Effects of a 12-Week Diet versus Diet plus Aerobic and Resistance Exercise Program on Acylated and Desacylated Ghrelin, and Ghrelin O-Acyltransferase in Adolescent Girls with Obesity
Abstract
1. Introduction
2. Materials and Methods
2.1. Trial Design
2.2. Study Participants
2.3. Randomisation and Follow-Up
2.4. Interventions
2.5. Baseline and Follow-Up Measurements
2.6. Sample Size
2.7. Statistical Analyses
3. Results
3.1. Baseline Characteristics of the Participants
3.2. Changes in Body Composition, Glucose Concentrations, and Lipid Profile
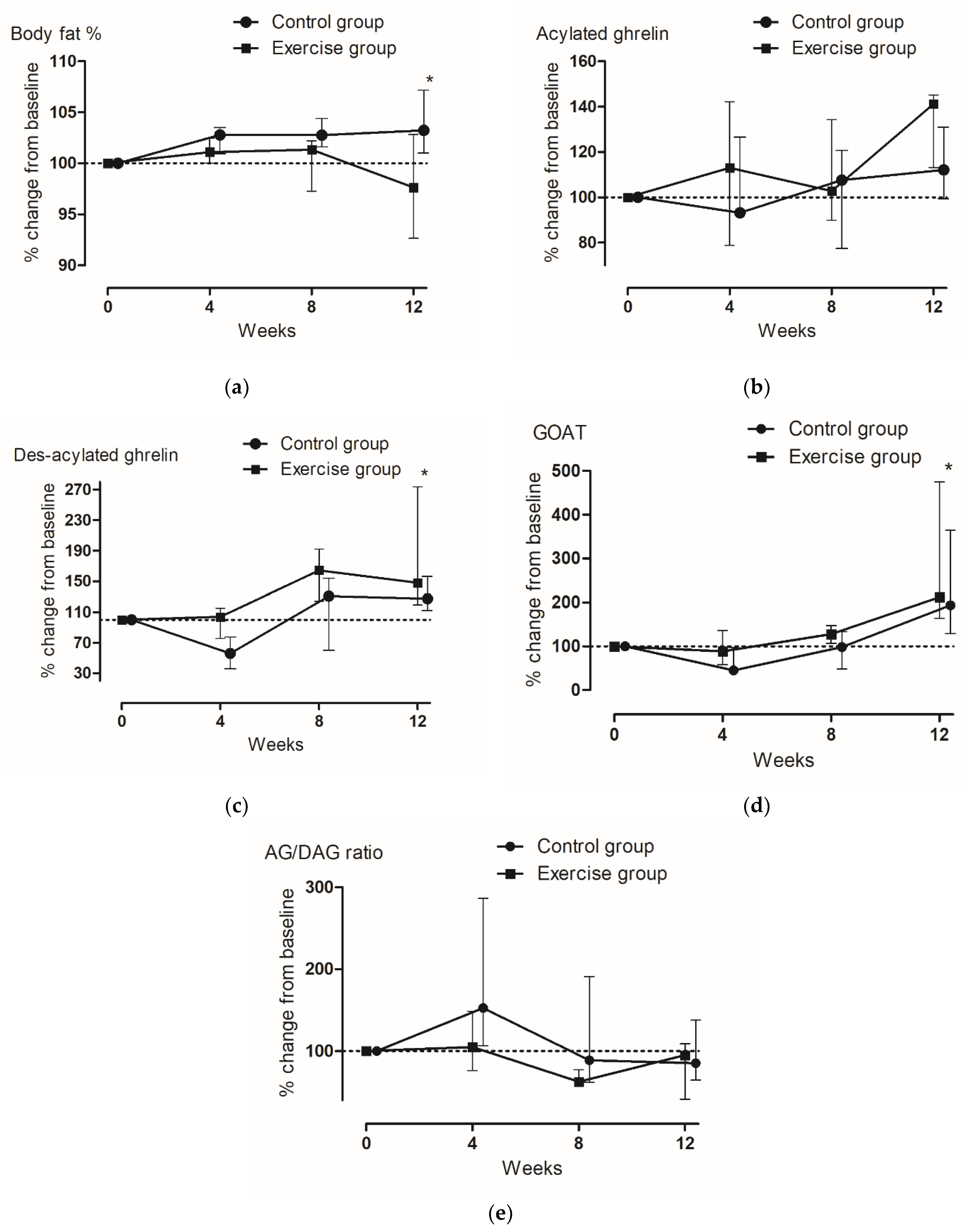
3.3. Changes in Gut Hormones
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef]
- Patton, G.C.; Coffey, C.; Carlin, J.B.; Sawyer, S.M.; Williams, J.; Olsson, C.A.; Wake, M. Overweight and obesity between adolescence and young adulthood: A 10-year prospective cohort study. J. Adolesc. Health 2011, 48, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Steinbeck, K.S.; Lister, N.B.; Gow, M.L.; Baur, L.A. Treatment of adolescent obesity. Nat. Rev. Endocrinol. 2018, 14, 331–344. [Google Scholar] [CrossRef] [PubMed]
- King, N.A.; Horner, K.; Hills, A.P.; Byrne, N.M.; Wood, R.E.; Bryant, E.; Caudwell, P.; Finlayson, G.; Gibbons, C.; Hopkins, M.; et al. Exercise, appetite and weight management: Understanding the compensatory responses in eating behaviour and how they contribute to variability in exercise-induced weight loss. Br. J. Sports Med. 2012, 46, 315–322. [Google Scholar] [CrossRef]
- Rumbold, P.L.; St Clair Gibson, A.; Allsop, S.; Stevenson, E.; Dodd-Reynolds, C.J. Energy intake and appetite following netball exercise over 5 days in trained 13–15 year old girls. Appetite 2011, 56, 621–628. [Google Scholar] [CrossRef]
- Mackelvie, K.J.; Meneilly, G.S.; Elahi, D.; Wong, A.C.; Barr, S.I.; Chanoine, J.P. Regulation of appetite in lean and obese adolescents after exercise: Role of acylated and desacyl ghrelin. J. Clin. Endocrinol. Metab. 2007, 92, 648–654. [Google Scholar] [CrossRef]
- Mani, B.K.; Shankar, K.; Zigman, J.M. Ghrelin’s Relationship to Blood Glucose. Endocrinology 2019, 160, 1247–1261. [Google Scholar] [CrossRef]
- Shlimun, A.; Unniappan, S. Ghrelin o-acyl transferase: Bridging ghrelin and energy homeostasis. Int. J. Pept. 2011, 2011, 217957. [Google Scholar] [CrossRef]
- Fernandez, G.; Cabral, A.; Cornejo, M.P.; De Francesco, P.N.; Garcia-Romero, G.; Reynaldo, M.; Perello, M. Des-Acyl Ghrelin Directly Targets the Arcuate Nucleus in a Ghrelin-Receptor Independent Manner and Impairs the Orexigenic Effect of Ghrelin. J. Neuroendocrinol. 2016, 28, 12349. [Google Scholar] [CrossRef]
- Akalu, Y.; Molla, M.D.; Dessie, G.; Ayelign, B. Physiological Effect of Ghrelin on Body Systems. Int. J. Endocrinol. 2020, 2020, 1385138. [Google Scholar] [CrossRef]
- Howick, K.; Griffin, B.T.; Cryan, J.F.; Schellekens, H. From Belly to Brain: Targeting the Ghrelin Receptor in Appetite and Food Intake Regulation. Int. J. Mol. Sci. 2017, 18, 273. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, S.; Kim, T.W.; Kim, H.H.; Jeon, T.Y.; Yoon, Y.S.; Oh, S.W.; Kwak, H.; Lee, J.G. Effects of exercise-induced weight loss on acylated and unacylated ghrelin in overweight children. Clin. Endocrinol. 2008, 68, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, B.; Furness, J.B. Novel and conventional receptors for ghrelin, desacyl-ghrelin, and pharmacologically related compounds. Pharmacol. Rev. 2014, 66, 984–1001. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Gómez-Ambrosi, J.; Catalán, V.; Becerril, S.; Sáinz, N.; Gil, M.J.; Silva, C.; Salvador, J.; Barba, J.; Colina, I.; et al. Association of plasma acylated ghrelin with blood pressure and left ventricular mass in patients with metabolic syndrome. J. Hypertens. 2010, 28, 560–567. [Google Scholar] [CrossRef]
- Cederberg, H.; Rajala, U.; Koivisto, V.M.; Jokelainen, J.; Surcel, H.M.; Keinänen-Kiukaanniemi, S.; Laakso, M. Unacylated ghrelin is associated with changes in body composition and body fat distribution during long-term exercise intervention. Eur. J. Endocrinol. 2011, 165, 243–248. [Google Scholar] [CrossRef]
- Barazzoni, R.; Aleksova, A.; Armellini, I.; Cattin, M.R.; Zanetti, M.; Carriere, C.; Giacca, M.; Dore, F.; Guarnieri, G.; Sinagra, G. Adipokines, ghrelin and obesity-associated insulin resistance in nondiabetic patients with acute coronary syndrome. Obesity 2012, 20, 2348–2353. [Google Scholar] [CrossRef]
- Kuppens, R.J.; Delhanty, P.J.; Huisman, T.M.; Van Der Lely, A.J.; Hokken-Koelega, A.C. Acylated and unacylated ghrelin during OGTT in Prader-Willi syndrome: Support for normal response to food intake. Clin. Endocrinol. 2016, 85, 488–494. [Google Scholar] [CrossRef]
- Dorling, J.; Broom, D.R.; Burns, S.F.; Clayton, D.J.; Deighton, K.; James, L.J.; King, J.A.; Miyashita, M.; Thackray, A.E.; Batterham, R.L.; et al. Acute and Chronic Effects of Exercise on Appetite, Energy Intake, and Appetite-Related Hormones: The Modulating Effect of Adiposity, Sex, and Habitual Physical Activity. Nutrients 2018, 10, 1140. [Google Scholar] [CrossRef]
- Prado, W.L.; Oyama, L.M.; Lofrano-Prado, M.C.; de Piano, A.; Stella, S.G.; Nascimento, C.M.; Carnier, J.; Caranti, D.A.; Tock, L.; Tufik, S.; et al. Alterations in downstream mediators involved in central control of eating behavior in obese adolescents submitted to a multidisciplinary therapy. J. Adolesc. Health 2011, 49, 300–305. [Google Scholar] [CrossRef]
- King, J.A.; Wasse, L.K.; Stensel, D.J.; Nimmo, M.A. Exercise and ghrelin. A narrative overview of research. Appetite 2013, 68, 83–91. [Google Scholar] [CrossRef]
- Leidy, H.J.; Gardner, J.K.; Frye, B.R.; Snook, M.L.; Schuchert, M.K.; Richard, E.L.; Williams, N.I. Circulating ghrelin is sensitive to changes in body weight during a diet and exercise program in normal-weight young women. J. Clin. Endocrinol. Metab. 2004, 89, 2659–2664. [Google Scholar] [CrossRef] [PubMed]
- Ouerghi, N.; Feki, M.; Bragazzi, N.L.; Knechtle, B.; Hill, L.; Nikolaidis, P.T.; Bouassida, A. Ghrelin Response to Acute and Chronic Exercise: Insights and Implications from a Systematic Review of the Literature. Sports Med. 2021, 51, 2389–2410. [Google Scholar] [CrossRef] [PubMed]
- Sieburg, M.A.; Cleverdon, E.R.; Hougland, J.L. Biochemical Assays for Ghrelin Acylation and Inhibition of Ghrelin O-Acyltransferase. In Protein Lipidation. Methods in Molecular Biology; Linder, M., Ed.; Humana: New York, NY, USA, 2019; Volume 2009, pp. 227–241. [Google Scholar]
- Ha, K.H.; Kim, D.J. Epidemiology of Childhood Obesity in Korea. Endocrinol. Metab. 2016, 31, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Moon, J.S. Secular Trends in Pediatric Overweight and Obesity in Korea. J. Obes. Metab. Syndr. 2020, 29, 12–17. [Google Scholar] [CrossRef]
- Park, J.; Ma, H.; Lee, Y.; Oh, H. Trends in Intervention Study for Childhood Obesity in Korea. Child Health Nurs. Res. 2017, 23, 81–90. [Google Scholar] [CrossRef][Green Version]
- Kim, J.H.; Yun, S.; Hwang, S.S.; Shim, J.O.; Chae, H.W.; Lee, Y.J.; Lee, J.H.; Kim, S.C.; Lim, D.; Yang, S.W.; et al. The 2017 Korean National Growth Charts for children and adolescents: Development, improvement, and prospects. Korean J. Pediatr. 2018, 61, 135–149. [Google Scholar] [CrossRef]
- Williams, N. The Borg Rating of Perceived Exertion (RPE) scale. Occup. Med. 2017, 67, 404–405. [Google Scholar] [CrossRef]
- Thompson, P.D.; Arena, R.; Riebe, D.; Pescatello, L.S.; American College of Sports Medicine. ACSM’s new preparticipation health screening recommendations from ACSM’s guidelines for exercise testing and prescription, ninth edition. Curr. Sports Med. Rep. 2013, 12, 215–217. [Google Scholar] [CrossRef]
- Stark, R.; Santos, V.V.; Geenen, B.; Cabral, A.; Dinan, T.; Bayliss, J.A.; Lockie, S.H.; Reichenbach, A.; Lemus, M.B.; Perello, M.; et al. Des-Acyl ghrelin and ghrelin O-acyltransferase regulate hypothalamic-pituitary-adrenal axis activation and anxiety in response to acute stress. Endocrinology 2016, 157, 3946–3957. [Google Scholar] [CrossRef]
- Barazzoni, R.; Gortan Cappellari, G.; Zanetti, M.; Klaus, K.A.; Semolic, A.; Johnson, M.L.; Nair, K.S. Higher unacylated ghrelin and insulin sensitivity following dietary restriction and weight loss in obese humans. Clin. Nutr. 2021, 40, 638–644. [Google Scholar] [CrossRef]
- Goebel-Stengel, M.; Hofmann, T.; Elbelt, U.; Teuffel, P.; Ahnis, A.; Kobelt, P.; Lambrecht, N.W.; Klapp, B.F.; Stengel, A. The ghrelin activating enzyme ghrelin-O-acyltransferase (GOAT) is present in human plasma and expressed dependent on body mass index. Peptides 2013, 43, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Satou, M.; Nishi, Y.; Yoh, J.; Hattori, Y.; Sugimoto, H. Identification and characterization of acyl-protein thioesterase 1/lysophospholipase I as a ghrelin deacylation/lysophospholipid hydrolyzing enzyme in fetal bovine serum and conditioned medium. Endocrinology 2010, 151, 4765–4775. [Google Scholar] [CrossRef] [PubMed]
- Ravussin, E.; Tschöp, M.; Morales, S.; Bouchard, C.; Heiman, M.L. Plasma ghrelin concentration and energy balance: Overfeeding and negative energy balance studies in twins. J. Clin. Endocrinol. Metab. 2001, 86, 4547–4551. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, W.; Chai, B.; Li, J.Y.; Wang, H.; Mulholland, M.W. Effect of des-acyl ghrelin on adiposity and glucose metabolism. Endocrinology 2008, 149, 4710–4716. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.D.; Nogueiras, R.; Andermann, M.L.; Andrews, Z.B.; Anker, S.D.; Argente, J.; Batterham, R.L.; Benoit, S.C.; Bowers, C.Y.; Broglio, F.; et al. Ghrelin. Mol. Metab. 2015, 4, 437–460. [Google Scholar] [CrossRef]
- Delhanty, P.J.; Sun, Y.; Visser, J.A.; van Kerkwijk, A.; Huisman, M.; Van Ijcken, W.F.; Swagemakers, S.; Smith, R.G.; Themmen, A.P.; Van Der Lely, A.J. Unacylated ghrelin rapidly modulates lipogenic and insulin signaling pathway gene expression in metabolically active tissues of GHSR deleted mice. PLoS ONE 2010, 5, e11749. [Google Scholar] [CrossRef]
- Dardzińska, J.A.; Kaska, Ł.; Proczko-Stepaniak, M.; Szymańska-Gnacińska, M.; Aleksandrowicz-Wrona, E.; Małgorzewicz, S. Fasting and postprandial acyl and desacyl ghrelin and the acyl/desacyl ratio in obese patients before and after different types of bariatric surgery. Wideochir. Inne. Tech. Maloinwazyjne. 2018, 13, 366–375. [Google Scholar] [CrossRef]
- Rigamonti, A.E.; Agosti, F.; De Col, A.; Marazzi, N.; Lafortuna, C.L.; Cella, S.G.; Muller, E.E.; Sartorio, A. Changes in plasma levels of ghrelin, leptin, and other hormonal and metabolic parameters following standardized breakfast, lunch, and physical exercise before and after a multidisciplinary weight-reduction intervention in obese adolescents. J. Endocrinol. Investig. 2010, 33, 633–639. [Google Scholar] [CrossRef]
- Jeon, T.Y.; Lee, S.; Kim, H.H.; Kim, Y.J.; Son, H.C.; Kim, D.H.; Sim, M.S. Changes in plasma ghrelin concentration immediately after gastrectomy in patients with early gastric cancer. J. Clin. Endocrinol. Metab. 2004, 89, 5392–5396. [Google Scholar] [CrossRef]
- Cummings, D.E.; Frayo, R.S.; Marmonier, C.; Aubert, R.; Chapelot, D. Plasma ghrelin levels and hunger scores in humans initiating meals voluntarily without time- and food-related cues. Am. J. Physiol. Endocrinol. Metab. 2004, 287, E297–E304. [Google Scholar] [CrossRef]
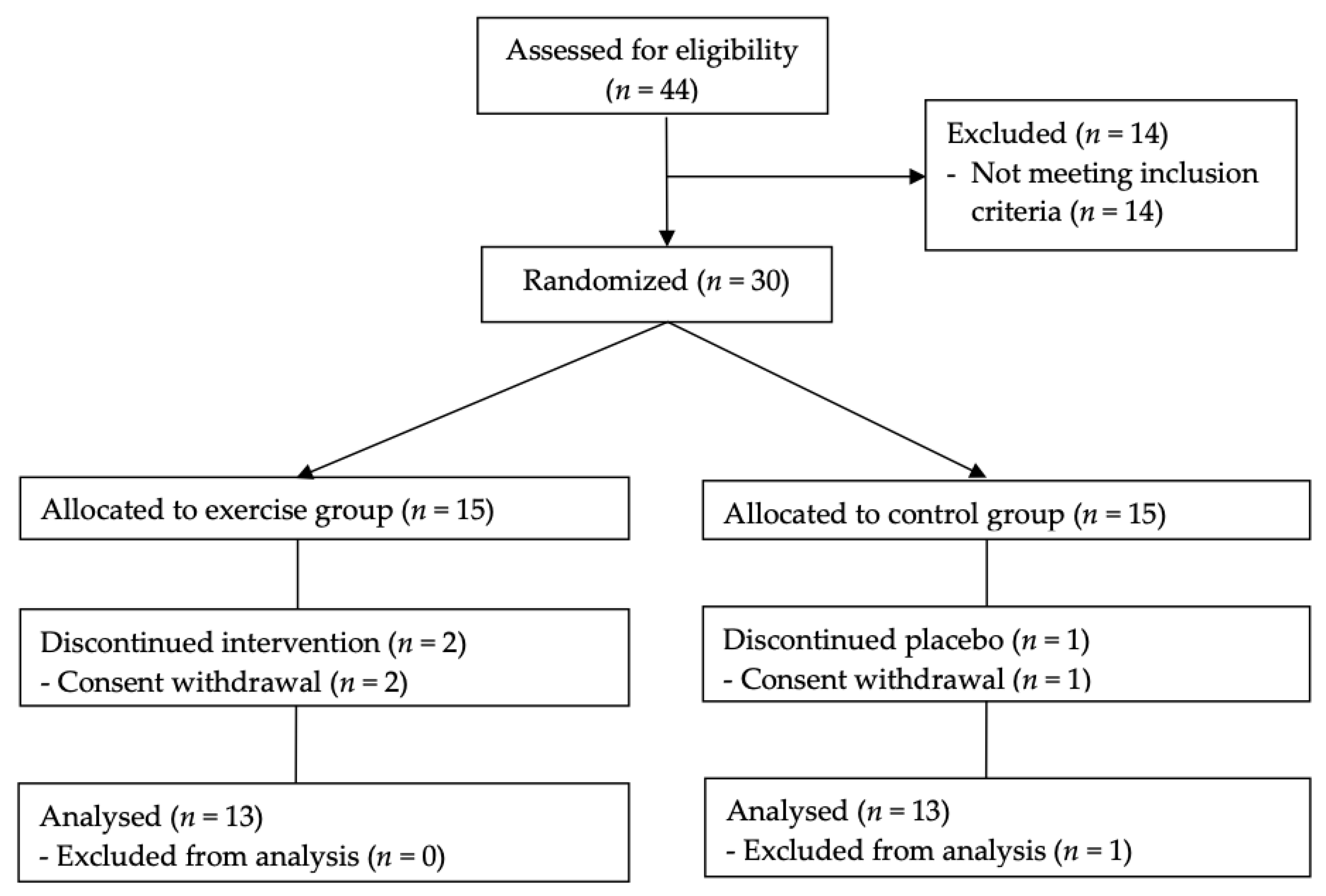
| Parameters | Exercise Group (n = 13) | Control Group (n = 13) | p |
|---|---|---|---|
| Age | |||
| 17 | 12 (92.3) | 8 (61.5) | |
| 18 | 1 (0.7) | 5 (38.5) | |
| Weight (kg) | 75.1 ± 8.2 | 78.0 ± 13.5 | 0.513 |
| BMI (kg/m2) | 27.1 (26.4–30.0) | 29.7 (26.3–33.4) | 0.397 * |
| WC (cm) | 85.0 ± 5.5 | 88.5 ± 9.0 | 0.236 |
| Body fat (%) | 33.9 ± 2.7 | 35.5 ± 3.5 | 0.189 |
| Lean body mass (kg) | 45.3 ± 3.4 | 45.3 ± 5.7 | 0.964 |
| TC (mg/dL) | 155.7 ± 45.2 | 131.9 ± 15.6 | 0.086 |
| Triglyceride (mg/dL) | 55.0 (45.0–87.0) | 52.0 (45.0–77.3) | 0.652 * |
| HDL-C (mg/dL) | 61.6 ± 15.4 | 57.0 ± 10.6 | 0.382 |
| LDL-C (mg/dL) | 79.9 ± 41.1 | 61.8 ± 14.7 | 0.148 |
| Glucose (mg/dL) | 88.0 (84.5–90.3) | 87.0 (76.8–91.5) | 0.567 |
| Total ghrelin (pg/mL) | 445.9 ± 236.0 | 799.5 ± 213.4 | 0.001 |
| AG (pg/mL) | 64.9 ± 16.5 | 81.7 ± 16.5 | 0.188 |
| DAG (pg/mL) | 381.0 ± 247.2 | 717.8 ± 59.8 | 0.001 |
| AG/DAG ratio | 0.27 ± 0.18 | 0.13 ± 0.09 | 0.002 |
| GOAT (ng/mL) | 9.3 (6.9–23.1) | 15.0 (9.5–22.5) | 0.535 |
| Parameters | Baseline | 4 Weeks | 8 Weeks | 12 Weeks | Within-Group | Between-Group | Group × Time | |
|---|---|---|---|---|---|---|---|---|
| Weight (kg) | EG | 75.1 ± 8.2 | 75.2 ± 7.9 | 75.1 ± 8.3 | 74.3 ± 8.5 | 0.538 | 0.412 | 0.114 |
| CG | 78.0 ± 13.5 | 78.5 ± 13.1 | 78.8 ± 13.6 | 79.2 ± 14.1 | ||||
| BMI (kg/m2) | EG | 27.1 (26.4–30.0) | 27.2 (26.5–29.4) | 27.1 (26.2–29.5) | 27.1 (25.9–29.5) | 0.542 | 0.163 | 0.097 |
| CG | 29.7 (26.3–33.4) | 28.9 (26.7–33.5) | 29.9 (26.4–33.5) | 30.0 (26.1–34.0) | ||||
| WC (cm) | EG | 85.0 ± 5.5 | 83.4 ± 6.7 | 83.7 ± 6.6 | 84.2 ± 7.5 | 0.345 | 0.259 | 0.543 |
| CG | 88.5 ± 9.0 | 88.0 ± 10.7 | 87.4 ± 8.2 | 86.5 ± 10.4 | ||||
| BF (%) | EG | 33.9 ± 2.7 | 34.1 ± 2.6 | 33.1 ± 2.6 | 33.3 ± 2.9 | 0.042 | 0.064 | 0.051 |
| CG | 35.5 ± 3.5 | 36.4 ± 3.4 | 36.4 ± 3.4 | 36.3 ± 3.7 | ||||
| LBM (kg) | EG | 45.3 ± 3.4 | 45.1 ± 3.2 | 45.3 ± 3.4 | 45.2 ± 3.4 | 0.473 | 0.936 | 0.581 |
| CG | 45.3 ± 5.7 | 45.1 ± 5.3 | 45.4 ± 5.8 | 45.5 ± 5.8 |
| Parameters | Baseline | 4 Weeks | 8 Weeks | 12 Weeks | Within-Group | Between-Group | Group × Time | |
|---|---|---|---|---|---|---|---|---|
| TC (mg/dL) | EG | 155.7 ± 45.2 | 139.4 ± 28.3 | 138.8 ± 32.9 | 161.2 ± 31.9 | 0.002 | 0.184 | 0.107 |
| CG | 131.9 ± 15.6 | 129.6 ± 12.1 | 138.2 ± 19.1 | 144.5 ± 24.9 | ||||
| Tryglyceride (mg/dL) | EG | 61.6 ± 15.4 | 48.9 ± 11.3 | 54.2 ± 14.6 | 55.2 ± 13.0 | 0.001 | 0.985 | 0.193 |
| CG | 57.0 ± 10.6 | 51.8 ± 10.4 | 53.4 ± 11.4 | 57.5 ± 7.7 | ||||
| HDL-C (mg/dL) | EG | 55.0 (45.0–87.0) | 45.0 (45.0–83.0) | 69.0 (51.8–93.0) | 80.0 (64.8–115.3) | 0.048 | 0.258 | 0.527 |
| CG | 52.0 (45.0–77.3) | 57.0 (45.0–71.8) | 64.0 (49.5–82.8) | 65.0 (48.8–95.5) | ||||
| LDL-C (mg/dL) | EG | 79.9 ± 41.1 | 76.0 ± 27.1 | 68.9 ± 33.1 | 86.4 ± 27.5 | 0.198 | 0.253 | 0.162 |
| CG | 61.8 ± 14.7 | 67.3 ± 11.2 | 70.8 ± 18.2 | 71.6 ± 21.7 | ||||
| Glucose (mg/dL) | EG | 88.0 (84.5–90.3) | 82.0 (79.8–85.3) | 88.0 (77.8–89.0) | 85.0 (83.0–100.8) | 0.236 | 0.187 | 0.026 |
| CG | 87.0 (76.8–91.5) | 81.0 (80.0–84.5) | 85.0 (81.0–96.3) | 79.0 (78.0–83.3) | ||||
| TG (pg/mL) | EG | 445.9 ± 247.2 | 449.4 ± 214.6 | 654.8 ± 277.8 | 737.0 ± 296.1 | 0.088 | 0.042 | 0.392 |
| CG | 799.5 ± 213.4 | 543.0 ± 342.4 | 1296.7 ± 539.1 | 1022.0 ± 298.1 | ||||
| AG (pg/mL) | EG | 64.9 ± 16.5 | 74.3 ± 32.3 | 76.4 ± 32.4 | 83.9 ± 34.7 | 0.017 | 0.372 | 0.707 |
| CG | 81.7 ± 16.5 | 84.5 ± 16.5 | 81.7 ± 16.5 | 94.4 ± 16.5 | ||||
| DAG (pg/mL) | EG | 381.0 ± 247.2 | 375.1 ± 214.6 | 578.3 ± 277.8 | 653.0 ± 297.1 | 0.092 | 0.048 | 0.386 |
| CG | 717.8 ± 215.8 | 458.5 ± 326.3 | 1214.0 ± 465.0 | 927.5 ± 299.1 | ||||
| AG/DAG ratio | EG | 0.27 ± 0.18 | 0.29 ± 0.21 | 0.18 ± 0.12 | 0.18 ± 0.13 | <0.001 | 0.902 | 0.915 |
| CG | 0.13 ± 0.09 | 0.24 ± 0.13 | 0.12 ± 0.07 | 0.11 ± 0.07 | ||||
| GOAT (ng/mL) | EG | 9.3 (6.7–23.8) | 8.1 (6.0–14.3) | 27.7 (8.3–35.7) | 44.8 (16.1–53.8) | <0.001 | 0.096 | 0.220 |
| CG | 15.0 (9.0–25.7) | 7.9 (6.3–15.2) | 20.1 (8.5–23.6) | 43.5 (26.2–46.2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.J.; Tak, Y.J.; Lee, S.Y.; Seo, J.P. Effects of a 12-Week Diet versus Diet plus Aerobic and Resistance Exercise Program on Acylated and Desacylated Ghrelin, and Ghrelin O-Acyltransferase in Adolescent Girls with Obesity. Int. J. Environ. Res. Public Health 2022, 19, 1480. https://doi.org/10.3390/ijerph19031480
Kim HJ, Tak YJ, Lee SY, Seo JP. Effects of a 12-Week Diet versus Diet plus Aerobic and Resistance Exercise Program on Acylated and Desacylated Ghrelin, and Ghrelin O-Acyltransferase in Adolescent Girls with Obesity. International Journal of Environmental Research and Public Health. 2022; 19(3):1480. https://doi.org/10.3390/ijerph19031480
Chicago/Turabian StyleKim, Hyun Jun, Young Jin Tak, Sang Yeoup Lee, and Jeong Pyo Seo. 2022. "Effects of a 12-Week Diet versus Diet plus Aerobic and Resistance Exercise Program on Acylated and Desacylated Ghrelin, and Ghrelin O-Acyltransferase in Adolescent Girls with Obesity" International Journal of Environmental Research and Public Health 19, no. 3: 1480. https://doi.org/10.3390/ijerph19031480
APA StyleKim, H. J., Tak, Y. J., Lee, S. Y., & Seo, J. P. (2022). Effects of a 12-Week Diet versus Diet plus Aerobic and Resistance Exercise Program on Acylated and Desacylated Ghrelin, and Ghrelin O-Acyltransferase in Adolescent Girls with Obesity. International Journal of Environmental Research and Public Health, 19(3), 1480. https://doi.org/10.3390/ijerph19031480







