Contemporary Research Progress on the Detection of Polycyclic Aromatic Hydrocarbons
Abstract
1. Introduction
2. The Spatial Distribution and Migration of PAHs
2.1. The Distribution, Migration and Harm of PAHs in Atmosphere
2.2. The Distribution, Migration and Harm of PAHs in Water
2.3. The Distribution, Migration and Harm of PAHs in Soil
3. Traditional Analytical Methods for PAHs
3.1. High Performance Liquid Chromatography (HPLC)
3.2. Gas Chromatography (GC)
3.3. Capillary Electrophoresis (CE)
3.4. Surface Enhanced Raman Spectroscopy (SERS)
3.5. Optical Spectrometry
3.6. Other Analytical Methods
4. Mass Spectrometry
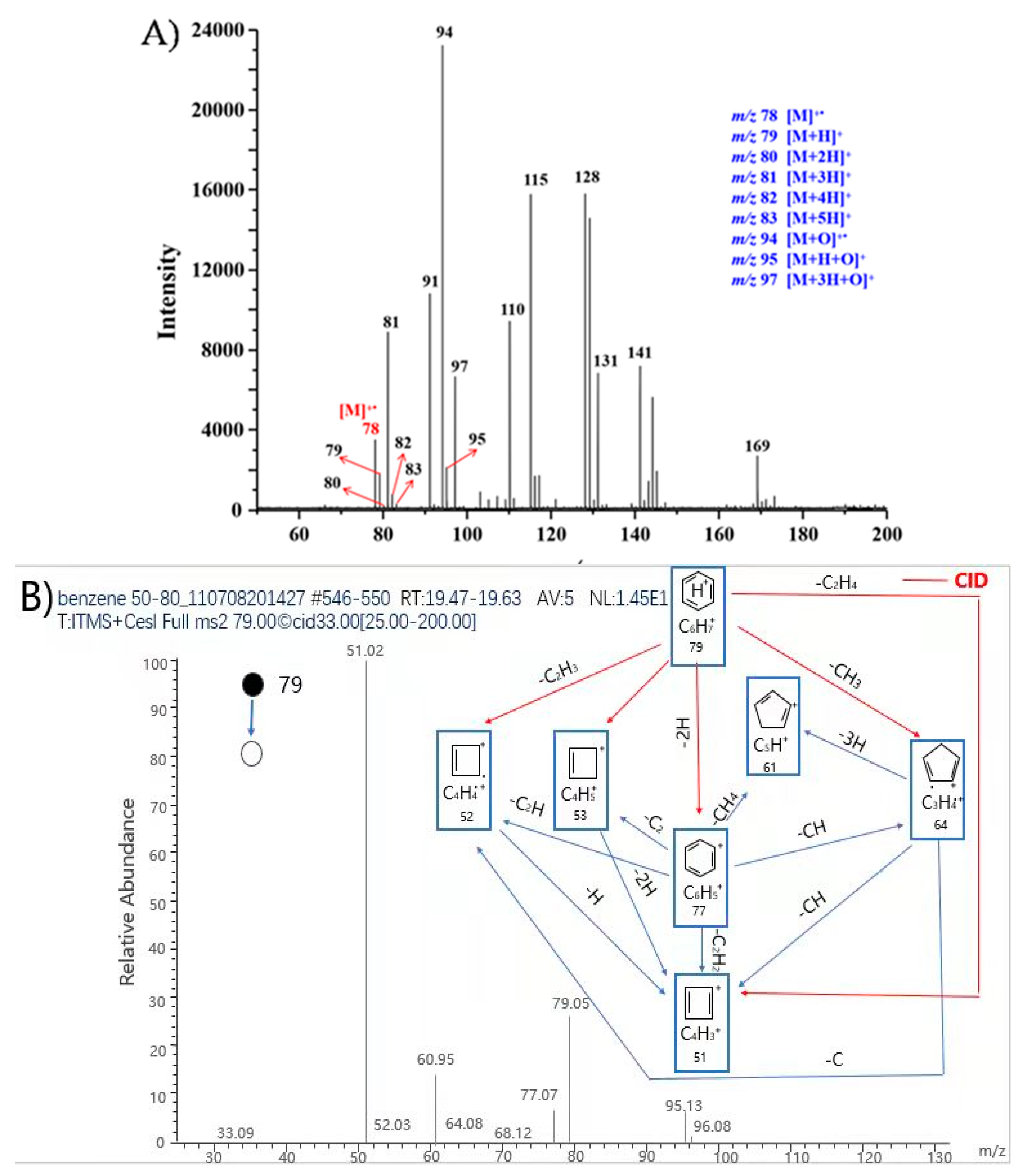
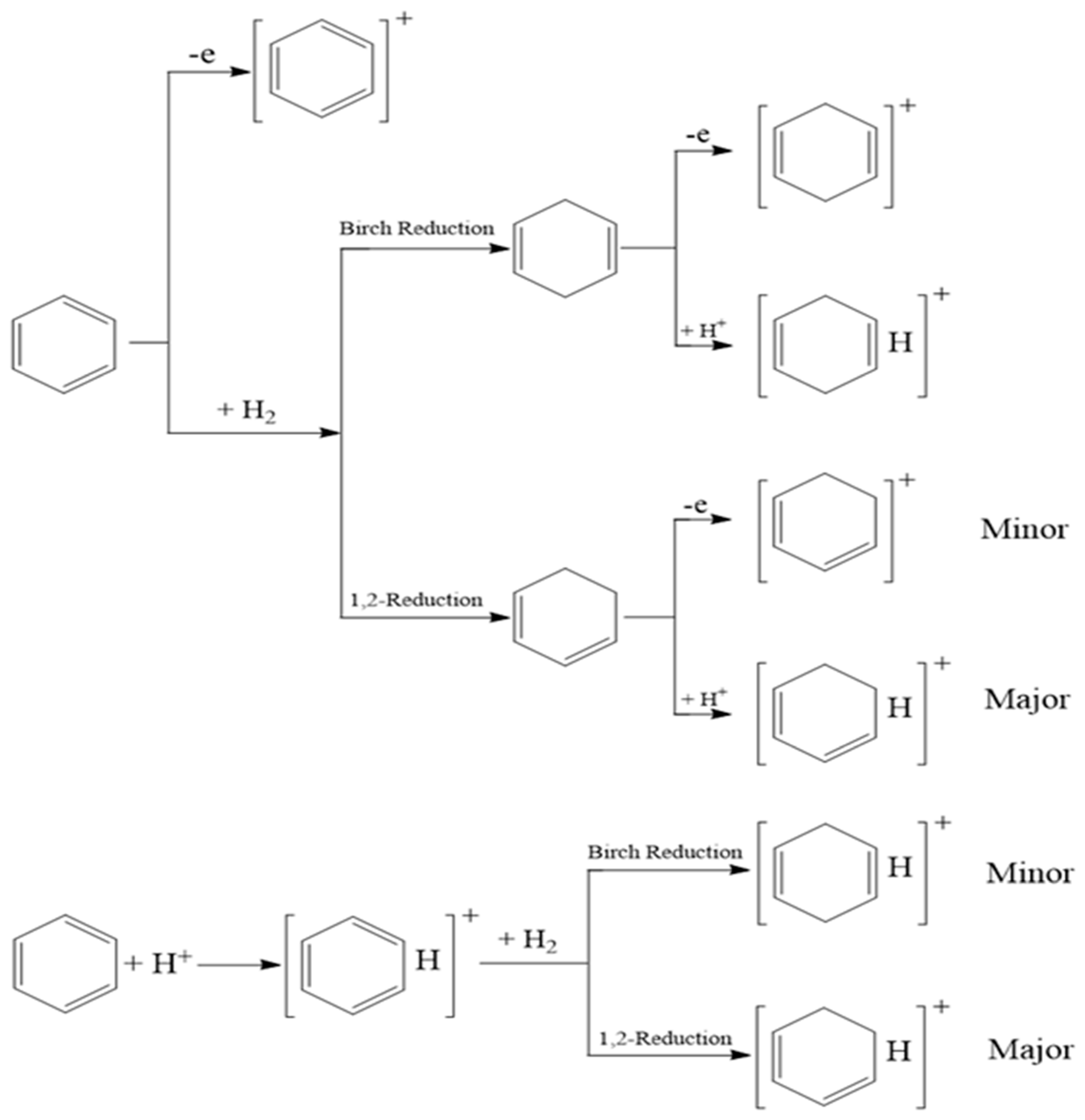
4.1. MPT Mass Spectra of Benzene
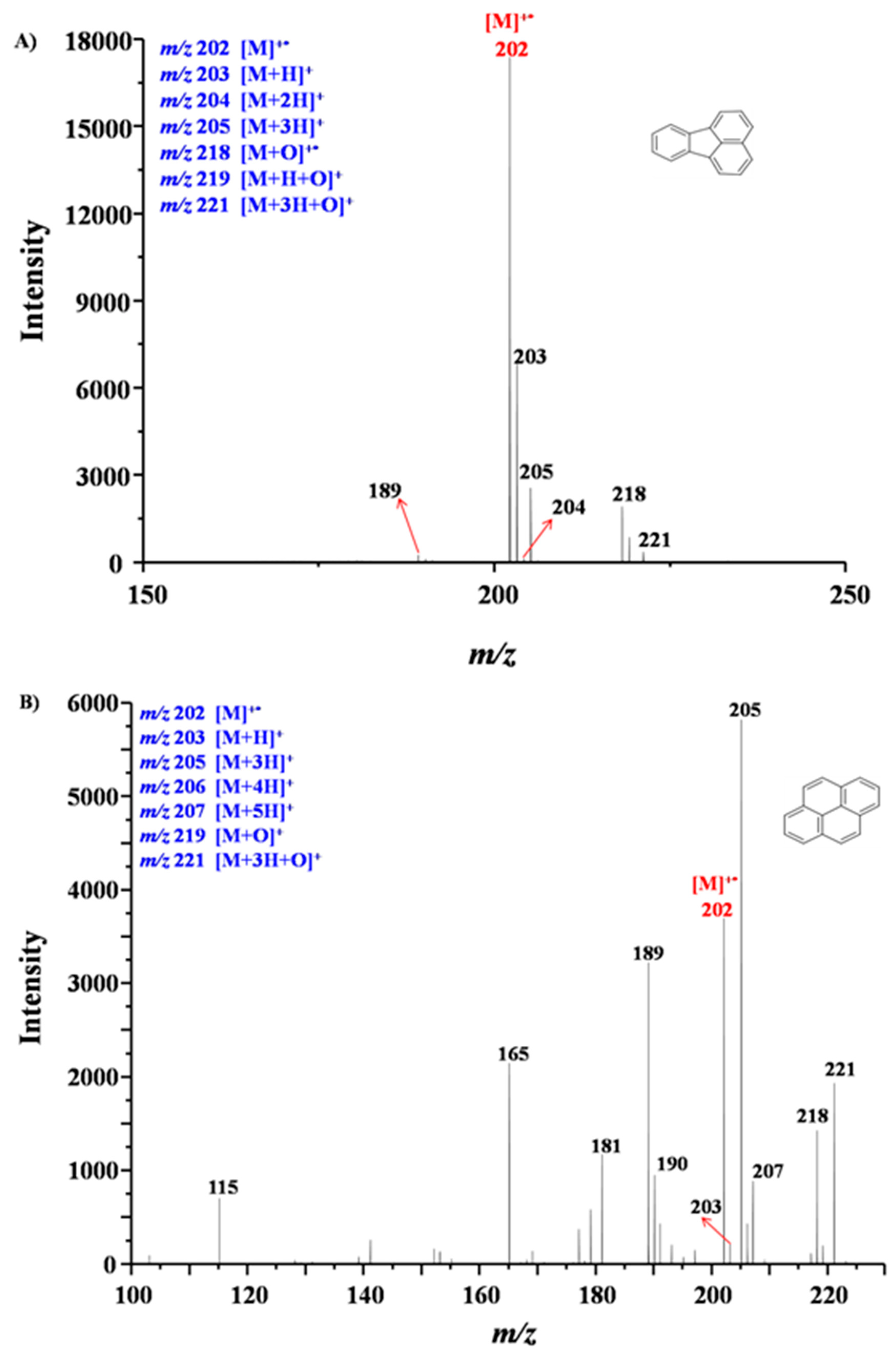
4.2. MPT Mass Spectra of Fluoranthene and Pyrene
5. Prospects
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ncube, S.; Madikizela, L.; Cukrowska, E.; Chimuka, L. Recent advances in the adsorbents for isolation of polycyclic aromatic hydrocarbons (PAHs) from environmental sample solutions. TrAC Trends Anal. Chem. 2018, 99, 101–116. [Google Scholar] [CrossRef]
- Kim, K.H.; Jahan, S.A.; Kabir, E.; Brown, R.J. A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environ. Int. 2013, 60, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Shafy, H.I.; Mansour, M.S.M. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt. J. Pet. 2016, 25, 1–17. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Y.; Lin, J.; Tang, N.; Hayakawa, K.; Maeda, T. Development of analytical methods for polycyclic aromatic hydrocarbons (PAHs) in airborne particulates: A review. J. Environ. Sci. 2007, 19, 1–11. [Google Scholar] [CrossRef]
- McGuire, B.A.; Loomis, R.A.; Burkhardt, A.M.; Lee, K.L.K.; Shingledecker, C.N.; Charnley, S.B.; Cooke, I.R.; Cordiner, M.A.; Herbst, E.; Kalenskii, S.; et al. Detection of two interstellar polycyclic aromatic hydrocarbons via spectral matched filtering. Science 2021, 371, 1265–1269. [Google Scholar] [CrossRef] [PubMed]
- Tielens, A.G. Interstellar Polycyclic Aromatic Hydrocarbon Molecules. Annu. Rev. Astron. Astrophys. 2008, 46, 289–337. [Google Scholar] [CrossRef]
- Ballantyne, B.; Marrs, T.; Syversen, T. General and Applied Toxicology; McGraw-Hill: New York, NY, USA, 2009. [Google Scholar]
- Klaassen, C.D. Casarett and Doull’s Toxicology: The Basic Science of Poisons; McGraw-Hill: New York, NY, USA, 2001. [Google Scholar]
- Mackay, D.; Callcott, D. PAHs and Related Compounds: Chemistry; Springer: Berlin/Heidelberg, Germany, 1998. [Google Scholar]
- Gelboin, H.V. Polycyclic Hydrocarbons and Cancer. In A Subsidiary of Harcourt Brace Jovanovich; Academic Press: New York, NY, USA; San Francisco, CA, USA; London, UK, 1978; Volume 1. [Google Scholar]
- Futagaki, S.K. Petroleum Refinery Wokers Exposure To PAHs at Fluid Catalytic Cracker, Coker, and Asphalt Procssing Units; U. S. Department of Health and Human Services Publica Health Service: Cincinnati, OH, USA, 1983.
- Zhang, J.Y.; Yu, F.; Yu, Y. Content and source apportionment of polycyclic aromatic hydrocarbons in surface soil in major areas of China. Ecol. Environ. Sci. 2017, 26, 1059–1067. [Google Scholar]
- Jiao, H.; Rui, X.; Wu, S.; Bai, Z.; Zhuang, X.; Huang, Z. Polycyclic Aromatic Hydrocarbons in the Dagang Oilfield (China): Distribution, Sources, and Risk Assessment. Int. J. Environ. Res. Public Health 2015, 12, 5775–5791. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yan, C.; Ding, X.; Wang, X.; Fu, Q.; Zhao, Q.; Zhang, Y.; Duan, Y.; Qiu, X.; Zheng, M. Sources and spatial distribution of particulate polycyclic aromatic hydrocarbons in Shanghai, China. Sci. Total Environ. 2017, 584–585, 307–317. [Google Scholar] [CrossRef]
- Frederica, P.; Deliang, T.; Robin, W.; Ann, L.S.; Wieslaw, J. DNA damage from polycyclic aromatic hydrocarbons measured by benzo[a]pyrene-DNA adducts in mothers and newborns from Northern Manhattan, the World Trade Center Area, Poland, and China. Cancer Epidemiol. Biomark. Prev. A Publ. Am. Assoc. Cancer Res. Cosponsored By Am. Soc. Prev. Oncol. 2005, 14, 3. [Google Scholar]
- Jedrychowski, W.; Perera, F.P.; Tang, D.; Stigter, L.; Mroz, E.; Flak, E.; Spengler, J.; Budzyn-Mrozek, D.; Kaim, I.; Jacek, R. Impact of barbecued meat consumed in pregnancy on birth outcomes accounting for personal prenatal exposure to airborne polycyclic aromatic hydrocarbons: Birth cohort study in Poland. Nutrition 2012, 28, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Drwal, E.; Rak, A.; Gregoraszczuk, E.L. Review: Polycyclic aromatic hydrocarbons (PAHs)—action on placental function and health risks in future life of newborns. Toxicology 2019, 411, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Zedeck, M.S. Polycyclic aromatic hydrocarbons: A review. J. Env. Pathol. Toxicol. 1980, 3, 537–567. [Google Scholar]
- Ramesh, A.; Harris, K.J.; Archibong, A.E. Chapter 40, Reproductive toxicity of polycyclic aromatic hydrocarbons. In Reproductive and Developmental Toxicology, 2nd ed.; Gupta, R.C., Ed.; Elsevier, Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Nie, S.W.; Xu, C.T. The research actualities of aryl hydrocarbon receptor and harm to human body. Med. Recapitul. 2011, 17, 24–26. [Google Scholar]
- Ghosal, D.; Ghosh, S.; Dutta, T.K.; Ahn, Y. Current State of Knowledge in Microbial Degradation of Polycyclic Aromatic Hydrocarbons (PAHs): A Review. Front. Microbiol. 2016, 7, 1369. [Google Scholar] [CrossRef]
- Zhang, T.; Zhou, W.; Jin, W.; Zhou, J.; Handberg, E.; Zhu, Z.; Chen, H.; Jin, Q. Direct desorption/ionization of analytes by microwave plasma torch for ambient mass spectrometric analysis. J. Mass Spectrom. 2013, 48, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Peng, Z.; Xie, M.; Fang, X.; Hong, Y.; Huang, Z.; Gao, W.; Zhou, Z.; Li, L.; Zhu, Z. Rapid analysis of tetracycline in honey by microwave plasma torch mass spectrometry with ablation samples. Anal. Methods 2020, 12, 535–543. [Google Scholar] [CrossRef]
- Yang, J.; Zheng, M.; Liu, Q.; Zhu, M.; Yang, C.; Zhang, Y.; Zhu, Z. The Study of Titanium and Zirconium Ions in Water by MPT-LTQ Mass Spectrometry in Negative Mode. Int. J. Environ. Res. Public Health 2017, 14, 1129. [Google Scholar] [CrossRef]
- Jiang, T.; Jiang, F.; Liu, H.; Yuan, L.; Mo, T.; Huang, Z.; Li, X.; Li, L.; Zhu, Z.; Zhou, Z. An easy and simple kilowatt-MPT-MS-based metal elements analysis method for rapid environmental water monitoring: An example from Poyang Lake of China. Arab. J. Chem. 2020, 13, 7939–7952. [Google Scholar] [CrossRef]
- Jiang, T.; Jiang, F.; Zhuo, Z.; Liu, H.; Hu, B.; Li, M.; Li, L.; Huang, Z.; Zhou, Z.; Zhu, Z. Comparative study on a kilowatt-MPT-MS-based method with two ion polarity modes for the inert palladium metal. Analyst 2021, 146, 1760–1771. [Google Scholar] [CrossRef]
- Jung, K.H.; Yan, B.; Moors, K.; Chillrud, S.N.; Perzanowski, M.S.; Whyatt, R.M.; Hoepner, L.; Goldstein, I.; Zhang, B.; Camann, D.; et al. Repeated exposure to polycyclic aromatic hydrocarbons and asthma: Effect of seroatopy. Ann. Allergy Asthma Immunol. Off. Publ. Am. Coll. Allergy Asthma Immunol. 2012, 109, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Sheng, G.; Tan, J.; Tang, X.; Fu, J. Phase partitioning of polycyclic aromatic hydrocarbons (PAHs) in the atmosphere. Acta Sci. Circumstantiae 2004, 1, 101–106. [Google Scholar]
- Ji, G.; Gu, J.; Guo, M.; Wu, G.; Shi, L. Pollution Characteristics and Health Risk Assessment of Atmospheric PAHs in Typical Areas in Nanjing City. Environ. Monit. 2021, 13, 87–92. [Google Scholar]
- Liu, S.; Lu, Y.; Wang, T.; Xie, S.; Jones, K.C.; Sweetman, A.J. Using gridded multimedia model to simulate spatial fate of Benzo[α]pyrene on regional scale. Environ. Int. 2014, 63, 53–63. [Google Scholar] [CrossRef]
- Wang, K.; Wang, W.; Liu, X.; Li, J.; Chen, Y.; Li, J.; Yang, W.; Ge, M. Research progress of intermediate volatility organic compounds. Environ. Chem. 2021, 40, 2960–2978. [Google Scholar]
- Gardner, B.; Hewitt, N.C.; Jones, C.K. PAHs in Air Adjacent to Two Inland Water Bodies. Environ. Sci. Technol. 1995, 29, 2405–2413. [Google Scholar] [CrossRef] [PubMed]
- Yue, M.; Gu, X.X.; Zou, H.; Zhu, R.H.; Su, W.B. The hazards and prevention of polycyclic aromatic hydrocarbons. J. Cap. Norm. Univ. Nat. Sci. Ed. 2003, 24, 40–44. [Google Scholar]
- Li, Y.; Wu, A.; Tong, M.; Luan, S.; Li, Z. Emission Characteristics of Gas- and Particle- Phase Polycyclic Aromatic Hydrocarbons from Cooking. Environ. Sci. 2021. [Google Scholar] [CrossRef]
- Sun, T.; Ye, B.; Wang, Y.Y.; Chen, Y. Pollution of PAHs in China’s Lakes and Its Source Apportionment: A Review. Environ. Sci. Technol. 2020, 43, 151–160. [Google Scholar]
- Edwards, N.T. Polycyclic Aromatic Hydrocarbons (PAH’s) in the Terrestrial Environment—A Review. J. Environ. Qual. 1983, 12, 427–441. [Google Scholar] [CrossRef]
- Ge, C.; Yu, H. The environmental behaviors of polycyclic aromatic hydrocarbons (PAHs) in the soil. Chin. J. Eco-Agric. 2016, 14, 162–165. [Google Scholar]
- Tian, Z.; Liu, X. Influence of polycyclic aromatic hydrocarbons (PAHs) from the environment on organisms and their bioremediation effect. Environ. Sci. Technol. 2018, 41, 79–89. [Google Scholar]
- Chiou, C.; McGroddy, S.; Kile, D. artition Characteristics of Polycyclic Aromatic Hydrocarbons on Soils and Sediments. Environ. Sci. Technol. 1998, 32, 264–269. [Google Scholar] [CrossRef]
- Shang, N.; Yu, H.; Li, M.; Qin, Y.; Huang, C.; Wang, Q. PAHs in leaves of camphor trees in cities’ green belts: Accumulation and source apportionment. Environ. Sci. Technol. 2021, 44, 91–98. [Google Scholar]
- Li, Y.T.; Li, F.B.; Chen, J.J.; Yang, G.Y.; Wan, H.F.; Zhang, T.B.; Zeng, X.D.; Liu, J.M. The concentrations, distribution and sources of PAHs in agricultural soils and vegetables from Shunde, Guangdong, China. Environ. Monit. Assess. 2008, 139, 61–76. [Google Scholar] [CrossRef]
- Liao, X.; Liu, Q.; Li, Y.; Gong, X.; Cao, H. Removal of polycyclic aromatic hydrocarbons from different soil fractions by persulfate oxidation. J. Environ. Sci. 2019, 78, 239–246. [Google Scholar] [CrossRef]
- Shen, Y. Study on Remediation of PAHs Pollution in Soil and Water Environment based on Biological Mud Method. Environ. Sci. Manag. 2019, 44, 111–114. [Google Scholar]
- Pandey, S.K.; Kim, K.; Brown, R.J.C. A review of techniques for the determination of polycyclic aromatic hydrocarbons in air. TrAC Trends Anal. Chem. 2011, 30, 1716–1739. [Google Scholar] [CrossRef]
- Christian, G.D.; Dasgupta, P.K.S.; Schug, K.A. Analytical Chemistry, 7th ed.; John Wiley & Sons Inc.: Hoboken: NJ, USA, 2014. [Google Scholar]
- Wang, J.L.; Zhang, N.H.; Ying, Y.; Tian, C.X.; Feng, L.; Wu, P.G.; Wang, Z.Y.; Han, J.L. Simultaneous determination of 16 polycyclic aromatic hydrocarbons insource water and tap water by performance liquid chromatography with ultraviolet detector tandem fluorescence detector combined with solid phase extraction. J. Hyg. Res. 2020, 49, 480–485. [Google Scholar]
- Yang, W.W.; Tan, S.Z.; Guo, Y.; Zhou, N. Determination of 15 Polycyclic Aromatic Hydrocarbons in Barbecued Meat by Molecularly Imprinted Solid-Phase Extraction Combined with High Performance Liquid Chromatography-Fluorescence Detection. Meat Res. 2018, 32, 47–52. [Google Scholar]
- Wang, X.; Feng, J.; Bu, Y.; Tian, Y.; Luo, C.; Sun, M. Mesoporous titanium oxide with high-specific surface area as a coating for in-tube solid-phase microextraction combined with high-performance liquid chromatography for the analysis of polycyclic aromatic hydrocarbons. J. Sep. Sci. 2017, 40, 2474–2481. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, T.; Wang, H.; Ye, Y.; He, S.H.; Cao, X.C. Rapid determination of 16 polycyclic aromatic hydrocarbons in soil by gas chromatography-mass spectrometry. Environ. Chem. 2020, 39, 2321–2324. [Google Scholar]
- Vu-Duc, N.; Thi, L.A.P.; Le-Minh, T.; Nguyen, L.; Nguyen-Thi, H.; Pham-Thi, L.; Doan-Thi, V.; Le-Quang, H.; Nguyen-Xuan, H.; Nguyen, T.T.; et al. Analysis of Polycyclic Aromatic Hydrocarbon in Airborne Particulate Matter Samples by Gas Chromatography in Combination with Tandem Mass Spectrometry (GC-MS/MS). J. Anal. Methods Chem. 2021, 2021, 6641326. [Google Scholar] [CrossRef] [PubMed]
- Sulej-Suchomska, A.M.; Polkowska, Ż.; Chmiel, T.; Dymerski, T.M.; Kokot, Z.J.; Namieśnik, J. Solid phase microextraction–comprehensive two-dimensional gas chromatography–time-of-flight mass spectrometry: A new tool for determining PAHs in airport runoff water samples. Anal. Methods 2016, 8, 4509–4520. [Google Scholar] [CrossRef]
- Manzano, C.; Hoh, E.; Simonich, S.L. Improved separation of complex polycyclic aromatic hydrocarbon mixtures using novel column combinations in GC x GC/ToF-MS. Environ. Sci. Technol. 2012, 46, 7677–7684. [Google Scholar] [CrossRef][Green Version]
- Dos Santos, R.R.; Vidotti Leal, L.D.; de Lourdes Cardeal, Z.; Menezes, H.C. Determination of polycyclic aromatic hydrocarbons and their nitrated and oxygenated derivatives in coffee brews using an efficient cold fiber-solid phase microextraction and gas chromatography mass spectrometry method. J. Chromatogr. A 2019, 1584, 64–71. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, X.; Liu, Y.; Fu, X. Determination of 16 polycyclic aromatic hydrocarbons in oily sludge by gas chromatography- mass spectrometry. J. Anal. Sci. 2017, 33, 86–90. [Google Scholar]
- Jira, W.; Ziegenhals, K.; Speer, K. Gas chromatography-mass spectrometry (GC-MS) method for the determination of 16 European priority polycyclic aromatic hydrocarbons in smoked meat products and edible oils. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2008, 25, 704–713. [Google Scholar] [CrossRef]
- Zakerian, R.; Bahar, S. Electrochemical exfoliation of pencil graphite for preparation of graphene coating as a new versatile SPME fiber for determination of polycyclic aromatic hydrocarbons by gas chromatography. Mikrochim. Acta 2019, 186, 861. [Google Scholar] [CrossRef] [PubMed]
- Harrison, D.J.; Karl, F.; Seiler, K.; Fan, Z.; Effenhauser, C.S.; Manz, A. Micromachining a miniaturized capillary electrophoresis-based chemical analysis system on a chip. Science 1993, 261, 895–897. [Google Scholar] [CrossRef] [PubMed]
- Towns, J.K.; Regnier, F.E. Capillary electrophoretic separations of proteins using nonionic surfactant coatings. Anal. Chem. 1991, 63, 1126–1132. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ni, X.J.; Zhang, J.Y.; Liu, Y.; Cao, Y.H. Detection of polycyclic aromatic hydrocarbons in cosmetics by reverse microemulsion capillary electrophoresis. Chin. J. Anal. Chem. 2015, 43, 81–86. [Google Scholar]
- do Rosário, P.M.; Nogueira, J.M. Combining stir bar sorptive extraction and MEKC for the determination of polynuclear aromatic hydrocarbons in environmental and biological matrices. Electroanalysis 2006, 27, 4694–4702. [Google Scholar] [CrossRef] [PubMed]
- Alzola, R.; Pons, B.; Bravo, D.; Arranz, A. Determination of Polynuclear aromatic hydrocabrons (PAHs) in sewage sludge by micellar electrokinetic capillary chromatography and HPLC-Fluorescence detection: A comparative study. Environ. Technol. 2008, 29, 1219–1228. [Google Scholar] [CrossRef]
- Qu, Q.; Wang, S.; Mangelings, D.; Wang, C.; Yang, G.; Hu, X.; Yan, C. Monolithic silica xerogel capillary column for separations in capillary LC and pressurized CEC. Electroanalysis 2009, 30, 1071–1076. [Google Scholar] [CrossRef]
- Ferey, L.; Delaunay, N.; Rutledge, D.N.; Cordella, C.B.; This, H.; Huertas, A.; Raoul, Y.; Gareil, P. Optimizing separation conditions of 19 polycyclic aromatic hydrocarbons by cyclodextrin-modified capillary electrophoresis and applications to edible oils. Talanta 2014, 119, 572–581. [Google Scholar] [CrossRef]
- Stockton, A.M.; Chiesl, T.N.; Scherer, J.R.; Mathies, R.A. Polycyclic aromatic hydrocarbon analysis with the Mars organic analyzer microchip capillary electrophoresis system. Anal. Chem. 2009, 81, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Raman, C.V.; Krishinan, K.S. A New Type of Secondary Radiation. Nature 1928, 121, 501–502. [Google Scholar] [CrossRef]
- Schlücker, S. Surface Enhanced Raman Spectroscopy: Analytical, Biophysical and Life Science Applications; Wile-VCH Verl. GmbH Co., KGaA: Weinheim, Germany, 2011. [Google Scholar]
- Popp, J.; Mayerhöfer, T. Surface-enhanced Raman spectroscopy. Anal. Bioanal. Chem. 2009, 394, 1717–1718. [Google Scholar] [CrossRef]
- Wang, S.; Wang, C.P.; Wu, G.Q.; Yan, X.Z.; Zhou, W.W.; Hong, Y.P.; Wang, C.R. Rapid detection of volatile polycyclic aromatic hydrocarbons using gold nano-films by coupling headspace solid phase solid phase extraction with surface-enhanced Raman spectroscopy. Chin. J. Anal. Lab. 2020, 39, 880–884. [Google Scholar]
- Zhang, M.; Zhang, X.; Shi, Y.E.; Liu, Z.; Zhan, J. Surface enhanced Raman spectroscopy hyphenated with surface microextraction for in-situ detection of polycyclic aromatic hydrocarbons on food contact materials. Talanta 2016, 158, 322–329. [Google Scholar] [CrossRef]
- Kwon, Y.H.; Sowoidnich, K.; Schmidt, H.; Kronfeldt, H.D. Application of calixarene to high active surface-enhanced Raman scattering (SERS) substrates suitable for in situ detection of polycyclic aromatic hydrocarbons (PAHs) in seawater. J. Raman Spectrosc. 2012, 43, 1003–1009. [Google Scholar] [CrossRef]
- Shen, Z.D.; Yang, Z.X.; Kong, X.M. Application of photonic crystal bio-silicon chromatographic chip in SERS detection of polycyclic aromatic hydrocarbons in edible oil. Spectrosc. Spectr. Anal. 2020, 40, 147–148. [Google Scholar]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer Science & Business Media: New Yorka, NY, USA, 1999. [Google Scholar]
- He, L.; Zhang, R.; Zhang, L. Constant- Wavelength Synchronous Fluorescence Spectrometry for the Determination of Polynuclear Aromatic hydrocarbons in Environmental Water Samples. Environ. Monit. China 2012, 28, 32–37. [Google Scholar]
- Cai1, Q.; Zhao, C.; Zhu, H.; Shen, Y.; Hou, H.; Tang, Y. Constant-wavelength synchronous fluorescence spectrometry for simultaneous and rapid determination of five polycyclic aromatic hydrocarbon residues in dairy products. Lumin. J. Biol. Chem. Lumin. 2021, 36, 353–359. [Google Scholar] [CrossRef]
- Wang, G.Y.; Tian, H.Q.; Niu, X.L.; Jia, S.M.; Liu, Y.R.; Chen, X.F.; Xie, Z.; Yang, D.Z.; Li, L.; Shi, G.F.; et al. Constant- energy synchronous fluorescence spectrum characteristics of 15 polycyclic aromatic hydrocarbons from atmospheric particulate matters. Acta Sci. Circumstantiae 2019, 39, 44–52. [Google Scholar]
- Wang, H.B.; Zhang, Y.J.; Xiao, X.; Yu, S.H.; Liu, W.Q. Application of excitation-emission matrix fluorescence combined with second-order calibration algorithm for the determination of five polycyclic aromatic hydrocarbons simultaneously in drinking. Anal. Methods Adv. Methods Appl. 2011, 3, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, L.; Luo, S.; Chen, B.; Li, J.; Lin, H.; Cai, Q.; Yao, S. Polycyclic aromatic hydrocarbon detection by electrochemiluminescence generating Ag/TiO(2) nanotubes. Anal. Chem. 2010, 82, 7357–7361. [Google Scholar] [CrossRef]
- Woodbury, W.B. Phosphorescence. Nature 1879, 20, 56. [Google Scholar] [CrossRef]
- Shoji, Y.; Ikabata, Y.; Wang, Q.; Nemoto, D.; Sakamoto, A.; Tanaka, N.; Seino, J.; Nakai, H.; Fukushima, T. Unveiling a New Aspect of Simple Arylboronic Esters: Long-Lived Room-Temperature Phosphorescence from the Heavy Atom-Free Molecules. J. Am. Chem. Soc. 2017, 139, 2728–2733. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.H. Principle and Application of Low-Temperature Phosphorescent Analysis; China Science Publishing & Media Ltd. (CSPM): Beijing, China, 2008. [Google Scholar]
- Díaz, B.C.; Schulman, S.G.; Carretero, A.S.; Blanco, C.C.; Gutiérrez, A.F. Facile Analysis Of Carbazole In Commercial Anthracene by Heavy Atom–Induced Room Temperature Phosphorescence. Polycycl. Aromat. Compd. 2004, 24, 65–74. [Google Scholar] [CrossRef]
- Carretero, A.S.; Cruces-Blanco, C.; Polo, M.S.; Gutierrez, A.F.; Fernandez-Nieves, A. Study of Microemulsion Composition Effect over Phosphorescence Emission of a Polycyclic Aromatic Compound. Polycycl. Aromat. Compd. 2003, 23, 2. [Google Scholar] [CrossRef][Green Version]
- Carretero, A.S.; Blanco, C.C.; Gutiérrez, A.F. Simultaneous microemulsion room temperature phosphorimetric determination of five polycyclic aromatic hydrocarbons by variable-angle synchronous scanning. Anal. Chim. Acta 1997, 353, 337–344. [Google Scholar] [CrossRef]
- Mansouri, E.; Yousefi, V.; Ebrahimi, V.; Eyvazi, S.; Hejazi, M.S.; Mahdavi, M.; Mesbahi, A.; Tarhriz, V. Overview of ultraviolet-based methods used in polycyclic aromatic hydrocarbons analysis and measurement. Sep. Sci. Plus 2020, 3, 112–120. [Google Scholar] [CrossRef]
- Luan, L.X.; Tang, X.Z.; Luo, X.; Tang, J. Research on simultaneous determination of monocyclic and polycyclic aromatic hydrocarbons in white oil by principal component regression method. Appl. Chem. Ind. 2008, 1, 104–106. [Google Scholar]
- Gottlieb, J.; Hötzl, H.; Huck, K.; Niessner, R. Field Screening Europe; Springer Science+Business Media: Dordrecht, The Netherlands, 1997. [Google Scholar]
- Rodriguez-Mozaz, S.; Lopez de Alda, M.J.; Barcelo, D. Biosensors as useful tools for environmental analysis and monitoring. Anal. Bioanal. Chem. 2006, 386, 1025–1041. [Google Scholar] [CrossRef]
- Shen, X.; Cui, Y.; Pang, Y.; Qian, H. Pre-concentration and in situ electrochemical sensing of 1-hydroxypyrene on an electrodeposited poly(3-methylthiophene) film modified electrode. J. Electroanal. Chem. 2012, 667, 1–6. [Google Scholar] [CrossRef]
- Moore, E.J.; Kreuzer, M.P.; Pravda, M.; Guilbault, G.G. Development of a Rapid Single-Drop Analysis Biosensor for Screening of Phenanthrene in Water Samples. Electroanalysis 2004, 16, 1653–1659. [Google Scholar] [CrossRef]
- Ni, Y.; Wang, P.; Song, H.; Lin, X.; Kokot, S. Electrochemical detection of benzo(a)pyrene and related DNA damage using DNA/hemin/nafion-graphene biosensor. Anal. Chim. Acta 2014, 821, 34–40. [Google Scholar] [CrossRef]
- Iqbal, S.M.; Bashir, R. Nanopores: Sensing and Fundamental Biological Interactions; Springer Science+Business Media: Dordrecht, The Netherlands, 2011. [Google Scholar]
- Perera, R.T.; Fleming, A.M.; Johnson, R.P.; Burrows, C.J.; White, H.S. Detection of benzo[a]pyrene-guanine adducts in single-stranded DNA using the alpha-hemolysin nanopore. Nanotechnology 2015, 26, 1–7. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.; Lu, W.; Wu, X.; Li, J. Molecular imprinting: Perspectives and applications. Chem. Soc. Rev. 2016, 45, 2137–2211. [Google Scholar] [CrossRef]
- Li, H.; Wang, L. Highly selective detection of polycyclic aromatic hydrocarbons using multifunctional magnetic-luminescent molecularly imprinted polymers. ACS Appl. Mater. Interfaces 2013, 5, 10502–10509. [Google Scholar] [CrossRef] [PubMed]
- Gross, J.H. Mass Spectrometry: A Textbook, 2nd ed.; Springer: Berlin, Germany, 2011. [Google Scholar]
- Hoffmann, E.D.; Stroobant, V. Mass Spectrometry: Principles and Applications, 3rd ed.; John Wiley & Sons Ltd.: Chichester, UK, 2007. [Google Scholar]
- Emmenegger, C.; Kalberer, M.; Morrical, B.; Zenobi, R. Quantitative analysis of polycyclic aromatic hydrocarbons in water in the low-nanogram per liter range with two-step laser mass spectrometry. Anal. Chem. 2003, 75, 4508–4513. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, X.; Hou, K.; Li, H.; Yan, M.; Yan, J. On-line monitoring of polycyclic aromatic hydrocarbons using time of flight-mass spectrometer. Chin. J. Anal. Chem. 2010, 38, 859–863. [Google Scholar]
- Graham, C.R.; Zheng, O.; Zoltan, T.; Wiseman, J.M. Detection Technologies. Ambient mass spectrometry. Science 2006, 311, 5767. [Google Scholar]
- Alberici, R.M.; Simas, R.C.; Sanvido, G.B.; Romao, W.; Lalli, P.M.; Benassi, M.; Cunha, I.B.; Eberlin, M.N. Ambient mass spectrometry: Bringing MS into the “real world”. Anal. Bioanal. Chem. 2010, 398, 265–294. [Google Scholar] [CrossRef]
- Takáts, Z.; Wiseman, J.M.; Gologan, B.; Cooks, R.G. Mass Spectrometry Sampling Under Ambient Conditions with Desorption Electrospray Ionization. Science 2004, 306, 471–473. [Google Scholar] [CrossRef] [PubMed]
- Cody, R.B.; Laramée, J.A.; Durst, H.D. Versatile new ion source for the analysis of materials in open air under ambient conditions. Anal. Chem. 2005, 77, 2297–2302. [Google Scholar] [CrossRef] [PubMed]
- Cody, R.B. Observation of Molecular Ions and Analysis of Nonpolar Compounds with the Direct Analysis in Real Time Ion Source. Anal. Chem. 2009, 81, 1101–1107. [Google Scholar] [CrossRef]
- Yang, S.; Ding, J.; Zheng, J.; Hu, B.; Jianqiang, L.; Huanwen, C.; Zhiquan, Z.; Xiaolin, Q. Detection of melamine in milk products by surface desorption atmospheric pressure chemical ionization mass spectrometry. Anal. Chem. 2009, 81, 2426–2436. [Google Scholar] [CrossRef]
- Chen, H.; Lai, J.-H.; Zhou, Y.; Huan, Y.; Li, J.; Xie, Z.; Wang, Z.; Luo, M.-B. Instrumentation and Characterization of Surface Desorption Atmospheric Pressure Chemical Ionization Mass Spectrometry. Chin. J. Anal. Chem. 2007, 35, 1233–1240. [Google Scholar] [CrossRef]
- Ding, J.; Gu, H.; Yang, S.; Li, M.; Li, J.; Chen, H. Selective detection of diethylene glycol in toothpaste products using neutral desorption reactive extractive electrospray ionization tandem mass spectrometry. Anal. Chem. 2009, 81, 8632–8638. [Google Scholar] [CrossRef]
- Ding, J.; Yang, S.; Liang, D.; Chen, H.; Wu, Z.; Zhanga, L.; Ren, Y. Development of extractive electrospray ionization ion trap mass spectrometry for in vivo breath analysis. Analyst 2009, 134, 2040–2050. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yang, S.; Li, M.; Hu, B.; Li, J.; Wang, J. Sensitive detection of native proteins using extractive electrospray ionization mass spectrometry. Angew. Chem. Int. Ed. 2010, 49, 3053–3056. [Google Scholar] [CrossRef] [PubMed]
- Na, N.; Zhang, C.; Zhao, M.; Zhang, S.; Yang, C.; Fang, X.; Zhang, X. Direct detection of explosives on solid surfaces by mass spectrometry with an ambient ion source based on dielectric barrier discharge. Int. J. Mass Spectrom. 2007, 42, 1079–1085. [Google Scholar] [CrossRef]
- Andrade, F.J.; Shelley, J.T.; Wetzel, W.C.; Webb, M.R.; Gamez, G.; Ray, S.J.; Hieftje, G.M. Atmospheric pressure chemical ionization source. 1. Ionization of compounds in the gas phase. Anal. Chem. 2008, 80, 2646–2653. [Google Scholar] [CrossRef]
- Smith, C.A.; Want, E.J.; O’Maille, G.; Abagyan, R.; Siuzdak, G. XCMS: Processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Anal. Chem. 2006, 78, 779–787. [Google Scholar] [CrossRef]
- Arjen, L. MetAlign: Interface-driven, versatile metabolomics tool for hyphenated full-scan mass spectrometry data preprocessing. Anal. Chem. 2009, 81, 3079–3088. [Google Scholar]
- Gu, H.; Pan, Z.; Xi, B.; Asiago, V.; Musselman, B.; Raftery, D. Principal component directed partial least squares analysis for combining nuclear magnetic resonance and mass spectrometry data in metabolomics: Application to the detection of breast cancer. Anal. Chim. Acta 2011, 686, 57–63. [Google Scholar] [CrossRef]
- Pan, Z.; Gu, H.; Talaty, N.; Chen, H.; Shanaiah, N.; Hainline, B.E.; Cooks, R.G.; Raftery, D. Principal component analysis of urine metabolites detected by NMR and DESI-MS in patients with inborn errors of metabolism. Anal. Bioanal. Chem. 2007, 387, 539–549. [Google Scholar] [CrossRef]
- Gu, H.; Yang, S.; Li, J.; Hu, B.; Chen, H.; Zhang, L.; Fei, Q. Geometry-independent neutral desorption device for the sensitive EESI-MS detection of explosives on various surfaces. Analyst 2010, 135, 779–788. [Google Scholar] [CrossRef]
- Wood, M.; Laloup, M.; Samyn, N.; del Mar Ramirez Fernandez, M.; de Bruijn, E.A.; Maes, R.A.; De Boeck, G. Recent applications of liquid chromatography-mass spectrometry in forensic science. J. Chromatogr. A 2006, 1130, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.J.; Shen, Z.; Blackledge, R.; Siuzdak, G. Desorption–ionization on silicon mass spectrometry:an application in forensics. Anal. Chim. Acta 2001, 442, 183–190. [Google Scholar] [CrossRef]
- Rieder, J.; Prazeller, P.; Boehler, M.; Lirk, P.; Lindinger, W.; Amann, A. Online Monitoring of Air Quality at the Postanesthetic Care Unit by Proton-Transfer-Reaction Mass Spectrometry. Anesth. Analg. 2001, 92, 2. [Google Scholar] [CrossRef]
- Harris, G.A.; Galhena, A.S.; Fernandez, F.M. Ambient sampling/ionization mass spectrometry: Applications and current trends. Anal. Chem. 2011, 83, 4508–4538. [Google Scholar] [CrossRef]
- Ratcliffe, L.V.; Rutten, F.J.M.; Barrett, D.A.; Whitmore, T.; Seymour, D.; Greenwood, C.; Aranda-Gonzalvo, Y.; Robinson, S.; McCoustra, M. Surface analysis under ambient conditions using plasma-assisted desorption/ionization mass spectrometry. Anal. Chem. 2007, 79, 6094–6101. [Google Scholar] [CrossRef]
- Harper, J.D.; Charipar, N.A.; Mulligan, C.C.; Zhang, X.; Cooks, R.G.; Ouyang, Z. Low-temperature plasma probe for ambient desorption ionization. Anal. Chem. 2008, 80, 9097–9104. [Google Scholar] [CrossRef]
- Symonds, J.M.; Galhena, A.S.; Fernández, F.M.; Orlando, T.M. Microplasma discharge ionization source for ambient mass spectrometry. Anal. Chem. 2010, 82, 621–627. [Google Scholar] [CrossRef]
- Li, X.; Wang, H.; Sun, W.; Ding, L. Desorption corona beam ionization coupled with a poly(dimethylsiloxane) substrate: Broadening the application of ambient ionization for water samples. Anal. Chem. 2010, 82, 9188–9193. [Google Scholar] [CrossRef]
- Jin, Q.; Yang, G.; Yu, A.; Liu, J.; Zhang, H.; Ben, Y.A. Novel Plasma Emission Source. J. Nat. Sci. Jinlin Univ. 1985, 1, 90–92. [Google Scholar]
- Qinhan, J.; Chu, Z.; Borer, M.W.; Hieftje, G.M. A microwave plasma torch assembly for atomic emission spectrometry. Spectrochim. Acta Part B At. Spectrosc. 1991, 46, 417–430. [Google Scholar]
- Jankowski, K.J.; Reszke, E. Microwave Induced Plasma Analytical Spectrometry; Royal Society of Chemistry: Cambridge, UK, 2011; Volume 12. [Google Scholar]
- Feng, G.; Huan, Y.; Cao, Y.; Wang, S.; Wang, X.; Jiang, J.; Yu, A.; Jin, Q.; Yu, H. Development of a miniature simultaneous MPT spectrometer. Microchem. J. 2004, 76, 17–22. [Google Scholar] [CrossRef]
- Duan, Y.; Su, Y.; Jin, Z.; Abeln, S.P. Design and development of a highly sensitive, field portable plasma source instrument for on-line liquid stream monitoring and real-time sample analysis. Rev. Sci. Instrum. 2000, 71, 1557–1563. [Google Scholar] [CrossRef]
- Jin, Q.; Wang, F.; Zhu, C.; Chambers, D.M.; Hieftjeg, G.M. Atomic Emission Detector for Gas Chromatography and Supercritical Fluid Chromatography. J. Anal. At. Spectrom. 1990, 5, 487–494. [Google Scholar] [CrossRef]
- Broekaert, J.A.C.; Bings, N.; Prokisch, C.; Seelig, M. A close-up of three microwave plasma sources in view of improved element-specific detection in liquid chromatography. Spectrochim. Acta Part B At. Spectrosc. 1998, 53, 2. [Google Scholar] [CrossRef]
- Pack, B.W.; Broekaert, J.A.; Guzowski, J.P.; Poehlman, J.; Hieftje, G.M. Determination of halogenated hydrocarbons by helium microwave plasma torch time-of-flight mass spectrometry coupled to gas chromatography. Anal. Chem. 1998, 70, 18. [Google Scholar] [CrossRef]
- Jiang, T.; Li, Y.; Zhao, X.J.; Xiao, S.J.; Yang, S.P.; Zhu, Z.Q. Direct Analysis of Aflatoxin B1 in Rice by Microwave Plasma Torch Mass Spectrometry. J. Instrum. Anal. 2016, 35, 1575–1580. [Google Scholar]
- Xiong, X.; Jiang, T.; Zhou, R.; Wang, S.; Zou, W.; Zhu, Z. Microwave plasma torch mass spectrometry for the direct detection of copper and molybdenum ions in aqueous liquids. J. Mass Spectrom. JMS 2016, 51, 369–377. [Google Scholar] [CrossRef]
- Xiong, X.; Chen, G.; Zhu, M.; Li, Y.; Yang, C.; Xie, K.; Zhu, Z. The study of bismuth ions in drinking water at ultratrace levels by a microwave plasma torch coupled with linear ion trap mass spectrometry. Anal. Methods 2018, 10, 1346–1352. [Google Scholar] [CrossRef]
- Xiong, X.; Jiang, T.; Qi, W.; Zuo, J.; Yang, M.; Fei, Q.; Xiao, S.; Yu, A.; Zhu, Z.; Chen, H. Some Rare Earth Elements Analysis by Microwave Plasma Torch Coupled with the Linear Ion Trap Mass Spectrometry. Int. J. Anal. Chem. 2015, 2015, 156509. [Google Scholar] [CrossRef]
- Jiang, T.; Xiong, X.; Wang, S.; Luo, Y.; Fei, Q.; Yu, A.; Zhu, Z. Direct Mass Spectrometric Analysis of Zinc and Cadmium in Water by Microwave Plasma Torch Coupled with a Linear Ion Trap Mass Spectrometer. Int. J. Mass Spectrom. 2016, 399–400, 33–39. [Google Scholar] [CrossRef]
- Na, N.; Xia, Y.; Zhu, Z.; Zhang, X.; Cooks, R.G. Birch reduction of benzene in a low-temperature plasma. Angew. Chem. Int. Ed. 2009, 48, 2017–2019. [Google Scholar] [CrossRef] [PubMed]
- Wang, S. Studies on the diagnoses and application of oxygenshielded argon microwave plasma torch (OS-ArMPT). Ph.D. Thesis, University of Jilin, Changchu, China, 2006. [Google Scholar]
- Na, N.; Zhao, M.; Zhang, S.; Yang, C.; Zhang, X. Development of a dielectric barrier discharge ion source for ambient mass spectrometry. J. Am. Soc. Mass Spectrom. 2007, 18, 1859–1862. [Google Scholar] [CrossRef]
- Rice, J.E.; Hosted, T.J.; Lavoie, J.E. Fluoranthene and pyrene enhance benzo[a]pyrene--DNA adduct formation in vivo in mouse skin. Cancer Lett. 1984, 24, 327–333. [Google Scholar] [CrossRef]
- Alam, M.S.; Harrison, R.M. Recent advances in the application of 2-dimensional gas chromatography with soft and hard ionisation time-of-flight mass spectrometry in environmental analysis. Chem. Sci. 2016, 7, 3968–3977. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Feng, S.; Lin, L.; Mao, S.; Lin, J.M. Emerging open microfluidics for cell manipulation. Chem. Soc. Rev. 2021, 50, 5333–5348. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, F.; He, Z.; Ma, Y.; Uchiyama, K.; Lin, J.M. A novel approach for precisely controlled multiple cell patterning in microfluidic chips by inkjet printing and the detection of drug metabolism and diffusion. Analyst 2016, 141, 2940–2947. [Google Scholar] [CrossRef]
- Liang, Y.Z.; Xu, Q.S. Instrumental Analysis of Complex Systems-White, Grya and Black Analytical Systems and Their Multivariate Methods; Chemical Industry Press Co., Ltd.: Beijing, China, 2012. [Google Scholar]
- Eckschlager, K.; Stepanek, V. Information Theory as Applied to Chemical analysis. J. Chem. Educ. 1977, 49(8), 1265–1267. [Google Scholar] [CrossRef]
- Kaufmann, K.; Zhu, C.; Rosengarten, A.S.; Maryanovsky, D.; Harrington, T.J.; Marin, E.; Vecchio, K.S. Crystal symmetry determination in electron diffraction using machine learning. Science 2020, 367, 564–568. [Google Scholar] [CrossRef]
- Leemann, S.C.; Liu, S.; Hexemer, A.; Marcus, M.A.; Melton, C.N.; Nishimura, H.; Sun, C. Demonstration of Machine Learning-Based Model-Independent Stabilization of Source Properties in Synchrotron Light Sources. Phys. Rev. Lett. 2019, 123, 194801. [Google Scholar] [CrossRef]
- Harkin, C.; Smith, K.W.; Cruickshank, F.L.; Mackay, C.L.; Flinders, B.; Heeren, R.M.A.; Moore, T.; Brockbank, S.; Cobice, D.F. On-tissue chemical derivatization in mass spectrometry imaging. In Mass Spectrometry Review; John Wiley & Sons Ltd.: Chichester, UK, 2021. [Google Scholar]
- Solon, E.; Groseclose, M.R.; Ho, S.; Tanaka, K.; Nakada, N.; Linehan, S.; Nishidate, M.; Yokoi, H.; Kaji, H.; Urasaki, Y.; et al. Imaging Mass Spectrometry (IMS) for drug discovery and development survey: Results on methods, applications and regulatory compliance. Drug Metab. Pharmacokinet. 2021, 43, 100438. [Google Scholar] [CrossRef] [PubMed]
- Zang, Q.; Wang, M.; Zhu, Y.; Wang, L.; Luo, Z.; Li, X.; He, J.; Zhang, R.; Abliz, Z. Enhanced On-Tissue Chemical Derivatization with Hydrogel Assistance for Mass Spectrometry Imaging. Anal. Chem. 2021, 93, 15373–15380. [Google Scholar] [CrossRef] [PubMed]
- Balluff, B.; Heeren, R.M.A.; Race, A.M. An overview of image registration for aligning mass spectrometry imaging with clinically relevant imaging modalities. J. Mass Spectrom. Adv. Clin. Lab 2022, 23, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Ogrinc, N.; Kruszewski, A.; Chaillou, P.; Saudemont, P.; Lagadec, C.; Salzet, M.; Duriez, C.; Fournier, I. Robot-Assisted SpiderMass for In Vivo Real-Time Topography Mass Spectrometry Imaging. Anal. Chem. 2021, 93, 14383–14391. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Yuan, L.; He, S.; Tao, H.; Xie, W.; Zhang, X.; Ren, X.; Jiang, T.; Li, L.; Zhu, Z. Contemporary Research Progress on the Detection of Polycyclic Aromatic Hydrocarbons. Int. J. Environ. Res. Public Health 2022, 19, 2790. https://doi.org/10.3390/ijerph19052790
Zhang Y, Yuan L, He S, Tao H, Xie W, Zhang X, Ren X, Jiang T, Li L, Zhu Z. Contemporary Research Progress on the Detection of Polycyclic Aromatic Hydrocarbons. International Journal of Environmental Research and Public Health. 2022; 19(5):2790. https://doi.org/10.3390/ijerph19052790
Chicago/Turabian StyleZhang, Yan, Limin Yuan, Shuli He, Huilin Tao, Wenlian Xie, Xinyu Zhang, Xiaolu Ren, Tao Jiang, Lihong Li, and Zhiqiang Zhu. 2022. "Contemporary Research Progress on the Detection of Polycyclic Aromatic Hydrocarbons" International Journal of Environmental Research and Public Health 19, no. 5: 2790. https://doi.org/10.3390/ijerph19052790
APA StyleZhang, Y., Yuan, L., He, S., Tao, H., Xie, W., Zhang, X., Ren, X., Jiang, T., Li, L., & Zhu, Z. (2022). Contemporary Research Progress on the Detection of Polycyclic Aromatic Hydrocarbons. International Journal of Environmental Research and Public Health, 19(5), 2790. https://doi.org/10.3390/ijerph19052790




