 13.0 | 11 | 11 | C, H and I |
| 8 | Hornby et al. (2008) [21] | 57 ± 10 | 57 ± 11 | 24 | 24 | C, H and I |
| 9 | Langhammer et al. (2010) † [22] | 74 ± 13.3 | 75 ± 10.4 | 18 | 16 | NR |
| 10 | Lau et al. (2011) † [23] | 69.5 ± 11.1 | 72.1 ± 9.2 | 13 | 13 | SA |
| 11 | Laufer et al. (2001) † [24] | 66.6 ± 7.2 | 69.3 ± 8.1 68 ± 7.6 (HOA) | 13 | 12 8 | SA, H and I |
| 12 | Lura et al. (2019) [25] | 63.8 ± 10.8 | 60.4 ± 16.1 | 18 | 20 | A |
| 13 | Mainka et al. (2018) † [26] | 65.6 ± 8.5 | 61.1 ± 8.6 | 13 | 11 | NR |
| 14 | McCain et al. (2008) [27] | 57.0 ± 17.6 | 61.6 ± 8.2 | 7 | 7 | A, H and I |
| 15 | Ribeiro et al. (2013) † [28] | 56.45 ± 8.31 | 58.33 ± 8.94 | 11 | 9 | C |
| 16 | Ribeiro et al. (2017) [29] | 57.0 | 60.0 | 19 | 19 | SA, H and I |
| 17 | Shin et al. (2017) [30] | 58.06 ± 6.00 | 58.06 ± 6.00 * | 17 | 17 * | C, H and I |
| 18 | Takao et al. (2015) † [31] | 59.1 ± 12.5 | 59.8 ± 6.3 | 10 | 8 | C, H and I |
| 19 | Ribeiro et al. (2020) [32] | 57.5 ± 11 | 60.0 ± 19 | 19 | 19 | C, H and I |
| Parkinson’s Disease | ||||||
| Int | Control | Int | Control | H and Y | ||
| 20 | Bello et al. (2012) † [33] | 59.45 ± 11.32 | 58 ± 9.38 | 11 | 11 | 1–3 |
| 21 | Cheng et al. (2017) † [34] | 65.8 ± 11.5 | 67.3 ± 6.4 | 12 | 12 | 1–2 |
| 22 | Cursino et al. (2018) † [35] | 63.29 ± 11.06 | 72 ± 10.52 | 7 | 7 | 1–3 |
| 23 | Fisher et al. (2008) † [36] | 64 ± 14.5 | 63.1 ± 11.5 | 10 | 10 | 1–2 |
| 24 | Frazzitta et al. (2009) [37] | 71 ± 8 | 71 ± 7 | 20 | 20 | 3 |
| 25 | Klamroth et al. (2016) [38] | 64.8 ± 10.3 | 64.2 ± 8.5 | 19 | 20 | 1–3.5 |
| 26 | Miyai et al. (2002) † [39] | 69.5 ± 1.9 | 69.8 ± 1.5 | 11 | 9 | 2.5–3 |
| 27 | Nadeau et al. (2013) † [40] | 64.0 ± 6.6 (Speed) 60.1 ± 6.8 (Mixed) | 64.3 ± 5.6 | 12 11 | 11 | 1–2 |
| 28 | Protas et al. (2005) † [41] | 71.3 ± 7.4 | 73.7 ± 8.5 | 9 | 9 | 2–3 |
| 29 | Schlick et al. (2015) † [42] | 71.2 ± 10.9 | 68.9 ± 6.8 | 6 | 7 | 2–4 |
| 30 | Steib et al. (2019) † [43] | 67.8 ± 8.2 | 62.5 ± 7.9 | 18 | 20 | 1–3.5 |
| 31 | Trigueiro et al. (2015) † [44] | 61.44 ± 11.91 (5% weight) 63.44 ± 8.79 (10% weight) | 61.89 ± 6.79 | 9 9 | 9 | 2–3 |
| 32 | Pelosin et al. (2020) [45] | 73.2 ± 3.6 | 71.9 ± 4.1 | 17 | 22 | 2–3 |
| Stroke | |||||
|---|---|---|---|---|---|
| Author, Year | Gait Parameter | Intervention | Effect Size (z), Overall Effect (95% CI) | I2 (%) | Weightage |
| Cho et al. 2014 [15] | Cadence | TT + VR | 6.238 *** 10.128 (6.946 to 13.310) | 11.5 | 6.51 |
| Cho et al. 2014 [15] | TT | 5.65 | |||
| Druzbicki et al. 2015 [16] | TT | 12.61 | |||
| Druzbicki et al. 2015 [16] | TT + VC | 25.00 ^ | |||
| Gama et al. 2015 [18] | TT | 5.90 | |||
| Gama et al. 2015 [18] | IncTT | 4.45 | |||
| Hase et al. 2011 [20] | TT | 5.95 | |||
| Langhammer et al. 2010 [22] | TT | 2.09 | |||
| Lau et al. 2011 [23] | SpTT | 6.49 | |||
| Lau et al. 2011 [23] | TT | 9.29 | |||
| Mainka et al. 2018 [26] | TT + RAS | 2.47 | |||
| Mainka et al. 2018 [26] | TT | 5.54 | |||
| Takao et al. 2015 [31] | BWSTT | 8.05 | |||
| Cho et al. 2014 [15] | Step Length | TT + VR | 2.255 * 8.670 (1.136 to 16.203) | 74.3 | 28.41 ^ |
| Cho et al. 2014 [15] | TT | 27.48 | |||
| Lau et al. 2011 [23] | SpTT | 8.21 | |||
| Lau et al. 2011 [23] | TT | 9.05 | |||
| Takao et al. 2015 [31] | BWSTT | 26.85 | |||
| Druzbicki et al. 2015 [16] | Step Length (Paretic) | TT + VC | 2.339 * 2.821 (0.457 to 5.184) | 0 | 29.48 |
| Druzbicki et al. 2015 [16] | TT | 30.29 ^ | |||
| Gama et al. 2015 [18] | IncTT | 8.41 | |||
| Gama et al. 2015 [18] | TT | 20.36 | |||
| Gama et al. 2017 [19] | TT | 7.68 | |||
| Hase et al. 2011 [20] | TT | 3.78 | |||
| Druzbicki et al. 2015 [16] | Step Length (Non-Paretic) | TT + VC | 0.291 0.381 (−2.188 to 2.950) | 0 | 30.32 ^ |
| Druzbicki et al. 2015 [16] | TT | 26.30 | |||
| Gama et al. 2015 [18] | IncTT | 13.29 | |||
| Gama et al. 2015 [18] | TT | 11.91 | |||
| Gama et al. 2017 [19] | TT | 7.59 | |||
| Hase et al. 2011 [20] | TT | 10.60 | |||
| Cho et al. 2014 [15] | Stride Length | TT + VR | 6.087 *** 6.748 (4.575 to 8.921) | 45.5 | 3.24 |
| Cho et al. 2014 [15] | TT | 2.79 | |||
| Gama et al. 2015 [18] | IncTT | 53.76 ^ | |||
| Gama et al. 2015 [18] | TT | 34.41 | |||
| Laufer et al. 2001 [24] | TT | 2.35 | |||
| Mainka et al. 2018 [26] | TT + RAS | 0.70 | |||
| Mainka et al. 2018 [26] | TT | 1.05 | |||
| Ribeiro et al. 2013 [32] | BWSTT | 1.69 | |||
| Hase et al. 2011 [20] | Control—Cadence | Prosth Walking | 0.900 3.658 (−4.307 to 11.623) | 0 | 45.76 ^ |
| Langhammer et al. 2010 [22] | Ground Walking | 30.16 | |||
| Takao et al. 2015 [31] | No Change | 40.33 | |||
| Parkinson’s Disease (PD) | |||||
|---|---|---|---|---|---|
| Author, Year | Gait Parameter | Intervention | Effect Size(z), Overall Effect (95% CI) | I2 (%) | Weightage |
| Bello et al. 2012 [33] | Cadence | TT | 0.991 2.051 (−2.005 to 6.107) | 59.9 | 18.67 ^ |
| Cheng et al. 2017 [34] | TT + (C path) | 11.25 | |||
| Fisher et al. 2008 [36] | BWSTT | 11.25 | |||
| Miyai et al. 2002 [39] | BWSTPertuT | 14.46 | |||
| Nadeau et al. 2013 [40] | SpTT | 7.51 | |||
| Nadeau et al. 2013 [40] | SpTT + IncTT | 14.09 | |||
| Protas et al. 2005 [41] | TT | 12.88 | |||
| Schlick et al. 2015 [42] | TT + VC | 4.47 | |||
| Schlick et al. 2015 [42] | TT | 5.42 | |||
| Cursino et al. 2018 [35] | Step Width | BWSTT | 0.367 0.005 (−0.022 to 0.031) | 74.7 | 49.98 ^ |
| Cursino et al. 2018 [35] | TT + AC | 49.98 ^ | |||
| Fisher et al. 2008 [36] | BWSTT | 0.02 | |||
| Nadeau et al. 2013 [40] | SpTT | 0.01 | |||
| Nadeau et al. 2013 [40] | SpTT + IncTT | 0.01 | |||
| Cheng et al. 2017 [34] | Step Length | TT + (C path) | 2.731 ** 3.982 (1.124 to 6.839) | 74.2 | 9.11 |
| Cursino et al. 2018 [35] | BWSTT | 19.19 | |||
| Cursino et al. 2018 [35] | TT + AC | 18.81 | |||
| Fisher et al. 2008 [36] | BWSTT | 8.36 | |||
| Trigueiro et al. 2015 [44] | TT + 5% L | 8.46 | |||
| Trigueiro et al. 2015 [44] | TT + 10% L | 13.74 | |||
| Pelosin et al. 2020 [45] | TT + VR | 22.33 ^ | |||
| Bello et al. 2012 [33] | Stride Length | TT | −2.535 * −3.706 (−6.571 to −0.841) | 96.8 | 17.06 |
| Fisher et al. 2008 [36] | BWSTT | 3.27 | |||
| Nadeau et al. 2013 [40] | SpTT | 2.06 | |||
| Nadeau et al. 2013 [40] | SpTT + IncTT | 3.07 | |||
| Schlick et al. 2015 [42] | TT + VC | 1.79 | |||
| Schlick et al. 2015 [42] | TT | 2.67 | |||
| Steib et al. 2019 [43] | TT | 29.36 ^ | |||
| Steib et al. 2019 [43] | TT + P | 29.35 | |||
| Trigueiro et al. 2015 [44] | TT + 5% L | 2.84 | |||
| Trigueiro et al. 2015 [44] | TT + 10% L | 8.54 | |||
| Bello et al. 2012 [33] | PD Control—Cadence | Ground Walking | 1.293 2.390 (−1.234 to 6.015) | 66.9 | 20.66 |
| Cheng et al. 2017 [34] | Trunk Exercises | 8.29 | |||
| Fisher et al. 2008 [36] | Educational Class | 11.87 | |||
| Fisher et al. 2008 [36] | Regular PT | 9.32 | |||
| Miyai et al. 2002 [39] | Regular PT | 24.76 ^ | |||
| Nadeau et al. 2013 [40] | RT + Tai Chi | 19.09 | |||
| Protas et al. 2005 [41] | No Change | 6.01 | |||
| Cursino et al. 2018 [35] | PD Control—Step Width | TT | −3.749 *** 0.020 (−0.031 to −0.010) | 0 | 99.99 ^ |
| Fisher et al. 2008 [36] | Educational Class | 0.00 | |||
| Fisher et al. 2008 [36] | Regular PT | 0.00 | |||
| Nadeau et al. 2013 [40] | RT + Tai Chi | 0.00 | |||
| Cheng et al. 2017 [34] | PD Control- Step Length | Trunk Exercises | 1.855 1.024 (−0.058 to 2.106) | 0 | 4.05 |
| Cursino et al. 2018 [35] | TT | 3.44 | |||
| Fisher et al. 2008 [36] | Educational Class | 1.65 | |||
| Fisher et al. 2008 [36] | Regular PT | 1.79 | |||
| Trigueiro et al. 2015 [44] | TT | 5.27 | |||
| Pelosin et al. 2020 [45] | TT | 83.80 ^ | |||
| Bello et al. 2012 [33] | PD Control- Stride Length | Ground Walking | 0.290 0.592 (−3.409 to 4.593) | 0 | 61.94 ^ |
| Fisher et al. 2008 [36] | Educational Class | 3.94 | |||
| Fisher et al. 2008 [36] | Regular PT | 9.90 | |||
| Nadeau et al. 2013 [40] | RT + Tai Chi | 5.67 | |||
| Trigueiro et al. 2015 [44] | TT | 18.56 | |||
| No. | Questions | Score |
|---|---|---|
| 1 | Was the study a randomized controlled trial? | 2 |
| 2 | Was the study population specified and defined? | 2 |
| 3 | Was sample size justification, power description, or variance and effect estimates provided? | 0.79 |
| 4 | Was there an active control group? | 1.90 |
| 5 | Was the treatment assessor or participant blinded? | 1.34 |
| 6 | Were the study groups baseline matched? | 1.41 |
| 7 | Were the exposure measures (independent variables) clearly defined, valid, reliable, and implemented consistently across all study participants? | 2 |
| 8 | Were the outcome measures (dependent variables) clearly defined, valid, reliable, and implemented consistently across all study participants? | 2 |
| 9 | Were potential confounding variables measured and adjusted statistically for their impact on the relationship between exposure(s) and outcome(s)? | 1.10 |
| 10 | Were the gait cycle parameters clearly defined and uniformly applied to all participants? | 2 |
| Total (maximum score = 20) | 16.55 | |
| SD | 0.45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bishnoi, A.; Lee, R.; Hu, Y.; Mahoney, J.R.; Hernandez, M.E. Effect of Treadmill Training Interventions on Spatiotemporal Gait Parameters in Older Adults with Neurological Disorders: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int. J. Environ. Res. Public Health 2022, 19, 2824. https://doi.org/10.3390/ijerph19052824
Bishnoi A, Lee R, Hu Y, Mahoney JR, Hernandez ME. Effect of Treadmill Training Interventions on Spatiotemporal Gait Parameters in Older Adults with Neurological Disorders: Systematic Review and Meta-Analysis of Randomized Controlled Trials. International Journal of Environmental Research and Public Health. 2022; 19(5):2824. https://doi.org/10.3390/ijerph19052824
Chicago/Turabian StyleBishnoi, Alka, Rachel Lee, Yang Hu, Jeannette R. Mahoney, and Manuel E. Hernandez. 2022. "Effect of Treadmill Training Interventions on Spatiotemporal Gait Parameters in Older Adults with Neurological Disorders: Systematic Review and Meta-Analysis of Randomized Controlled Trials" International Journal of Environmental Research and Public Health 19, no. 5: 2824. https://doi.org/10.3390/ijerph19052824
APA StyleBishnoi, A., Lee, R., Hu, Y., Mahoney, J. R., & Hernandez, M. E. (2022). Effect of Treadmill Training Interventions on Spatiotemporal Gait Parameters in Older Adults with Neurological Disorders: Systematic Review and Meta-Analysis of Randomized Controlled Trials. International Journal of Environmental Research and Public Health, 19(5), 2824. https://doi.org/10.3390/ijerph19052824








