Lung Cancer Imaging: Screening Result and Nodule Management
Abstract
1. Introduction
2. Materials and Methods
2.1. Publication Search
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
3. Results
3.1. Included Studies
3.2. Patient Characteristics for Screening Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Mazzei, M.A.; Guerrini, S.; Genovese, E.A.; Voltolini, L.; Mazzei, F.G.; Volterrani, L.; Macarini, L. Accuratezza della TC multistrato con approccio multiparametrico nel restaging di pazienti affetti da carcinoma polmonare non a piccole cellule con positività mediastinica sottoposti a chemioterapia neoadiuvante [Accuracy of multislice CT in restaging patients with non-small cell lung carcinoma after neoadjuvant chemotherapy using a multiparametric approach]. Recenti Prog. Med. 2012, 103, 465–470. (In Italian) [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Center, M.M. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol. Biomark. Prev. 2010, 19, 1893–1907. [Google Scholar] [CrossRef]
- Mazzei, M.A.; Contorni, F.; Gentili, F.; Guerrini, S.; Mazzei, F.G.; Pinto, A.; Cioffi Squitieri, N.; Sisinni, A.G.; Paolucci, V.; Romeo, R.; et al. Incidental and Underreported Pleural Plaques at Chest CT: Do Not Miss Them-Asbestos Exposure Still Exists. BioMed Res. Int. 2017, 2017, 6797826. [Google Scholar] [CrossRef] [PubMed]
- Volterrani, L.; Guerrini, S.; Zanfrini, E.; Grassi, A.; Addamo, E.; Mathieu, F.; Gentili, F.; Bellan, C.; Spina, D.; Mazzei, M.A.; et al. HRCT predictors of GGO surgical resection: Histopathological and molecular correlation in the era of lung sparing surgery. Lung Cancer 2022. [Google Scholar] [CrossRef]
- Paolucci, V.; Romeo, R.; Sisinni, A.G.; Scancarello, G.; Volterrani, L.; Mazzei, M.A.; Barabesi, L.; Sartorelli, P. Asbestos exposure biomarkers in the follow-up of asbestos-exposed workers. Ind. Health 2018, 56, 249–254. [Google Scholar] [CrossRef]
- Mazzei, M.A.; Sartorelli, P.; Bagnacci, G.; Gentili, F.; Sisinni, A.G.; Fausto, A.; Mazzei, F.G.; Volterrani, L. Occupational Lung Diseases: Underreported Diagnosis in Radiological Practice. Semin. Ultrasound CT MR 2019, 40, 36–50. [Google Scholar] [CrossRef]
- Saghir, Z.; Dirksen, A.; Ashraf, H.; Bach, K.S.; Brodersen, J.; Clementsen, P.F.; Døssing, M.; Hansen, H.; Kofoed, K.F.; Larsen, K.R.; et al. CT screening for lung cancer brings forward early disease. The randomised Danish Lung Cancer Screening Trial: Status after five annual screening rounds with low-dose CT. Thorax 2012, 67, 296–301. [Google Scholar] [CrossRef]
- Infante, M.; Cavuto, S.; Lutman, F.R.; Passera, E.; Chiarenza, M.; Chiesa, G.; Brambilla, G.; Angeli, E.; Aranzulla, G.; Chiti, A.; et al. Long-Term Follow-up Results of the DANTE Trial, a Randomized Study of Lung Cancer Screening with Spiral Computed Tomography. Am. J. Respir. Crit. Care Med. 2015, 191, 1166–1175. [Google Scholar] [CrossRef]
- Horeweg, N.; Scholten, E.T.; de Jong, P.A.; van der Aalst, C.M.; Weenink, C.; Lammers, J.W.; Nackaerts, K.; Vliegenthart, R.; ten Haaf, K.; Yousaf-Khan, U.A.; et al. Detection of lung cancer through low-dose CT screening (NELSON): A prespecified analysis of screening test performance and interval cancers. Lancet Oncol. 2014, 15, 1342–1350. [Google Scholar] [CrossRef]
- National Lung Screening Trial Research Team; Aberle, D.R.; Adams, A.M.; Berg, C.D.; Black, W.C.; Clapp, J.D.; Fagerstrom, R.M.; Gareen, I.F.; Gatsonis, C.; Marcus, P.M.; et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [CrossRef] [PubMed]
- Infante, M.; Sestini, S.; Galeone, C.; Marchianò, A.; Lutman, F.R.; Angeli, E.; Calareso, G.; Pelosi, G.; Sozzi, G.; Silva, M.; et al. Lung cancer screening with low-dose spiral computed tomography: Evidence from a pooled analysis of two Italian randomized trials. Eur. J. Cancer Prev. 2017, 26, 324–329. [Google Scholar] [CrossRef] [PubMed]
- De Koning, H.J.; van der Aalst, C.M.; de Jong, P.A.; Scholten, E.T.; Nackaerts, K.; Heuvelmans, M.A.; Lammers, J.J.; Weenink, C.; Yousaf-Khan, U.; Horeweg, N.; et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N. Engl. J. Med. 2020, 382, 503–513. [Google Scholar] [CrossRef]
- Xu, D.M.; Gietema, H.; de Koning, H.; Vernhout, R.; Nackaerts, K.; Prokop, M.; Weenink, C.; Lammers, J.W.; Groen, H.; Oudkerk, M.; et al. Nodule management protocol of the NELSON randomised lung cancer screening trial. Lung Cancer 2006, 54, 177–184. [Google Scholar] [CrossRef]
- Mazzei, F.G.; Volterrani, L.; Guerrini, S.; Cioffi Squitieri, N.; Sani, E.; Bettini, G.; Pozzessere, C.; Mazzei, M. Reduced time CT perfusion acquisitions are sufficient to measure the permeability surface area product with a deconvolution method. BioMed Res. Int. 2014, 2014, 573268. [Google Scholar] [CrossRef]
- Mazzei, M.A.; Cioffi Squitieri, N.; Guerrini, S.; Di Crescenzo, V.; Rossi, M.; Fonio, P.; Mazzei, F.G.; Volterrani, L. La perfusione con TC nella caratterizzazione del nodulo polmonare solitario: Possibilità e limiti in uno studio preliminare [Quantitative CT perfusion measurements in characterization of solitary pulmonary nodules: New insights and limitations]. Recenti Prog. Med. 2013, 104, 430–437. (In Italian) [Google Scholar] [CrossRef]
- Mazzei, M.A.; Squitieri, N.C.; Sani, E.; Guerrini, S.; Imbriaco, G.; Di Lucia, D.; Guasti, A.; Mazzei, F.G.; Volterrani, L. Differences in perfusion CT parameter values with commercial software upgrades: A preliminary report about algorithm consistency and stability. Acta Radiol. 2013, 54, 805–811. [Google Scholar] [CrossRef]
- Cicero, G.; Ascenti, G.; Albrecht, M.H.; Blandino, A.; Cavallaro, M.; D’Angelo, T.; Carerj, M.L.; Vogl, T.J.; Mazziotti, S. Extra-abdominal dual-energy CT applications: A comprehensive overview. Radiol. Med. 2020, 125, 384–397. [Google Scholar] [CrossRef]
- MacMahon, H.; Naidich, D.P.; Goo, J.M.; Lee, K.S.; Leung, A.; Mayo, J.R.; Mehta, A.C.; Ohno, Y.; Powell, C.A.; Prokop, M.; et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology 2017, 284, 228–243. [Google Scholar] [CrossRef]
- Digumarthy, S.R.; Mendoza, D.P.; Lin, J.J.; Chen, T.; Rooney, M.M.; Chin, E.; Sequist, L.V.; Lennerz, J.K.; Gainor, J.F.; Shaw, A.T.; et al. Computed Tomography Imaging Features and Distribution of Metastases in ROS1-rearranged Non-Small-cell Lung Cancer. Clin. Lung Cancer 2020, 21, 153–159. [Google Scholar] [CrossRef]
- Mendoza, D.P.; Heeger, A.; Mino-Kenudson, M.; Lanuti, M.; Shepard, J.A.; Sequist, L.V.; Digumarthy, S.R. Clinicopathologic and Longitudinal Imaging Features of Lung Cancer Associated with Cystic Airspaces: A Systematic Review and Meta-Analysis. AJR Am. J. Roentgenol. 2021, 216, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Jett, J.R. Limitations of screening for lung cancer with low-dose spiral computed tomography. Clin. Cancer Res. 2005, 11, 4988s–4992s. [Google Scholar] [CrossRef] [PubMed]
- Volterrani, L.; Mazzei, M.A.; Banchi, B.; Voltolini, L.; La Sala, F.; Carbone, S.F.; Ricci, V.; Gotti, G.; Zompatori, M. MSCT multi-criteria: A novel approach in assessment of mediastinal lymph node metastases in non-small cell lung cancer. Eur. J. Radiol. 2011, 79, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Gould, M.K.; Donington, J.; Lynch, W.R.; Mazzone, P.J.; Midthun, D.E.; Naidich, D.P.; Wiener, R. Evaluation of individuals with pulmonary nodules: When is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013, 143, e93S–e120S. [Google Scholar] [CrossRef] [PubMed]
- Callister, M.E.; Baldwin, D.R.; Akram, A.R.; Barnard, S.; Cane, P.; Draffan, J.; Franks, K.; Gleeson, F.; Graham, R.; Malhotra, P.; et al. British Thoracic Society Pulmonary Nodule Guideline Development Group; British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the investigation and management of pulmonary nodules. Thorax 2015, 70, ii1–ii54, Erratum in Thorax 2015, 70, 1188. [Google Scholar] [CrossRef] [PubMed]
- American College of Radiology. Lung CT Screening Reporting and Data System (Lung-RADS). Available online: www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/Lung-Rads (accessed on 13 April 2020).
- Mazzone, P.J.; Gould, M.K.; Arenberg, D.A.; Chen, A.C.; Choi, H.K.; Detterbeck, F.C.; Farjah, F.; Fong, K.M.; Iaccarino, J.M.; Janes, S.M.; et al. Management of Lung Nodules and Lung Cancer Screening During the COVID-19 Pandemic: CHEST Expert Panel Report. Radiol. Imaging Cancer 2020, 2, e204013. [Google Scholar] [CrossRef]
- Pedersen, J.H.; Ashraf, H.; Dirksen, A.; Bach, K.; Hansen, H.; Toennesen, P.; Thorsen, H.; Brodersen, J.; Skov, B.G.; Døssing, M.; et al. The Danish randomized lung cancer CT screening trial-overall design and results of the prevalence round. J. Thorac. Oncol. 2009, 4, 608–614. [Google Scholar] [CrossRef]
- Becker, N.; Motsch, E.; Gross, M.L.; Eigentopf, A.; Heussel, C.P.; Dienemann, H.; Schnabel, P.A.; Pilz, L.; Eichinger, M.; Optazaite, D.E.; et al. Randomized study on early detection of lung cancer with MSCT in Germany: Study design and results of the first screening round. J. Cancer Res. Clin. Oncol. 2012, 138, 1475–1486. [Google Scholar] [CrossRef]
- Lopes Pegna, A.; Picozzi, G.; Mascalchi, M.; Maria Carozzi, F.; Carrozzi, L.; Comin, C.; Spinelli, C.; Falaschi, F.; Grazzini, M.; Innocenti, F.; et al. ITALUNG Study Research Grou. Design, recruitment and baseline results of the ITALUNG trial for lung cancer screening with low-dose CT. Lung Cancer 2009, 64, 34–40. [Google Scholar] [CrossRef]
- Franceschini, D.; Bruni, A.; Borghetti, P.; Giaj-Levra, N.; Ramella, S.; Buffoni, L.; Badellino, S.; Andolina, M.; Comin, C.; Vattemi, E.; et al. Is multidisciplinary management possible in the treatment of lung cancer? A report from three Italian meetings. Radiol. Med. 2020, 125, 214–219. [Google Scholar] [CrossRef]
- Hein, P.A.; Romano, V.C.; Rogalla, P.; Klessen, C.; Lembcke, A.; Bornemann, L.; Dicken, V.; Hamm, B.; Bauknecht, H.C. Variability of semiautomated lung nodule volumetry on ultralow-dose CT: Comparison with nodule volumetry on standard-dose CT. J. Digit. Imaging 2010, 23, 8–17. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Christe, A.; Torrente, J.C.; Lin, M.; Yen, A.; Hallett, R.; Roychoudhury, K.; Schmitzberger, F.; Vock, P.; Roos, J. CT screening and follow-up of lung nodules: Effects of tube current-time setting and nodule size and density on detectability and of tube current-time setting on apparent size. AJR Am. J. Roentgenol. 2011, 197, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Gartenschläger, M.; Schweden, F.; Gast, K.; Westermeier, T.; Kauczor, H.; von Zitzewitz, H.; Thelen, M. Pulmonary nodules: Detection with low-dose vs conventional-dose spiral CT. Eur. Radiol. 1998, 8, 609–614. [Google Scholar] [CrossRef]
- Karabulut, N.; Törü, M.; Gelebek, V.; Gülsün, M.; Ariyürek, O.M. Comparison of low-dose and standard-dose helical CT in the evaluation of pulmonary nodules. Eur. Radiol. 2002, 12, 2764–2769. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Dabiri, B.; Hammer, M.M. Micronodular lung disease on high-resolution CT: Patterns and differential diagnosis. Clin. Radiol. 2021, 76, 399–406. [Google Scholar] [CrossRef]
- Abbritti, M.; Mazzei, M.A.; Bargagli, E.; Refini, R.M.; Penza, F.; Perari, M.G.; Volterrani, L.; Rottoli, P. Utility of spiral CAT scan in the follow-up of patients with pulmonary Langerhans cell histiocytosis. Eur. J. Radiol. 2012, 81, 1907–1912. [Google Scholar] [CrossRef]
- Godoy, M.C.; Naidich, D.P. Overview and strategic management of subsolid pulmonary nodules. J. Thorac. Imaging 2012, 27, 240–248. [Google Scholar] [CrossRef]
- Thunnissen, F.B.; Schuurbiers, O.C.; den Bakker, M.A. A critical appraisal of prognostic and predictive factors for common lung cancers. Histopathology 2006, 48, 779–786. [Google Scholar] [CrossRef]
- Volterrani, L.; Mazzei, M.A.; Scialpi, M.; Carcano, M.; Carbone, S.F.; Ricci, V.; Guazzi, G.; Lupattelli, L. Three-dimensional analysis of pulmonary nodules by MSCT with Advanced Lung Analysis (ALA1) software. Radiol. Med. 2006, 111, 343–354. [Google Scholar] [CrossRef]
- Mazzei, M.A.; Scialpi, M.; Mazzei, F.G.; Giacobone, G.; Volterrani, L. Three-dimensional volumetric assessment with thoracic CT: A reliable approach for noncalcified lung nodules? Radiology 2010, 254, 634. [Google Scholar] [CrossRef][Green Version]
- Binczyk, F.; Prazuch, W.; Bozek, P.; Polanska, J. Radiomics and artificial intelligence in lung cancer screening. Transl. Lung Cancer Res. 2021, 10, 1186–1199. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; Granata, V.; Mazzei, M.A.; Meglio, N.D.; Roscio, D.D.; Moroni, C.; Monti, R.; Cappabianca, C.; Picone, C.; Neri, E.; et al. Quantitative imaging decision support (QIDSTM) tool consistency evaluation and radiomic analysis by means of 594 metrics in lung carcinoma on chest CT scan. Cancer Control 2021, 28, 1073274820985786. [Google Scholar] [CrossRef] [PubMed]
- Nardone, V.; Tini, P.; Pastina, P.; Botta, C.; Reginelli, A.; Carbone, S.F.; Giannicola, R.; Calabrese, G.; Tebala, C.; Guida, C.; et al. Radiomics predicts survival of patients with advanced non-small cell lung cancer undergoing PD-1 blockade using Nivolumab. Oncol. Lett. 2020, 19, 1559–1566. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Yang, Z.; Gong, L.; Jiang, S.; Wang, L.; Zhang, H. Classification of lung nodules based on CT images using squeeze-and-excitation network and aggregated residual transformations. Radiol. Med. 2020, 125, 374–383. [Google Scholar] [CrossRef]
- Volterrani, L.; Mazzei, M.A.; Fedi, M.; Scialpi, M. Computed tomography perfusion using first pass methods for lung nodule characterization: Limits and implications in radiologic practice. Invest. Radiol. 2009, 44, 124. [Google Scholar] [CrossRef]
- Mazzei, M.A.; Preda, L.; Cianfoni, A.; Volterrani, L. CT perfusion: Technical developments and current and future applications. BioMed Res. Int. 2015, 2015, 397521. [Google Scholar] [CrossRef]

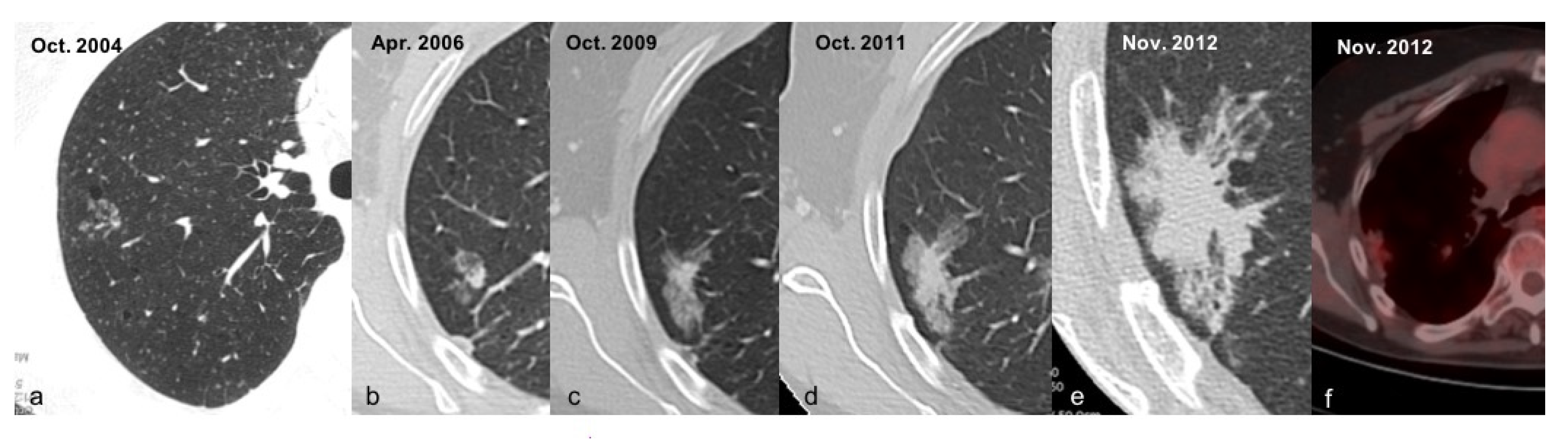
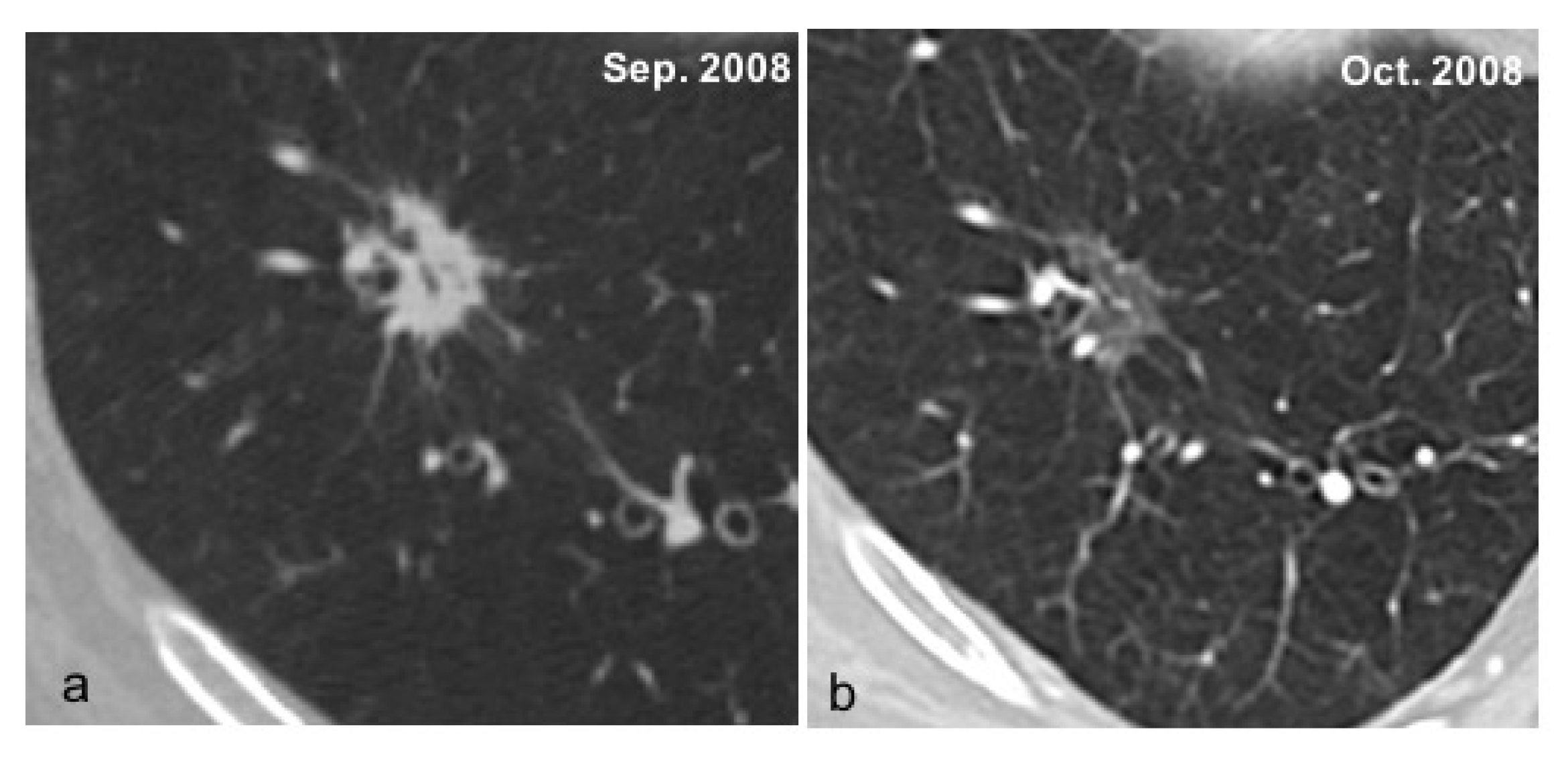
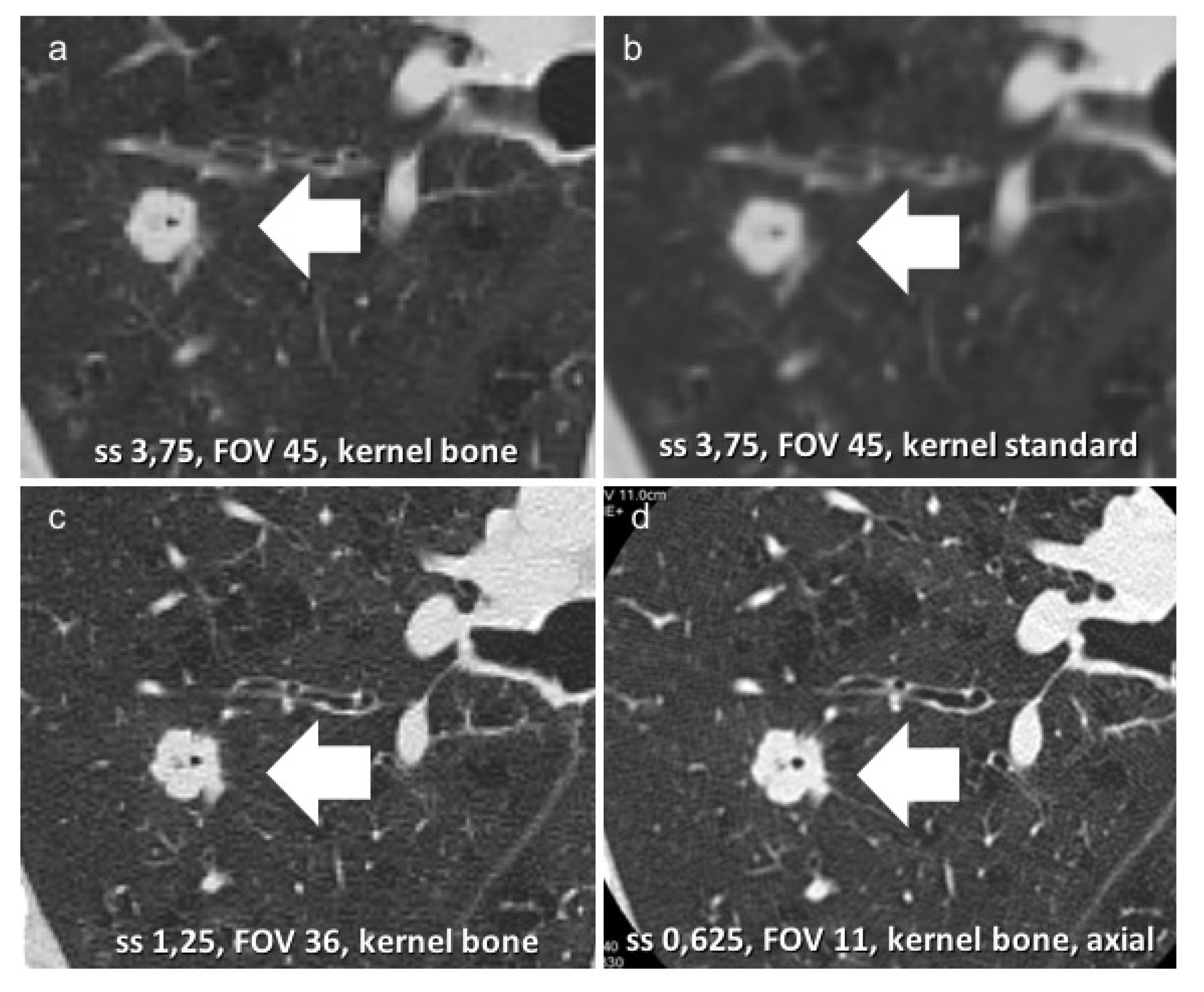
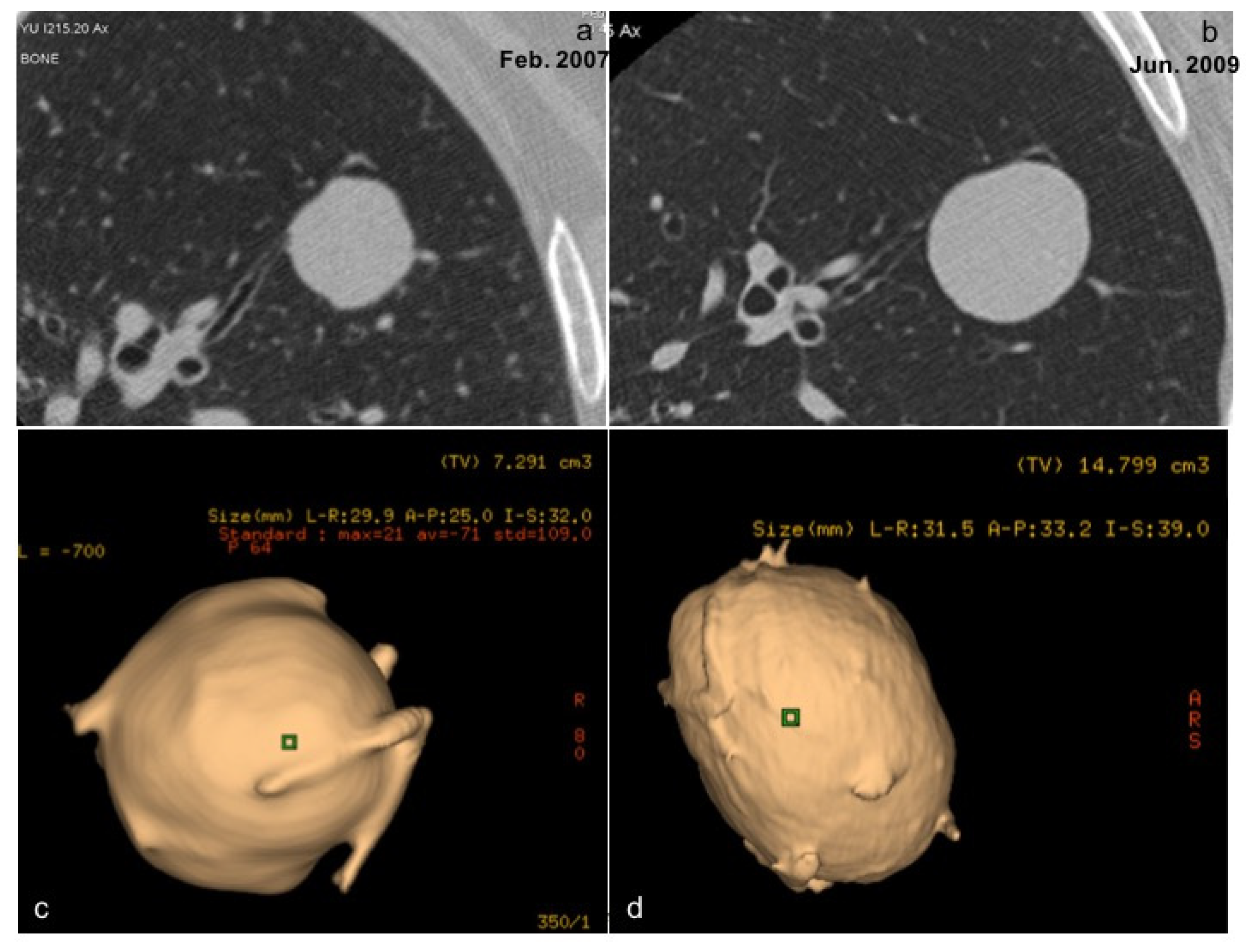
| De Koning 2020 [13] | Infante 2017 [12] | Infante 2015 [9] | Horeweg 2014 [10] | Aberle 2011 [11] | |
|---|---|---|---|---|---|
| Mean Age | 58 yo | 61 yo | 64 yo | 58 yo | NR |
| Male Sex | All | 2890 | NR | 5999 | 15,770 |
| Smoking status * | All | 2344 | 714 | 3959 | 12,862 |
| Mean p/y ** | 38 | 40 | 47.3 | 38 | NR |
| Patients | 6583 | 3640 | 1264 | 7155 | 27,722 |
| Nodule Dimensions | The Fleischner Society [19] | American College of Chest Physicians [24] | British Thoracic Society [25] | Lung CT Screening Reporting and Data System * [26] |
|---|---|---|---|---|
| <6 mm | LR, no FU HR, 12 mo FU | LR, ≤4 mm no FU LR, >4–6 mm or HR, ≤4 mm, 12 mo FUHR, >4–6 mm, 6–12 mo FU | <5 mm, no FU5–6 mm, 12–24 mo FU | <6 mm, AS (cat 2) |
| ≥6 mm to 8 mm | LR and HR, 6–12 mo FU, then re-evaluate | LR, 6–12 mo FU HR, 3–6 mo FU | 3 mo FU then 12 mo FU | ≥6 mm or new nodules 4–6 mm, 6 mo LDCT (cat 3) |
| ≥8 mm | CT or PET/CT at 3 mo | <5% risk, 3 mo FU; 5–65% risk, PET/CT and/or biopsy; >65% risk, treatment | <10% risk, surveillance; >10% risk, PET/CT or consider resection | 8–15 mm, 3 mo LDCT (cat 4A) >15 mm (cat 4B) |
| Nodule Dimensions | The Fleischner Society [19] | American College of Chest Physicians [24] | British Thoracic Society [25] | Lung CT Screening Reporting and Data System * [26] |
|---|---|---|---|---|
| <6 mm | <6 mm, GG or PS, no FU; if multiple, 3–6 mo LDCT FU | <6 mm, GG, no FU | <5 mm, no FU | 30 mm or more, GG, AS (cat 2) 6 mm, PS, AS (cat 2) if new 6 mo LDCT (cat 3) |
| ≥6 mm to 8 mm | ≥6 mm, GG, 6–12 mo FU; PS, 3–6 mo FUIf multiple, 3–6 mo FU | ≥6 mm GG, 12 mo FU; PS, ≤8 mm, 3, 12, and 24 mo FU | ≥5 mm, 3-mo LDCT than re-evaluate | ≥30 mm, GG or new 6 mo LDCT (cat 3) 6–8 mm, PS, 3 mo LDCT (cat 4A) |
| ≥8 mm | / | If solid, 3 mo FU | / | ≥8 mm, PS, (cat 4B) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerrini, S.; Del Roscio, D.; Zanoni, M.; Cameli, P.; Bargagli, E.; Volterrani, L.; Mazzei, M.A.; Luzzi, L. Lung Cancer Imaging: Screening Result and Nodule Management. Int. J. Environ. Res. Public Health 2022, 19, 2460. https://doi.org/10.3390/ijerph19042460
Guerrini S, Del Roscio D, Zanoni M, Cameli P, Bargagli E, Volterrani L, Mazzei MA, Luzzi L. Lung Cancer Imaging: Screening Result and Nodule Management. International Journal of Environmental Research and Public Health. 2022; 19(4):2460. https://doi.org/10.3390/ijerph19042460
Chicago/Turabian StyleGuerrini, Susanna, Davide Del Roscio, Matteo Zanoni, Paolo Cameli, Elena Bargagli, Luca Volterrani, Maria Antonietta Mazzei, and Luca Luzzi. 2022. "Lung Cancer Imaging: Screening Result and Nodule Management" International Journal of Environmental Research and Public Health 19, no. 4: 2460. https://doi.org/10.3390/ijerph19042460
APA StyleGuerrini, S., Del Roscio, D., Zanoni, M., Cameli, P., Bargagli, E., Volterrani, L., Mazzei, M. A., & Luzzi, L. (2022). Lung Cancer Imaging: Screening Result and Nodule Management. International Journal of Environmental Research and Public Health, 19(4), 2460. https://doi.org/10.3390/ijerph19042460








