One Health Action against Human Fascioliasis in the Bolivian Altiplano: Food, Water, Housing, Behavioural Traditions, Social Aspects, and Livestock Management Linked to Disease Transmission and Infection Sources
Abstract
1. Introduction
- Human inhabitants are also obliged to adapt to such extreme altitudinal rural environment; thus, they mainly depend on livestock, because plant cultures require a lot of effort and produce low, insufficient yields, useful only for subsistence; sheep, cattle, pigs, and donkeys are the main species owned by families, and the four species most infected by F. hepatica in that area [27];
- Climate characteristics including (i) the lack of marked differences of temperature throughout the whole year and (ii) the high altitudinal evapotranspiration [32] leading lymnaeid populations to mainly inhabit permanent freshwater collections, together with the long infection capacity kept by the metacercariae under low temperatures [33], assure a permanent transmission with human and animal infection risk throughout the whole year, i.e., lack of seasonality;
- The transmission is markedly enhanced when compared with lowlands [34], because (i) the infected snails follow a longer patent period, with a cercarial shedding process being twice as long, (ii) the production of cercariae by each snail is pronouncedly higher, (iii) the infected lymnaeids survive longer [35], and (iv) the uterus of the adult stage is shorter, favoring a continuous shedding [36,37].
2. Materials and Methods
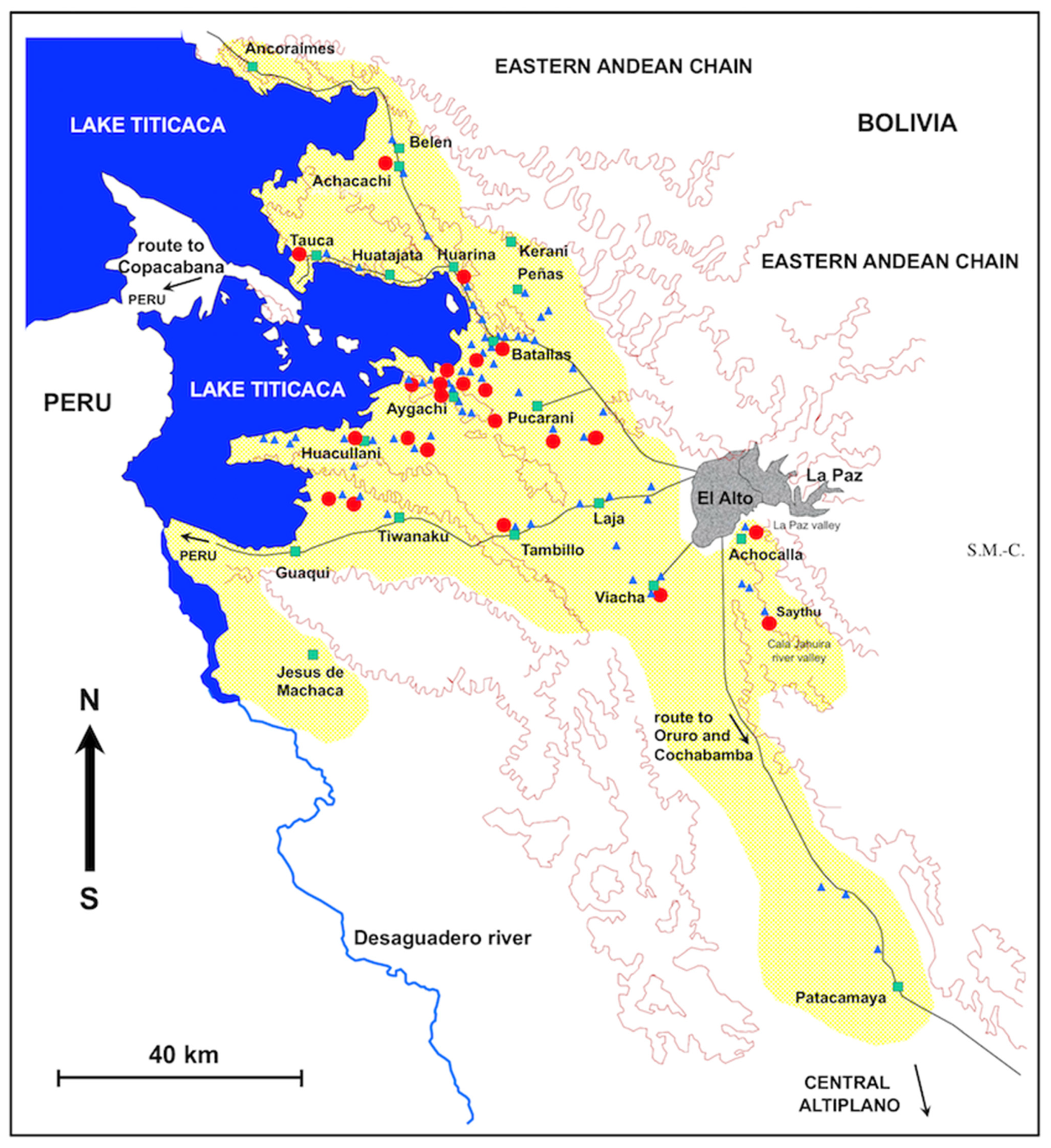
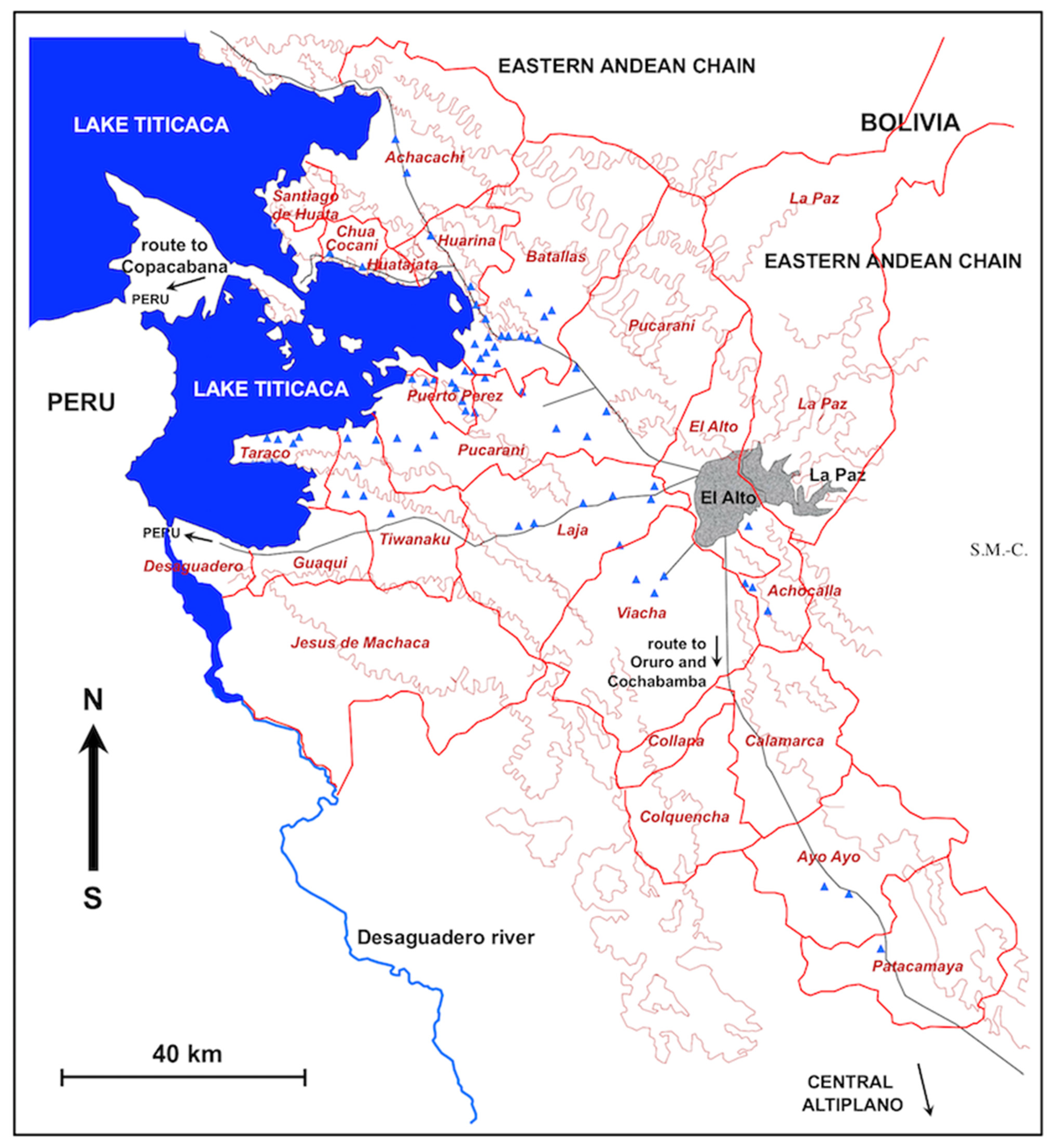
2.1. Study Area
- Assessing the distribution of lymnaeid snails allowing for liver fluke transmission: Lymnaeid snails were differentiated from other freshwater snails by their pair of triangular tentacles with darkly pigmented eyes at their bases and their typical small, smooth, and dextral conical shell. In the Northern Altiplano, there are no other snails in the freshwater collections which may be confused with lymnaeids [51]. To distinguish between lymnaeid species with liver fluke transmission capacity, i.e., lymnaeid intermediate hosts or vectors, and non-transmitting lymnaeid species potentially present because known to also inhabit Andean high altitude areas [22], lymnaeids from each population collected were molecularly characterized by the sequencing of two ribosomal DNA markers (ITS-2 and ITS-1) and two mitochondrial DNA markers (16S and cox1). These genetic analyses demonstrated that a single, genetically monomorphic lymnaeid vector species is present in the Northern Bolivian Altiplano and is the responsible of fascioliasis transmission in this endemic area, namely Galba truncatula [46].
- Analysing the geographical extent of fasciolid infection in cattle: Cattle was the livestock species selected for this assessment because (i) this ruminant reservoir is present and distributed throughout the Northern Bolivian Altiplano and has proved to significantly participate in the transmission of the disease [47]; (ii) it is the most important species among the livestock owned by each Altiplanic Aymara family [27]; and (iii) cattle is the most appropriate livestock species for such a geographical extent assessment because egg output in this animal only lasts for a short time, a high production of eggs has a duration of only a few weeks, resistance in this ruminant is acquired already from the first infection, and consequently fascioliasis in cattle is self-limiting with the majority of flukes being eliminated in the initial 9–12 months, a host–parasite interaction pronouncedly different from the long-lasting infection and egg shedding in sheep [9].
- Verifying that lymnaeid snail presence and cattle infection was geographically followed by human infection: To assess the geographical overlap of human infection with the distribution of the lymnaeid vector and cattle infection, liver fluke infection in 5–15-year-old schoolchildren was coprologically diagnosed [26], because (i) primary rural schools are present and distributed throughout the Northern Bolivian Altiplano, (ii) each one of these schools receives children from its surrounding zone, and (iii) faecal output of eggs indicates active infections, whereas serological tests do not necessarily indicate such [52].
2.2. Field Prospections for Transmission Foci
2.3. Suspicious Infective Food Habit Assessments
- Cutusuma, located inside a zone where human infection widely occurs, relatively far from the shore of the Lake Titicaca, along the way from Batallas to Aygachi (Figure 1); a total of 194 persons participated, comprising small children to adult subjects, including mainly school children.
- Tauca, located at the shore of Lake Titicaca, in the road from Huarina to Copacabana (Figure 1), in a zone where human infection shows lower prevalence; a total of 100 persons participated, comprising small children to adult subjects, including mainly school children.
2.4. Ethnographic Fieldwork Methods Used
2.5. Literature Search
2.6. Statistical Analyses
3. Results and Discussion
3.1. Extent of the Endemic Area and its Strategic Importance
- The first corridor includes the localities of Pucarani and Batallas, and extends from El Alto in the East up to the shore of the Lake Titicaca in the West, with a northern prolongation through Huarina up to Achacachi and Belen and another westward prolongation along the shore of the Lake up to Tauca. The most recent prospection has demonstrated that lymnaeids have colonized the northward sub-corridor of Peñas and Kerani, where such snails were never found in previous surveys.
- The second corridor extends from Tambillo in the East up to Aygachi, Huacullani and the shore of the Lake Titicaca in the West. The lymnaeids have recently colonized the mountainous hills between Huacullani and the third corridor following the route to Tiwanaku.
- The third corridor concerns the flatland presenting Tiwanaku and Guaqui as main localities and also extends up to the shore of Lake Titicaca in the West.
- It is the main exit for and from the capital La Paz through the recently improved westward road to the city of El Alto.
- The international and national airport is located in El Alto.
- It includes the two terrestrial roads to the Peru border crossings through (i) the northern way of Batallas, Tiquina, and Copacabana, and (i) the southern way of Laja, Guaqui, and Desaguadero.
- It includes the terrestrial roads to Cochabamba, Oruro, Sucre, and other southern parts of the country.
- It includes Lake Titicaca, of undoubted interest from the touristic and naturalistic points of view, including native fauna and flora, landscapes, and opportunities for boating and fishing.
- It includes archeological sites such as the unique ruins of Tiwanaku and the old temple centre of the Aymara empire, of evident touristic, ethnical, and historical attraction, including age-old customs and traditions, folkloric events, etc.
3.2. Population at Risk, Community Development and Mortality Rates
3.3. Characteristics of the Transmission Foci
- The decreasing slope from the Eastern Andean Chain (with altitudes up to more than 6000 m) down to the Lake Titicaca (at 3820 m a.s.l.), as indeed all freshwater collections in the endemic area, whether superficial or phreatic, giving rise to efflorescence, come from this chain;
- The distance from the Eastern Andean Chain explains the gradual disappearance of transmission foci when far away, such as in the southern part of the Tiwanaku corridor; the long Kheto river allows for the southward spread up to Patacamaya;
3.4. Food Infection Sources
3.5. Water Infection Sources
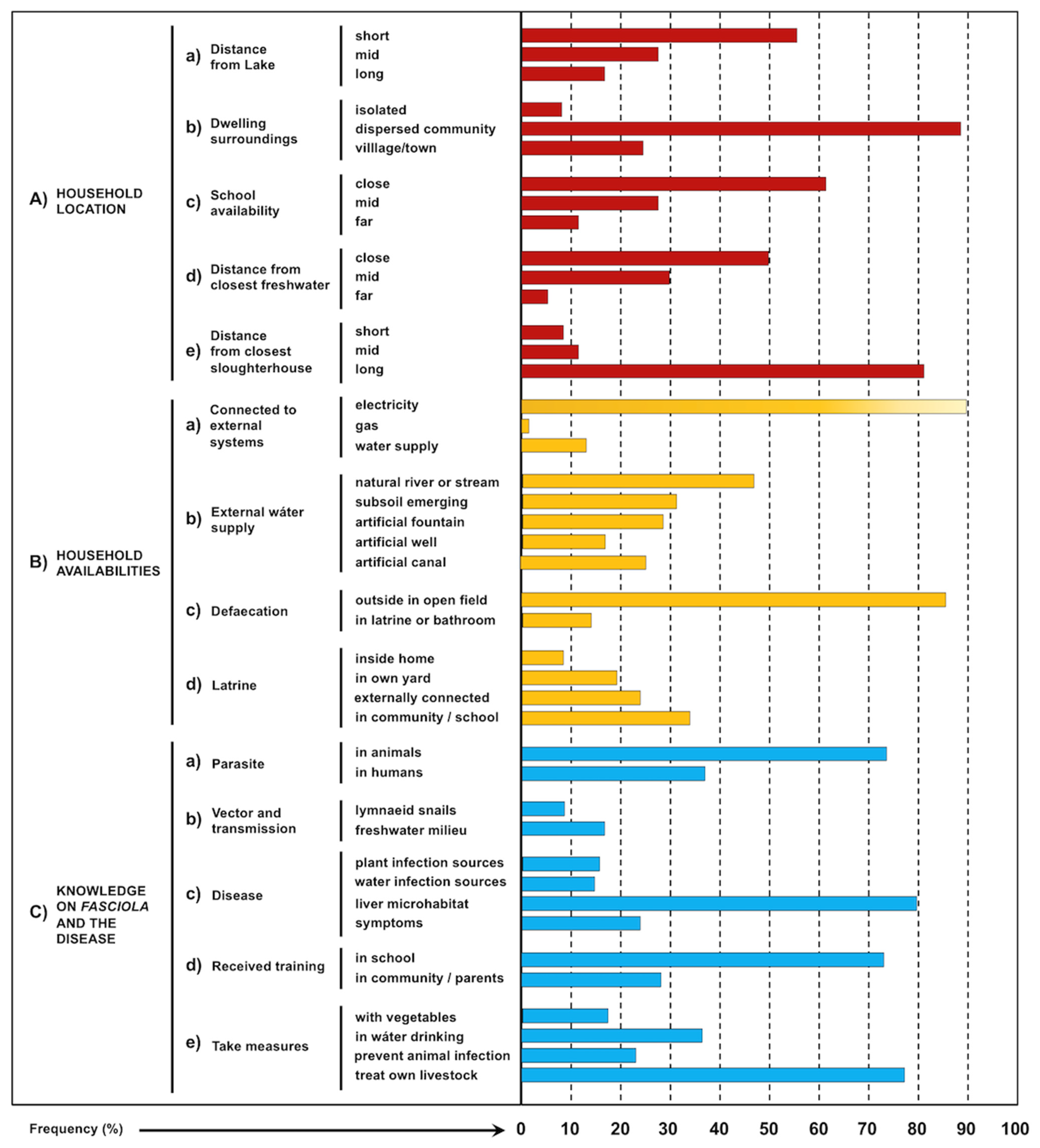
3.6. Household Characteristics
3.7. Knowledge of Fasciola and the Disease
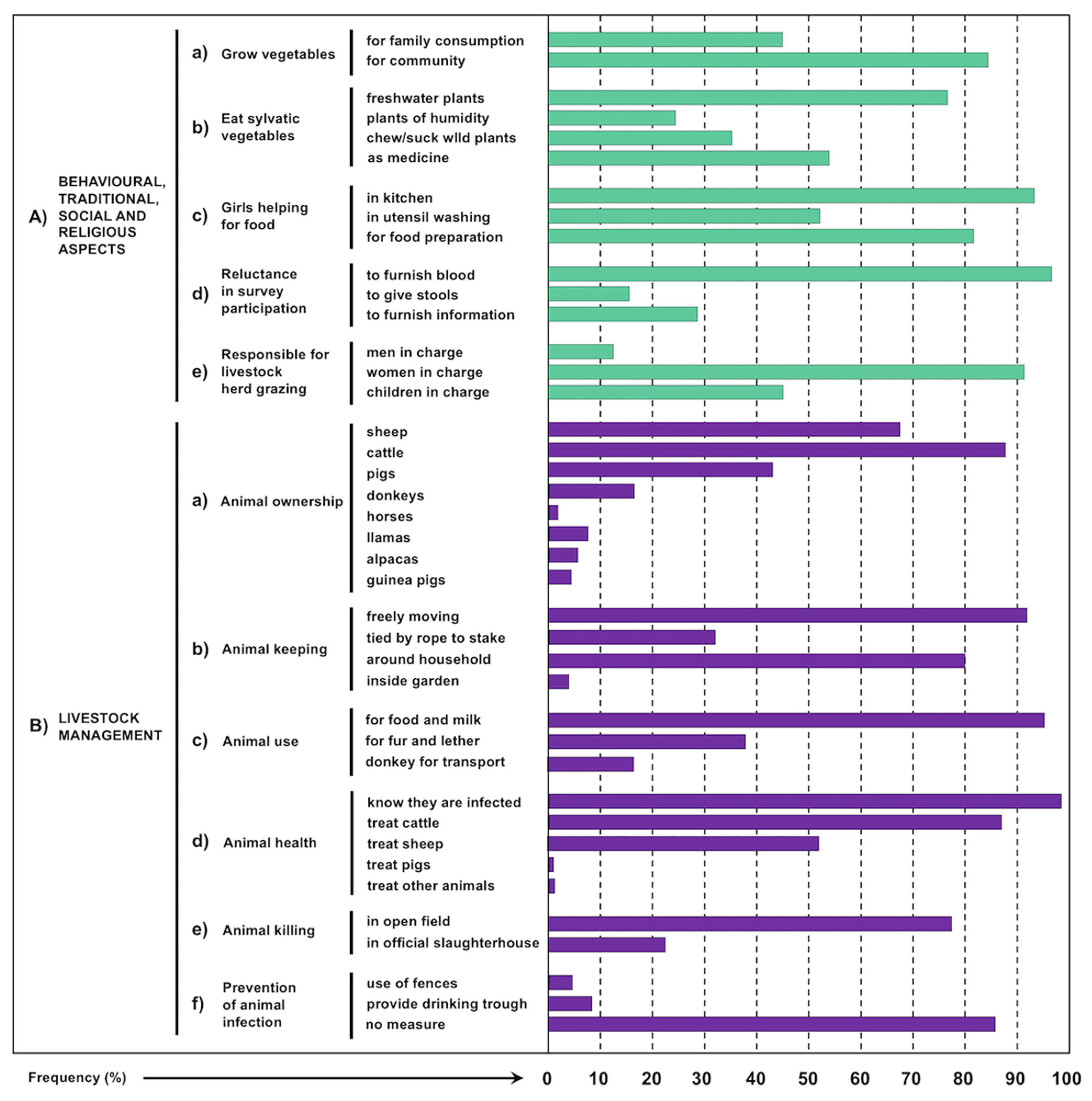
3.8. Behavioural, Traditional, Social and Religious Aspects
- Vegetables they grow in reduced crops (Figure 6H and Figure 8B,C). The very high altitude hostile environment including dry and cold climate play an important limiting factor for agricultural production [119], although elderly Aymara persons still keep valuable knowledge acquired by previous generations about crop strains more resistant to long dry periods [120]. Therefore, crop purpose is mostly to feed families living in communities (Figure 9(Aa)) and whose consumption varies according to the rainy and dry seasons. Seeds and vegetables grown in crops may be stored in a specific room which is strictly in charge of women.
- As complements they use edible tubers and sylvatic plants (with preference for tender leaves) used as condiments and which are consumed fresh and only rarely stocked for short time (Figure 5B–G). Indeed, a wide traditional knowledge on edible sylvatic plants whose leaves, stems and flowers are consumed tender appears in the culture of native ethic groups living in rural communities throughout the Americas since pre-Hispanic times. The additional use of several of these plants as in house medicinal remedies for diseases of the digestive system and liver should be highlighted [121,122]. In the Northern Altiplano, inhabitants are used to consume sylvatic plants, among which the most tender and tasty growing in freshwater and humid environments, whether as food condiments or traditional medicine remedies. The habit to chew and/or suck juicy sylvatic plants, as the stems of “totorillas”, is recognized by many children (Figure 9(Ab)).
- Familiar livestock husbandry allows to obtain fresh meat from the animals they breed and slaughter, above all from cattle, sheep and pigs that are the main three livestock species owned by the families. Donkeys are used for good transport [49]. Llamas and alpacas are very few throughout the endemic area, although several may be seen in the northern part, as in the subcorridor of Peñas and the farms of Belen [50], and meat of llama is sporadically consumed.
- Other agricultural products and industrialized foods are sometimes obtained by exchange in the local markets of the area, exchange activities which appear to gradually increase recently due to the increase in dry periods making family crops difficult, pronounced effort-needing, and less productive [123].
3.9. Livestock Management
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hayward, A.D.; Skuce, P.J.; McNeilly, T.N. The influence of liver fluke infection on production in sheep and cattle: A meta-analysis. Int. J. Parasitol. 2021, 51, 913–924. [Google Scholar] [CrossRef] [PubMed]
- Mas-Coma, S.; Agramunt, V.H.; Valero, M.A. Neurological and ocular fascioliasis in humans. Adv. Parasitol. 2014, 84, 27–149. [Google Scholar]
- Gonzalez-Miguel, J.; Valero, M.A.; Reguera-Gomez, M.; Mas-Bargues, C.; Bargues, M.D.; Simon-Martin, F.; Mas-Coma, S. Numerous Fasciola plasminogen-binding proteins may underlie blood-brain barrier leakage and explain neurological disorder complexity and heterogeneity in the acute and chronic phases of human fascioliasis. Parasitology 2019, 146, 284–298. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.G.; Mott, K.E. Progress in assessment of morbidity due to Fasciola hepatica infection: A review of recent literature. Trop. Dis. Bull. 1990, 87, R1–R38. [Google Scholar]
- Mas-Coma, S. Human fascioliasis emergence risks in developed countries: From individual patients and small epidemics to climate and global change impacts. Enf. Emerg. Microbiol. Clín. 2020, 38, 253–256. [Google Scholar]
- Rondelaud, D.; Dreyfuss, G.; Vignoles, P. Clinical and biological abnormalities in patients after fasciolosis treatment. Med. Mal. Infect. 2006, 36, 466–468. [Google Scholar] [CrossRef] [PubMed]
- Bargues, M.D.; Artigas, P.; Khoubbane, M.; Flores, R.; Glöer, P.; Rojas-Garcia, R.; Ashrafi, K.; Falkner, G.; Mas-Coma, S. Lymnaea schirazensis, an overlooked snail distorting fascioliasis data: Genotype, phenotype, ecology, worldwide spread, susceptibility, applicability. PLoS ONE 2011, 6, e24567. [Google Scholar] [CrossRef]
- Valero, M.A.; Bargues, M.D.; Khoubbane, M.; Artigas, P.; Quesada, C.; Berinde, L.; Ubeira, F.M.; Mezo, M.; Hernandez, J.L.; Agramunt, V.H.; et al. Higher physiopathogenicity by Fasciola gigantica than by the genetically close F. hepatica: Experimental long-term follow-up of biochemical markers. Trans. Roy. Soc. Trop. Med. Hyg. 2016, 110, 55–66. [Google Scholar] [CrossRef]
- Mas-Coma, S.; Valero, M.A.; Bargues, M.D. Fasciola, lymnaeids and human fascioliasis, with a global overview on disease transmission, epidemiology, evolutionary genetics, molecular epidemiology and control. Adv. Parasitol. 2009, 69, 41–146. [Google Scholar] [PubMed]
- Bargues, M.D.; Valero, M.A.; Trueba, G.A.; Fornasini, M.; Villavicencio, A.E.; Guamán, R.; De Elias-Escribano, A.; Perez-Crespo, I.; Artigas, P.; Mas-Coma, S. Multi-marker genotyping and CIAS morphometric phenotyping of Fasciola gigantica-sized flukes from Ecuador, with an analysis of the Radix absence in the New World and the evolutionary lymnaeid snail vector filter. Animals 2021, 11, 2495. [Google Scholar] [CrossRef] [PubMed]
- Fox, N.J.; White, P.C.L.; McClean, C.J.; Marion, G.; Evans, A.; Hutchings, M.R. Predicting impacts of climate change on Fasciola hepatica risk. PLoS ONE 2011, 6, e16126. [Google Scholar] [CrossRef]
- Afshan, K.; Fortes-Lima, C.A.; Artigas, P.; Valero, M.A.; Qayyum, M.; Mas-Coma, S. Impact of climate change and man-made irrigation systems on the transmission risk, long-term trend and seasonality of human and animal fascioliasis in Pakistan. Geospat. Health 2014, 8, 317–334. [Google Scholar] [CrossRef]
- Mas-Coma, S.; Bargues, M.D.; Valero, M.A. Human fascioliasis infection sources, their diversity, incidence factors, analytical methods and prevention measures. Parasitology 2018, 145, 1665–1699. [Google Scholar] [CrossRef] [PubMed]
- Girones, N.; Valero, M.A.; Garcia-Bodelon, M.A.; Chico-Calero, M.I.; Punzon, C.; Fresno, M.; Mas-Coma, S. Immune suppression in advanced chronic fascioliasis: An experimental study in a rat model. J. Infect. Dis. 2007, 195, 1504–1512. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Sustaining the Drive to Overcome the Global Impact of Neglected Tropical Diseases; Department of Control of Neglected Tropical Diseases, World Health Organization: Geneva, Switzerland, 2013; pp. 1–128. [Google Scholar]
- World Health Organization. Ending the Neglect to Attain the Sustainable Development Goals. A Road Map for Neglected Tropical Diseases 2021–2030; World Health Organization: Geneva, Switzerland, 2020; pp. 1–47. Available online: https://www.who.int/neglected_diseases/Ending-the-neglect-to-attain-the-SDGs--NTD-Roadmap.pdf (accessed on 23 July 2020).
- Zumaquero-Rios, J.L.; Sarracent-Perez, J.; Rojas-Garcia, R.; Rojas-Rivero, L.; Martinez-Tovilla, Y.; Valero, M.A.; Mas-Coma, S. Fascioliasis and intestinal parasitoses affecting schoolchildren in Atlixco, Puebla State, Mexico: Epidemiology and treatment with nitazoxanide. PLoS Negl. Trop. Dis. 2013, 7, e2553. [Google Scholar] [CrossRef] [PubMed]
- Apt, W.; Aguilera, X.; Vega, F.; Zulantay, I.; Retamal, C.; Apt, P.; Sandoval, J. Fascioliasis en la población rural de las provincias de Curico, Talca y Linares. Rev. Méd. Chile 1992, 120, 621–626. [Google Scholar]
- Artigas, P.; Bargues, M.D.; Mera y Sierra, R.; Agramunt, V.H.; Mas-Coma, S. Characterisation of fascioliasis lymnaeid intermediate hosts from Chile by DNA sequencing, with emphasis on Lymnaea viator and Galba truncatula. Acta Trop. 2011, 120, 245–257. [Google Scholar] [CrossRef]
- Mera y Sierra, R.; Agramunt, V.H.; Cuervo, P.; Mas-Coma, S. Human fascioliasis in Argentina: Retrospective overview, critical analysis and baseline for future research. Parasit. Vectors 2011, 4, 104. [Google Scholar] [CrossRef] [PubMed]
- Bargues, M.D.; Malandrini, J.B.; Artigas, P.; Soria, C.C.; Velasquez, J.N.; Carnevale, S.; Mateo, L.; Khoubbane, M.; Mas-Coma, S. Human fascioliasis endemic areas in Argentina: Multigene characterisation of the lymnaeid vectors and climatic-environmental assessment of the transmission pattern. Parasit. Vectors 2016, 9, 306. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bargues, M.D.; Gonzalez, C.; Artigas, P.; Mas-Coma, S. A new baseline for fascioliasis in Venezuela: Lymnaeid vectors ascertained by DNA sequencing and analysis of their relationships with human and animal infection. Parasit. Vectors 2011, 4, 200. [Google Scholar] [CrossRef]
- Gonzalez, L.C.; Esteban, J.G.; Bargues, M.D.; Valero, M.A.; Ortiz, P.; Naquira, C.; Mas-Coma, S. Hyperendemic human fascioliasis in Andean valleys: An altitudinal transect analysis in children of Cajamarca province, Peru. Acta Trop. 2011, 120, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Esteban, J.G.; Gonzalez, C.; Bargues, M.D.; Angles, R.; Sanchez, C.; Naquira, C.; Mas-Coma, S. High fascioliasis infection in children linked to a man-made irrigation zone in Peru. Trop. Med. Int. Health 2002, 7, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Angles, R.; Strauss, W.; Ramirez, S.; Esteban, J.G.; Mas-Coma, S. Human fascioliasis in Bolivia: Coprological surveys in different provinces of the Department of La Paz. Res. Rev. Parasitol. 1997, 57, 33–37. [Google Scholar]
- Esteban, J.G.; Flores, A.; Angles, R.; Mas-Coma, S. High endemicity of human fascioliasis between Lake Titicaca and La Paz valley, Bolivia. Trans. Roy. Soc. Trop. Med. Hyg. 1999, 93, 151–156. [Google Scholar] [CrossRef]
- Mas-Coma, S.; Angles, R.; Esteban, J.G.; Bargues, M.D.; Buchon, P.; Franken, M.; Strauss, W. The Northern Bolivian Altiplano: A region highly endemic for human fascioliasis. Trop. Med. Int. Health 1999, 4, 454–467. [Google Scholar] [CrossRef]
- Hillyer, G.V.; Soler de Galanes, M.; Rodriguez-Perez, J.; Bjorland, J.; Silva de Lagrava, M.; Ramirez Guzman, S.; Bryan, R.T. Use of the Falcon Assay Screening Test–Enzyme-Linked Immunosorbent Assay (FAST-ELISA) and the Enzyme-Linked Immunoelectrotransfer Blot (EITB) to determine the prevalence of human Fascioliasis in the Bolivian Altiplano. Am. J. Trop. Med. Hyg. 1992, 46, 603–609. [Google Scholar] [CrossRef]
- Strauss, W.; Angles, R.; Esteban, J.G.; Mas-Coma, S. Human fascioliasis in Bolivia: Serological surveys in Los Andes province of the Department of La Paz. Res. Rev. Parasitol. 1997, 57, 109–113. [Google Scholar]
- O’Neill, S.M.; Parkinson, M.; Strauss, W.; Angles, R.; Dalton, J.P. Immunodiagnosis of Fasciola hepatica (Fascioliasis) in a human population in the Bolivian Altiplano using purified cathepsin L cysteine proteinase. Amer. J Trop. Med. Hyg. 1998, 58, 417–423. [Google Scholar] [CrossRef]
- Valero, M.A.; Girones, N.; Garcia-Bodelon, M.A.; Periago, M.V.; Chico-Calero, I.; Khoubbane, M.; Fresno, M.; Mas-Coma, S. Anaemia in advanced chronic fasciolosis. Acta Trop. 2008, 108, 35–43. [Google Scholar] [CrossRef]
- Fuentes, M.V.; Valero, M.A.; Bargues, M.D.; Esteban, J.G.; Angles, R.; Mas-Coma, S. Analysis of climatic data and forecast indices for human fascioliasis at very high altitude. Ann. Trop. Med. Parasitol. 1999, 93, 835–850. [Google Scholar] [CrossRef]
- Valero, M.A.; Mas-Coma, S. Comparative infectivity of Fasciola hepatica metacercariae from isolates of the main and secondary reservoir animal host species in the Bolivian Altiplano high human endemic region. Folia Parasitol. 2000, 47, 17–22. [Google Scholar] [CrossRef]
- Bargues, M.D.; Gayo, V.; Sanchis, J.; Artigas, P.; Khoubbane, M.; Birriel, S.; Mas-Coma, S. DNA multigene characterization of Fasciola hepatica and Lymnaea neotropica and its fascioliasis transmission capacity in Uruguay, with historical correlation, human report review and infection risk analysis. PLoS Negl. Trop. Dis. 2017, 11, e0005352. [Google Scholar] [CrossRef]
- Mas-Coma, S.; Funatsu, I.R.; Bargues, M.D. Fasciola hepatica and lymnaeid snails occurring at very high altitude in South America. Parasitology 2001, 123, S115–S127. [Google Scholar] [CrossRef]
- Valero, M.A.; Panova, M.; Mas-Coma, S. Developmental differences in the uterus of Fasciola hepatica between livestock liver fluke populations from Bolivian highland and European lowlands. Parasitol. Res. 2001, 87, 337–342. [Google Scholar] [CrossRef]
- Valero, M.A.; Panova, M.; Perez-Crespo, I.; Khoubbane, M.; Mas-Coma, S. Correlation between egg-shedding and uterus development in Fasciola hepatica human and animal isolates: Applied implications. Vet. Parasitol. 2011, 183, 79–86. [Google Scholar] [CrossRef]
- Valero, M.A.; Girones, N.; Reguera-Gomez, M.; Perez-Crespo, I.; Lopez-Garcia, M.P.; Quesada, C.; Bargues, M.D.; Fresno, M.; Mas-Coma, S. Impact of fascioliasis reinfection on Fasciola hepatica egg shedding: Relationship with the immune-regulatory response. Acta Trop. 2020, 209, 105518. [Google Scholar] [CrossRef]
- Valero, M.A.; Perez-Crespo, I.; Chillon-Marinas, C.; Khoubbane, M.; Quesada, C.; Reguera-Gomez, M.; Mas-Coma, S.; Fresno, M.; Girones, N. Fasciola hepatica reinfection potentiates a mixed Th1/Th2/Th17/Treg response and correlates with the clinical phenotypes of anemia. PLoS ONE 2017, 12, e0173456. [Google Scholar] [CrossRef]
- Aldridge, A.; O’Neill, S.M. Fasciola hepatica tegumental antigens induce anergic like T cells via dendritic cells in a mannose receptor dependent manner. Eur. J. Immunol. 2016, 46, 1180–1192. [Google Scholar] [CrossRef] [PubMed]
- Esteban, J.G.; Flores, A.; Aguirre, C.; Strauss, W.; Angles, R.; Mas-Coma, S. Presence of very high prevalence and intensity of infection with Fasciola hepatica among Aymara children from the Northern Bolivian Altiplano. Acta Trop. 1997, 66, 1–14. [Google Scholar] [CrossRef]
- Esteban, J.G.; Flores, A.; Angles, R.; Strauss, W.; Aguirre, C.; Mas-Coma, S. A population-based coprological study of human fascioliasis in a hyperendemic area of the Bolivian Altiplano. Trop. Med. Int. Health 1997, 2, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Villegas, F.; Angles, R.; Barrientos, R.; Barrios, G.; Valero, M.A.; Hamed, K.; Grueningr, H.; Ault, S.K.; Montresor, A.; Engels, D.; et al. Administration of triclabendazole is safe and effective in controlling fascioliasis in an endemic community of the Bolivian Altiplano. PLoS Negl. Trop. Dis. 2012, 6, e1720. [Google Scholar] [CrossRef]
- Valero, M.A.; Periago, M.V.; Perez-Crespo, I.; Angles, R.; Villegas, F.; Aguirre, C.; Strauss, W.; Espinoza, J.R.; Herrera, P.; Terashima, A.; et al. Field evaluation of a coproantigen detection test for fascioliasis diagnosis and surveillance in human hyperendemic areas of Andean countries. PLoS Negl. Trop. Dis. 2012, 6, e1812. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, P.; Schmitt, E.K.; Chen, C.W.; Samantray, S.; Venishetty, V.K.; Hughes, D. Triclabendazole in the treatment of human fascioliasis: A review. Trans. Roy. Soc. Trop. Med. Hyg. 2019, 113, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Bargues, M.D.; Artigas, P.; Angles, R.; Osca, D.; Duran, P.; Buchon, P.; Gonzales-Pomar, R.K.; Pinto-Mendieta, J.; Mas-Coma, S. Genetic uniformity, geographical spread and anthropogenic habitat modifications of lymnaeid vectors found in a One Health initiative in the highest human fascioliasis hyperendemic of the Bolivian Altiplano. Parasit Vectors 2020, 13, 171. [Google Scholar] [CrossRef] [PubMed]
- Mas-Coma, S.; Buchon, P.; Funatsu, I.R.; Angles, R.; Artigas, P.; Valero, M.A.; Bargues, M.D. Sheep and cattle reservoirs in the highest human fascioliasis hyperendemic area: Experimental transmission capacity, field epidemiology and control within a One Health initiative in Bolivia. Front. Vet. Sci. 2020, 7, 583204. [Google Scholar] [CrossRef] [PubMed]
- Mas-Coma, S.; Funatsu, I.R.; Angles, R.; Buchon, P.; Mas-Bargues, C.; Artigas, P.; Valero, M.A.; Bargues, M.D. Domestic pig prioritized in one health action against fascioliasis in human endemic areas: Experimental assessment of transmission capacity and epidemiological evaluation of reservoir role. One Health 2021, 13, 100249. [Google Scholar] [CrossRef] [PubMed]
- Mas-Coma, S.; Buchon, P.; Funatsu, I.R.; Angles, R.; Mas-Bargues, C.; Artigas, P.; Valero, M.A.; Bargues, M.D. Donkey fascioliasis within a One Health control action: Transmission capacity, field epidemiology, and reservoir role in a human hyperendemic area. Front. Vet. Sci. 2020, 7, 591384. [Google Scholar] [CrossRef] [PubMed]
- Mas-Coma, S.; Cafrune, M.M.; Funatsu, I.R.; Mangold, A.J.; Angles, R.; Buchon, P.; Fantozzi, M.C.; Artigas, P.; Valero, M.A.; Bargues, M.D. Fascioliasis in llama, Lama glama, in Andean endemic areas: Experimental transmission capacity by the high altitude snail vector Galba truncatula and epidemiological analysis of its reservoir role. Animals 2021, 11, 2693. [Google Scholar] [CrossRef] [PubMed]
- Bargues, M.D.; Angles, R.; Coello, J.; Artigas, P.; Funatsu, I.R.; Cuervo, P.F.; Buchon, P.; Mas-Coma, S. One Health initiative in the Bolivian Altiplano human fascioliasis hyperendemic area: Lymnaeid biology, population dynamics, microecology and climatic factor influences. Braz. J. Vet. Parasitol. 2021, 30, e025620. [Google Scholar] [CrossRef] [PubMed]
- Mas-Coma, S.; Bargues, M.D.; Valero, M.A. Diagnosis of human fascioliasis by stool and blood techniques: Update for the present global scenario. Parasitology 2014, 141, 1918–1946. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, L.S. An ether sedimentation technique for routine stool examinations. Bull. U.S. Army Med. Depart. 1948, 8, 326. [Google Scholar]
- Katz, N.; Chaves, A.; Pellegrino, J. A simple device for quantitative stool thick-smear technique in Schistosomiasis mansoni. Rev. Inst. Med. Trop. Sao Pãulo 1972, 14, 397–402. [Google Scholar] [PubMed]
- Ash, L.R.; Orihel, T.C.; Savioli, L. Bench Aids for the Diagnosis of Intestinal Parasites; World Health Organization: Geneva, Switzerland, 1994; pp. 1–23. [Google Scholar]
- Ander-Egg, E. Técnicas de Investigación Social, 24th ed.; Editorial Lumen, Colección Política, Servicios y Trabajo Social: Buenos Aires, Argentina, 1995; pp. 1–436. [Google Scholar]
- INE. Bolivia: Proyecciones de Población, Según Departamento y Municipio, 2012–2022, Revisión 2020; Instituto Nacional de Estadística, Estado Plurinacional de Bolivia: La Paz, Bolivia, 2020. Available online: https://www.ine.es/dyngs/INEbase/es/categoria.htm?c=Estadistica_P&cid=1254735572981 (accessed on 9 August 2021).
- Buchon, P.; Cuenca, H.; Quiton, A.; Camacho, A.M.; Mas-Coma, S. Fascioliasis in cattle in the human high endemic region of the Bolivian Northern Altiplano. Res. Rev. Parasitol. 1997, 57, 71–83. [Google Scholar]
- Mas-Coma, S.; Angles, R.; Strauss, W.; Esteban, J.G.; Oviedo, J.A.; Buchon, P. Human fascioliasis in Bolivia: A general analysis and a critical review of existing data. Res. Rev. Parasitol. 1995, 55, 73–93. [Google Scholar]
- Fuentes, M.V.; Malone, J.B.; Mas-Coma, S. Validation of a mapping and predicting model for human fasciolosis transmission in Andean very high altitude endemic areas using remote sensing data. Acta Trop. 2001, 79, 87–95. [Google Scholar] [CrossRef]
- Brady, M.T.; O’Neill, S.M.; Dalton, J.P.; Mills, K.H. Fasciola hepatica suppresses a protective Th1 response against Bordetella Pertussis. Infect. Immun. 1999, 67, 5372–5378. [Google Scholar] [CrossRef] [PubMed]
- Valero, M.A.; Navarro, M.; Garcia-Bodelon, M.A.; Marcilla, A.; Morales, M.; Garcia, J.E.; Hernandez, J.L.; Mas-Coma, S. High risk of bacterobilia in advanced experimental chronic fasciolosis. Acta Trop. 2006, 100, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, J.R.; Terashima, A.; Herrera-Velit, P.; Marcos, L.A. Fasciolosis humana y animal en el Perú: Impacto en la economía de las zonas endémicas. Rev. Peru. Med. Exp. Salud Públ. 2010, 27, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Sanjinés Goytia, J.; Bermejo, A.; Revollo, M.; Gutiérrez, R.; Pacheco, A. Diagnostico Ambiental del Sistema Titicaca-Desaguadero-Poopo-Salar de Coipasa (Sistema TDPS) Bolivia-Perú; División de Aguas Continentales, Programa de las Naciones Unidas para el Medio Ambiente, Departamento de Desarrollo Regional y Medio Ambiente, Secretaría General de la Organización de los Estados Americanos: Washington, DC, USA, 1996; pp. 1–136. Available online: https://www.oas.org/usde/publications/Unit/oea31s/ (accessed on 26 July 2021).
- Llanos Cervantes, E. El embarazo en mujeres aymaras migrantes. Un estudio en zonas urbanopopulares al oeste de La Paz. In Mujeres de los Andes: Condiciones de Vida y Salud; Defossez, A.C., Fassin, D., Viveros, M., Eds.; Institut Français d’Études Andines (IFEA), Travaux de l’IFEA: Lima, Peru, 1992; pp. 111–140. [Google Scholar] [CrossRef]
- Morales Anaya, R.; Aguilar, A.M.; Pinto, G. Desarrollo y Pobreza en Bolivia: Análisis de la Situación del Niño y la Mujer; UNICEF: La Paz, Bolivia, 1984; pp. 1–285. [Google Scholar]
- De Meer, K.; Bergman, R.; Kusner, J.S.; Voorhoeve, H.W.A. Differences in physical growth of Aymara and Quechua children living at high altitude in Peru. Amer. J. Phys. Anthropol. 1993, 90, 59–75. [Google Scholar] [CrossRef]
- Stinson, S. The physical growth of high altitude Bolivian Aymara children. Amer. J. Phys. Anthropol. 1980, 52, 377–385. [Google Scholar] [CrossRef]
- Morales, R.; Aguilar, A.M.; Calzadilla, A. Undernutrition in Bolivia: Geography and Culture Matter; Paper No. R-492; Inter-American Development–Bank; Latin American Research Network: Washington, DC, USA, 2005; pp. 1–24. [Google Scholar]
- Teran, G.; Cuna, W.; Brañez, F.; Persson, K.E.M.; Rottenberg, M.E.; Nylen, S.; Rodriguez, C. Differences in nutritional and health status in school children from the highlands and lowlands of Bolivia. Am. J. Trop. Med. Hyg. 2018, 98, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Diaz, B.; Gallegos, D.; Murillo, F.; Lenart, V.L.; Weidman, W.H.; Goldsmith, R.I. Disease and Disability Among the Aymara. In The Aymara. Studies in Human Biology; Schull, W.J., Rothhammer, F., Eds.; Springer: Dordrecht, The Netherlands, 1990; Volume 2, pp. 101–131. [Google Scholar] [CrossRef]
- Pinilla, C.; Paz Calderon, D. Sobre un caso de parasitosis por fasciola hepática. El Hospital, La Paz. Rev. Méd. Costa Rica 1978, 45, 65–70. [Google Scholar]
- Pinilla, C. Veinte casos de Fascioliasis humana en La Paz. Semana Médica, Buenos Aires. Prensa Médica La Paz 1962, 14, 104–106. [Google Scholar]
- Hartmann, L.F.; Patiño, F. Distomatosis extrahepática. Prensa Médica La Paz 1962, 14, 104–106. [Google Scholar]
- Ueno, H.; Morales, G. Fasciolicidal activity of diamphenetide and niclofolan against Fasciola hepatica in sheep in the Altiplano Region of Bolivia. Nat. Inst. Anim. Health Quart. 1973, 13, 75–79. [Google Scholar]
- Ueno, H.; Arandia, R.; Morales, G.; Medina, G. Fascioliasis of livestock and snail host for Fasciola in the Altipiano region of Bolivia. Nat. Inst. Anim. Health Quart. 1975, 15, 61–67. [Google Scholar]
- Flores Serna, A.F.; Estevez Martini, R. Fasciolasis en la ciudad de La Paz. Cuad. Hosp. Clínicas La Paz 1988, 34, 14–18. [Google Scholar]
- Muñoz Ortiz, V.; Laura, N. High contamination with enteroparasites of vegetables expedited in the markets of the city La Paz, Bolivia. Biofarbo Organo Of. Del Col. De Bioquímica Y Farm. De Boliv. 2008, 16, 1–8. [Google Scholar]
- Vela Pacheco, E. Fascioliasis hepato-biliar en la infancia. Rev. Soc. Bol. Pediatr. La Paz 1988, 27, 397–404. [Google Scholar]
- Centellas Guevara, M.R. Inmunofluorescencia Indirecta en el Diagnóstico de la Distomatosis Hepática Humana; Tesis de Grado, Facultad de Farmacia y Bioquímica, Universidad Mayor de San Andrés (UMSA): La Paz, Bolivia, 1987; pp. 1–76. [Google Scholar]
- Villca, M.E.; Cruz, D.; Alco, C. Experiencias en el control de la Fascioliasis hepática 1988–1989. Informe de la organización Foster Parents Plan del Plan Altiplano La Paz a la Jefatura Nacional de Zoonosis del Ministerio de Previsión Social y Salud Publica, La Paz. Res. Rev. Parasitol. 1995, 55, 73–93. [Google Scholar]
- Caceres Vega, E. Fasciola hepatica–«Enfermedad y Pobreza Campesina»; Acción un Maestro Más, Imprenta Metodista: La Paz, Bolivia, 1989; pp. 1–201. [Google Scholar]
- Justiniano, J.G.; Arce Lema, J. Resolución Biministerial No. 0597 de 4 de Agosto de 1989 por la que se declara de interés la Vigilancia y Control de la Fascioliasis, Teniasis-Cisticercosis e Hidatidosis en las áreas epizoendémicas del territorio nacional y se crea el “Comité técnico de Vigilancia y Control de las Zoonosis”; Ministerio de Previsión Social y Salud Pública y Ministerio de Asuntos Campesinos y Agropecuarios: La Paz, Bolivia, 1989; pp. 1–2.
- De, N.V.; Le, T.H.; Agramunt, V.H.; Mas-Coma, S. Early postnatal and preschool age infection by Fasciola spp.: Report of five cases from Vietnam and worldwide review. Am. J. Trop. Med. Hyg. 2020, 103, 1578–1589. [Google Scholar] [CrossRef] [PubMed]
- Ubeira, F.M.; Muiño, L.; Valero, M.A.; Periago, M.V.; Perez-Crespo, I.; Mezo, M.; Gonzalez-Warleta, M.; Romaris, F.; Paniagua, E.; Cortizo, S.; et al. MM3-ELISA detection of Fasciola hepatica coproantigens in preserved human stool samples. Am. J. Trop. Med. Hyg. 2009, 81, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Mas-Coma, S. Human fascioliasis. In Waterborne Zoonoses: Identification, Causes and Control; Cotruvo, J.A., Dufour, A., Rees, G., Bartram, J., Carr, R., Cliver, D.O., Craun, G.F., Fayer, R., Gannon, V.P.J., Eds.; World Health Organization (WHO): Geneva, Switzerland; IWA Publishing: London, UK, 2004; pp. 305–322. [Google Scholar]
- Oviedo, J.A.; Bargues, M.D.; Mas-Coma, S. Lymnaeid snails in the human fascioliasis high endemic zone of the Northern Bolivian Altiplano. Res. Rev. Parasitol. 1995, 55, 35–43. [Google Scholar]
- Doumenge, J.P.; Mott, K.E.; Cheung, C.; Villenave, D.; Chapuis, O.; Perrin, M.F.; Reaud-Thomas, G. Atlas of the Global Distribution of Schistosomiasis; CEGET-CNRS, Talence and OMS/WHO: Geneva, Switzerland; Presses Universitaires de Bordeaux: Talence, France, 1987; pp. 1–398. [Google Scholar]
- Bjorland, J.; Bryan, R.T.; Strauss, W.; Hillyer, G.V.; McAuley, J.B. An outbreak of acute fascioliasis among Aymara Indians in the Bolivian Altiplano. Clin. Infect. Dis. 1995, 21, 1228–1233. [Google Scholar] [CrossRef] [PubMed]
- Lorini, J.; Liberman, M. El clima de la provincia Aroma del departamento de La Paz, Bolivia. Ecol. Boliv. 1983, 4, 19–29. [Google Scholar]
- Roche, M.A.; Bourges, J.; Cortes, J.; Mattos, R. Climatología e hidrología de la cuenca del Lago Titicaca. In El Lago Titicaca. Síntesis del Conocimiento Limnológico Actual; Dejoux, C., Iltis, A., Eds.; ORSTOM-HISBOL: La Paz, Bolivia, 1991; pp. 83–104. [Google Scholar]
- Iltis, A.; Carmouze, J.P.; Lemoalle, J. Características físico-químicas del agua. In El Lago Titicaca. Síntesis del Conocimiento Limnológico Actual; Dejoux, C., Iltis, A., Eds.; ORSTOM-HISBOL: La Paz, Bolivia, 1991; pp. 107–113. [Google Scholar]
- Dejoux, C. Los moluscos. In El Lago Titicaca. Síntesis del Conocimiento Limnológico Actual; Dejoux, C., Iltis, A., Eds.; ORSTOM-HISBOL: La Paz, Bolivia, 1991; pp. 321–343. [Google Scholar]
- Althaus, H. Biologische Abwasserreinigung mit Flechtbinsen. Das Gas. Wasserfach 1966, 17, 486–488. [Google Scholar]
- Seidel, K. Pflanzenbiologische Methoden zur Gewaessersanierung und zur Grundwasseranreicherung. Schr. Der Obersten Nat. Des Saarl. 1978, 4, 49–69. [Google Scholar]
- Periago, M.V.; Valero, M.A.; Artigas, P.; Agramunt, V.H.; Bargues, M.D.; Curtale, F.; Mas-Coma, S. Very high fascioliasis intensities in schoolchildren of Nile Delta governorates: The Old World highest burdens found in lowlands. Pathogens 2021, 10, 1210. [Google Scholar] [CrossRef]
- Curtale, F.; Mas-Coma, S.; Hassanein, Y.A.E.W.; Barduagni, P.; Pezzotti, P.; Savioli, L. Clinical signs and household characteristics associated with human fascioliasis among rural population in Egypt: A case-control study. Parassitologia 2003, 45, 5–11. [Google Scholar]
- Rondelaud, D. Données épidémiologiques sur la distomatose humaine à Fasciola hepatica L. dans la région du Limousin, France. Les plantes consommées et les limnées vectrices. Ann. Parasitol. Hum. Comp. 1980, 55, 394–405. [Google Scholar] [CrossRef]
- Rivera, M.; Galetovic, A.; Licuime, R.; Gómez-Silva, B. A microethnographic and ethnobotanical approach to Llayta consumption among Andes feeding practices. Foods 2018, 7, 202. [Google Scholar] [CrossRef] [PubMed]
- Jurado, B.; Fuertes, C.M.; Tomas, G.E.; Ramos, E.; Arroyo, J.L.; Cáceres, J.R.; Inocente, M.A.; Alvarado, B.; Rivera, B.M.; Ramirez, M.A.; et al. Estudio fisicoquímico, microbiológico y toxicolóico de los polisacáridos del Nostoc commune y Nostoc sphaericum. Rev. Peru. Quím. Ing. Quím. 2014, 17, 15–22. [Google Scholar]
- Raymundo, L.A.; Maco Flores, V.; Terashima, A.; Samalvides, F.; Miranda, E.; Tantalean, M.; Espinoza, J.R.; Gotuzzo, E. Hiperendemicidad de Fasciolosis humana en el Valle del Mantaro, Perú: Factores de riesgo de la infeccion por Fasciola hepatica. Rev. Gastroenterol. Peru 2004, 24, 158–164. [Google Scholar] [PubMed]
- Valero, M.A.; Perez-Crespo, I.; Khoubbane, M.; Artigas, P.; Panova, M.; Ortiz, P.; Maco, V.; Espinoza, J.R.; Mas-Coma, S. Fasciola hepatica phenotypic characterisation in Andean human endemic areas: Valley versus altiplanic patterns analysed in liver flukes from sheep from Cajamarca and Mantaro, Peru. Infect. Genet. Evol. 2012, 12, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Bargues, M.D.; Artigas, P.; Khoubbane, M.; Ortiz, P.; Naquira, C.; Mas-Coma, S. Molecular characterisation of Galba truncatula, Lymnaea neotropica and L. schirazensis from Cajamarca, Peru and their potential role in transmission of human and animal fascioliasis. Parasit. Vector 2012, 5, 174. [Google Scholar] [CrossRef] [PubMed]
- Bardales-Valdivia, J.N.; Bargues, M.D.; Hoban-Vergara, C.; Bardales-Bardales, C.; Goicoechea-Portal, C.; Bazan-Zurita, H.; Del Valle-Mendoza, J.; Ortiz, P.; Mas-Coma, S. Spread of the fascioliasis endemic area assessed by seasonal follow-up of rRNA ITS-2 sequenced lymnaeid populations in Cajamarca, Peru. One Health 2021, 13, 100265. [Google Scholar] [CrossRef] [PubMed]
- Valencia, M.N.; Pariona, D.A.; Huamán, A.M.; Miranda, M.F.; Quintanilla, C.S.; Gonzales, A.A. Seroprevalencia de fasciolosis en escolares y en ganado vacuno en la provincia de Huancavelica, Perú. Rev. Peru. Med. Exp. Salud Públ. 2005, 22, 96–102. [Google Scholar]
- Marcos, L.A.; Maco, V.; Samalvides, F.; Terashima, A.; Espinoza, J.R.; Gotuzzo, E. Risk factors for Fasciola hepatica infection in children: A case control study. Trans. Roy. Soc. Trop. Med. Hyg. 2006, 100, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Blancas, G.; Terashima, A.; Maguiña, C.; Lujan, L.; Alvarez, H.; Casanova, R.T. Fasciolosis humana y compromiso gastrointestinal: Estudio de 277 pacientes en el Hospital Nacional Cayetano Heredia. 1970–2002. Rev. Gastroenterol. Perú 2004, 24, 143–157. [Google Scholar]
- Amraie, E.; Farsani, M.K.; Sadeghi, L.; Khsan, T.N.; Babadi, V.Y.; Adavi, Z. The effects of aqueous extract of alfalfa on blood glucose and lipids in alloxan-induced diabetic rats. Intervent. Med. Appl. Sci. 2015, 7, 124–128. [Google Scholar] [CrossRef]
- Alanoca, V.; Apaza, J.; Calderon, A.; Ticona, C.; Maquera, Y. Gastronomy and industrialized food in the Aymara communities of the Pilcuyo District, El Collao Province-Ilave-Puno-Peru. Am. Scient. Res. J. Eng. Technol. Sci. 2020, 71, 153–163. [Google Scholar]
- Esteban, J.G.; Aguirre, C.; Flores, A.; Strauss, W.; Angles, R.; Mas-Coma, S. High Cryptosporidium prevalences in healthy Aymara children from the Northern Bolivian Altiplano. Am. J. Trop. Med. Hyg. 1998, 58, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Leelayoova, S.; Rangsinn, R.; Taamasri, P.; Naaglor, T.; Thathaisong, U.; Mungthin, M. Evidence of waterborne transmission of Blastocystis hominis. Am. J. Trop. Med. Hyg. 2004, 70, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Bargues, M.D.; Funatsu, I.R.; Oviedo, J.A.; Mas-Coma, S. Natural water, an additional source for human infection by Fasciola hepatica in the Northern Bolivian Altiplano. Parassitologia 1996, 38, 251. [Google Scholar]
- Bargues, M.D.; Oviedo, J.A.; Funatsu, I.R.; Mas-Coma, S. The human host, a viable definitive host for Fasciola hepatica in the Northern Bolivian Altiplano. Parassitologia 1996, 38, 252. [Google Scholar]
- Rivera-Jacinto, M.; Rodriguez-Ulloa, C.; Rojas-Huaman, Y.; Valdivia-Melendez, Y.; Saucedo-Duran, T. Conocimientos, actitudes y prácticas sobre Fascioliasis en madres de una zona rural andina del Norte peruano. Rev. Peru. Med. Exp. Salud. 2010, 27, 59–62. [Google Scholar]
- Rodríguez, C.; Rivera, M.; Del Valle, J.; Cerna, C.; Hoban, C.; Chilón, S.; Ortiz, P. Risk factors for human fascioliasis in schoolchildren in Baños del Inca, Cajamarca, Peru. Trans. Roy. Soc. Trop. Med. Hyg. 2018, 112, 216–222. [Google Scholar] [CrossRef]
- Fernandez Juarez, G. Enfermedad, moda y cuerpo social en el altiplano aymara: Un «boceto» de inspiración colonial sobre modelos de identidad en los Andes. Rev. Esp. Antropol. Am. 1998, 28, 259–281. [Google Scholar]
- Mamani, L.D.O. Medicina tradicional Aimara-Perú. Comuniaccion 2013, 1, 1–11. [Google Scholar]
- Fernandez Juarez, G. Aymaras De Bolivia Entre La Tradicion Y El Cambio Cultural; Colección Hombre y Ambiente No. 67–68; Ediciones Abya-Yala: Quito, Ecuador, 2002; pp. 1–224. [Google Scholar]
- Kim, S.W.; Kashiwazaki, H.; Imai, H.; Moji, K.; Orias-Rivera, J. Food consumption and energy expenditure of Aymara in a herding community of the Bolivian Altiplano. J. Human Ergol. 1991, 20, 181–197. [Google Scholar]
- Keleman Saxena, A.; Cadima Fuentes, X.; Gonzales Herbas, R.; Humphries, D.L. Indigenous food systems and climate change: Impacts of climatic shifts on the production and processing of native and traditional crops in the Bolivian Andes. Front. Public Health 2016, 4, 20. [Google Scholar] [CrossRef]
- Balcázar-Quiñones, A.; White-Olascoaga, L.; Chávez-Mejía, C.; Zepeda-Gómez, C. Los quelites: Riqueza de especies y conocimiento tradicional en la comunidad otommí de San Pedro Arriba, Temoaya, Estado de Mexico. Polibotanica 2020, 49, 219–242. [Google Scholar] [CrossRef]
- Mateos-Maces, L.; Chávez-Servia, J.L.; Vera-Guzmán, A.M.; Aquino-Bolaños, E.N.; Alba-Jiménez, J.E.; Villagómez-González, B.B. Edible leafy plants from Mexico as sources of antioxidant compounds, and their nutritional, nutraceutical and antimicrobial potential: A review. Antioxidants 2020, 9, 541. [Google Scholar] [CrossRef]
- Baldinelli, G.M. Agrobiodiversity conservation as a coping strategy: Adapting to climate change in the northern highlands of Bolivia. Cons. J. Sustain. Dev. 2014, 11, 153–166. [Google Scholar]
- Apaza, J.; Alacona, V.; Ticona, C.; Calderon, A.; Maquera, Y. Educación y alimentación en las comunidades aymaras de Puno. Comuniaccion: Rev. De Investig. En Comun. Y Desarro. 2019, 10, 36–46. [Google Scholar]
- Mera y Sierra, R.; Neira, G.; Bargues, M.D.; Cuervo, P.F.; Artigas, P.; Logarzo, L.; Cortiñas, G.; Ibaceta, D.E.J.; Lopez Garrido, A.; Bisutti, E.; et al. Equines as reservoirs of human fascioliasis: Transmission capacity, epidemiology and pathogenicity in Fasciola hepatica infected mules. J. Helminthol. 2020, 94, e189. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.M.; van Rensburg, L.J.; van Wyk, J.A. Fasciola in horses in the Republic of South Africa: A single natural case of Fasciola hepatica and the failure to infest ten horses either with F. hepatica or Fasciola gigantica. Onderstepoort J. Vet. Res. 1988, 55, 157–163. [Google Scholar]
- Dorchies, P. La fasciolose des équidés, une infestation parasitaire inhabituelle. Bul. Groupem. Techn. Vét. 2010, 57, 71–78. [Google Scholar]
- Fuentes, M.V.; Coello, J.R.; Bargues, M.D.; Valero, M.A.; Esteban, J.G.; Fons, R.; Mas-Coma, S. Small mammals (Lagomorpha and Rodentia) and fascioliasis transmission in the Northern Bolivian Altiplano endemic zone. Res. Rev. Parasitol. 1997, 57, 115–121. [Google Scholar]
- Arias-Pacheco, C.; Lucas, J.R.; Rodriguez, A.; Cordoba, D.; Lux-Hoppe, E. Economic impact of the liver condemnation of cattle infected with Fasciola hepatica in the Peruvian Andes. Trop. Anim. Health Prod. 2020, 52, 1927–1932. [Google Scholar] [CrossRef] [PubMed]
- Grock, R.; Morales, G.; Vaca, J.L.; Mas-Coma, S. Fascioliasis in sheep in the human high endemic region of the Northern Bolivian Altiplano. Res. Rev. Parasitol. 1998, 58, 95–101. [Google Scholar]







| Family | Species | Aymara Name * | Local Spanish Name * | Presence in 30 Freshwater Collections Inhabited by lymnaeids | |
|---|---|---|---|---|---|
| No. | Percentage | ||||
| Cyanophyta | |||||
| Nostocales | Nostoc sphaericum | Llaytha | Cochayuyo Cushuro Alga parda | 3 | 10% |
| Monocotyledonea | |||||
| Cyperaceae | Schoenoplectus californicus spp. tatora | Chullu Matara | Tallo de totora | 3 | 10% |
| Sakha Matara | Bulbo-raiz de totora | 3 | 10% | ||
| Eleocharis spp. | Joskosko | Totorilla | 20 | 66.7% | |
| Eleocharis albabracteata | Joskosko | Totorilla | 1 | 3.3% | |
| Juncaceae | Juncus spp. | Joskosko | Totorilla | 19 | 63.3% |
| Juncus andicola | Joskosko | Totorilla | 1 | 3.3% | |
| Juncus bufonius | Joskosko | Totorilla | 2 | 6.7% | |
| Juncus ebracteatus | Joskosko | Totorilla | 11 | 36.7% | |
| Juncus cf. microcephala | Joskosko | Totorilla | 1 | 3.3% | |
| Dicotyedonea | |||||
| Juncaginaceae | Lilaea spp. | Joskosko | Totorilla | 14 | 46.7% |
| Cruciferae | Rorippa (= Nasturtium) nana | Okororo | Berro | 1 | 3.3% |
| Rorippa (= Nasturtium) nasturtium-aquaticum | Okororo | Berro | 1 | 3.3% | |
| Umbelliferae | Hydrocotyle ranunculoides | Okororo | Berro | 21 | 70.0% |
| Scrophulariaceae | Mimulus glabratus | Okororo | Berro Flor de mono | 9 | 30.0% |
| Compositae | Cotula mexicana | Okororo ^ | Berro ^ Botones de latón mexicano | 18 | 60.0% |
| Taraxacum spp. | Qhanapaku | Diente de león | 6 | 20.0% | |
| Taraxacum officinale | Qhanapaku | Diente de león | 2 | 6.7% | |
| Province | Municipality | Human Population * Proyections for Years | No. Transmission Foci Studied ** | Human Localities with Infected Subjects | Local Human Prevalences $ | |||
|---|---|---|---|---|---|---|---|---|
| 2012 | 2022 | Ethnographically Analysed ** | Other Localities Reported ^ | E.V. | Mean | |||
| URBAN ZONE | ||||||||
| MURILLO | La Paz | 845,719 | 956,732 | 0 | 0 | 0 | (0) & | 0 |
| El Alto | 916,434 | 1,109,048 | 0 | 0 | 1 | 0.2 | 0.2 | |
| TOTAL URBAN | 1,762,153 | 2,065,780 | 0 | 0 | 1 | 0.0–0.2 | 0.1 | |
| RURAL ZONE | ||||||||
| MURILLO | Achocalla | 17,869 | 19,995 | 4 | 2 | 0 | 5.3–9.5 | 7.4 |
| TOTAL MURILLO | 1,780,022 | 2,085,775 | 4 | 2 | 0 | 0.2–9.5 | 5.0 | |
| OMASUYOS | Achacachi | 39,763 | 35,964 | 2 | 1 | 0 | 1.1–6.5 | 3.8 |
| Santiago de Huata | 8171 | 7921 | 0 | 0 | 0 | 0 | 0 | |
| Chua Cocani | 4965 | 5503 | 2 | 1 | 0 | 1.1–3.6 | 2.35 | |
| Huatajata | 4001 | 4579 | 0 | 0 | 0 | 0 | 0 | |
| Huarina | 7013 | 7532 | 3 | 1 | 0 | 6.3 | 6.3 | |
| TOTAL OMASUYOS | 63,913 | 61,499 | 7 | 3 | 0 | 1.1–6.5 | 3.72 | |
| LOS ANDES | Batallas | 15,952 | 14,892 | 15 | 2 | 4 | 12.6–72.0 | 48.78 |
| Puerto Perez | 7509 | 7670 | 7 | 4 | 0 | 7.0–30.3 | 15.55 | |
| Pucarani | 24,679 | 24,795 | 11 | 7 | 2 | 2.7–28.2 | 15.82 | |
| Laja | 19,343 | 17,670 | 6 | 1 | 1 | 5.9–47.7 | 26.8 | |
| TOTAL LOS ANDES | 67,483 | 65,027 | 39 | 14 | 7 | 2.7–72.0 | 26.28 | |
| INGAVI | Taraco | 6336 | 6491 | 4 | 0 | 0 | 0 | 0 |
| Tiwanaku | 10,728 | 9908 | 6 | 3 | 0 | 7.3–38.2 | 23.65 | |
| Guaqui | 7113 | 7672 | 0 | 0 | 0 | 0 | 0 | |
| Desaguadero | 8768 | 7394 | 0 | 0 | 0 | -- | -- | |
| Jesus de Machaca | 13,270 | 12,067 | 0 | 0 | 0 | -- | -- | |
| Viacha | 89,772 | 85,703 | 4 | 1 | 1 | 1.3 | 1.3 | |
| TOTAL INGAVI | 135,987 | 129,235 | 14 | 4 | 1 | 1.3–38.2 | 20.46 | |
| AROMA | Collana | 4373 | 3900 | 0 | 0 | 0 | -- | -- |
| Colquencha | 8999 | 8157 | 0 | 0 | 0 | -- | -- | |
| Calamarca | 11,599 | 10,836 | 0 | 0 | 0 | -- | -- | |
| Ayo Ayo | 7914 | 8359 | 2 | 0 | 0 | -- | -- | |
| Patacamaya | 21,566 | 22,303 | 1 | 0 | 0 | -- | -- | |
| TOTAL AROMA | 54,451 | 53,555 | 3 | 0 | 0 | -- | -- | |
| TOTAL RURAL | 339,703 | 329,311 | 67 | 23 | 8 | 1.1–72.0 | 14.46 | |
| Vegetable Consumption | Positive Answers in Total Participant Subjects No./% | Positive Answers in Infected Subjects No./% | |||||
|---|---|---|---|---|---|---|---|
| Aymara name | Spanish translation * | Males | Females | P | Males N = 20 | Females N = 22 | P |
| CUTUSUMA 194 participants: 100 males and 94 females | |||||||
| Chullu | tallo de totora | 81/81.00% | 88/93.61% | 0.0100 | 17/85.00% | 20/90.91% | ns |
| Joskosko | totorillas | 51/51.00% | 63/67.02% | 0.0287 | 13/65.00% | 14/63.63% | ns |
| Okororo | berros | 37/37.00% | 33/35.11% | ns | 9/45.00% | 3/13.64% | 0.0400 |
| Sakha | bulbo de totora | 64/64.00% | 72/76.70% | ns | 13/65.00% | 14/63.63% | ns |
| Llaytha | alga parda | 63/64.00% | 63/67.02% | ns | 13/65.00% | 15/68.18% | ns |
| TAUCA 100 participants: 52 males and 48 females | |||||||
| Chullu | tallo de totora | 45/86.54% | 43/89.58% | ns | - | - | - |
| Joskosko | totorillas | 16/30.77% | 21/43.75% | ns | - | - | - |
| Okororo | berros | 22/42.31% | 30/62.50% | 0.0483 | - | - | - |
| Sakha | bulbo de totora | 28/53.85% | 23/47.92% | ns | - | - | - |
| Llaytha | alga parda | 16/30.77% | 10/20.83% | ns | - | - | - |
| Vegetable Consumption | Positive Answers in Total Participant Subjects No./% | Positive Answers in Infected Subjects No./% | |||||
|---|---|---|---|---|---|---|---|
| Aymara name | Spanish translation * | Children | Adults | P | Children N = 39 | Adults N = 3 | P |
| CUTUSUMA 194 participants: 135 children and 59 adults | |||||||
| Chullu | tallo de totora | 115/85.19% | 54/91.52% | ns | 34/87.18% | 3/100% | ns |
| Joskosko | totorillas | 78/57.78% | 36/61.02% | ns | 24/61.54% | 3/100% | ns |
| Okororo | berros | 42/31.11% | 28/47.46% | 0.0350 | 10/25.64% | 2/66.66% | ns |
| Sakha | bulbo de totora | 88/65.19% | 48/81.36% | 0.0268 | 24/61.54% | 3/100% | ns |
| Llaytha | alga parda | 81/60.00% | 45/76.27% | 0.0337 | 25/64.10% | 3/100% | ns |
| TAUCA 100 participants: 72 children and 28 adults | |||||||
| Chullu | tallo de totora | 63/86.54% | 25/89.28% | ns | - | - | - |
| Joskosko | totorillas | 23/30.77% | 14/50.00% | ns | - | - | - |
| Okororo | berros | 32/42.31% | 20/71.43% | 0.0249 | - | - | - |
| Sakha | bulbo de totora | 35/53.85% | 16/57.14% | ns | - | - | - |
| Llaytha | alga parda | 18/30.77% | 8/28.57% | ns | - | - | - |
| Model 1 | Model 2 | ||||
|---|---|---|---|---|---|
| Coefficient | Significance | Coefficient | Significance | ||
| Data from 99 Children | |||||
| Gender (females) N/% | 49/49.5% | 1.826 | ns | 1.939 | ns |
| Age (years old) Average ± SD (min-max) | 8.6 ± 2.9 (2–15) | 1.455 | 0.047 | 1.527 | 0.046 |
| Weight (kg) Average ± SD (min-max) | 24.49 ± 7.5 (8–53) | 0.856 | 0.036 | 0.853 | 0.049 |
| Fascioliasis infection N/% | 39/39.4% | ||||
| Vegetable Consumption * | |||||
| Chullu/tallo de totora N (%) | 86/86.9% | 0.749 | ns | ||
| Joskosko/totorillas N (%) | 55/55.6% | 1.344 | ns | ||
| Okororo/berros N (%) | 30/30.3% | 0.596 | ns | ||
| Sakha/bulbo de totora N (%) | 63/63.6% | 0.601 | ns | ||
| Llaytha/alga parda N (%) | 55/55.6% | 1.641 | ns | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angles, R.; Buchon, P.; Valero, M.A.; Bargues, M.D.; Mas-Coma, S. One Health Action against Human Fascioliasis in the Bolivian Altiplano: Food, Water, Housing, Behavioural Traditions, Social Aspects, and Livestock Management Linked to Disease Transmission and Infection Sources. Int. J. Environ. Res. Public Health 2022, 19, 1120. https://doi.org/10.3390/ijerph19031120
Angles R, Buchon P, Valero MA, Bargues MD, Mas-Coma S. One Health Action against Human Fascioliasis in the Bolivian Altiplano: Food, Water, Housing, Behavioural Traditions, Social Aspects, and Livestock Management Linked to Disease Transmission and Infection Sources. International Journal of Environmental Research and Public Health. 2022; 19(3):1120. https://doi.org/10.3390/ijerph19031120
Chicago/Turabian StyleAngles, René, Paola Buchon, M. Adela Valero, M. Dolores Bargues, and Santiago Mas-Coma. 2022. "One Health Action against Human Fascioliasis in the Bolivian Altiplano: Food, Water, Housing, Behavioural Traditions, Social Aspects, and Livestock Management Linked to Disease Transmission and Infection Sources" International Journal of Environmental Research and Public Health 19, no. 3: 1120. https://doi.org/10.3390/ijerph19031120
APA StyleAngles, R., Buchon, P., Valero, M. A., Bargues, M. D., & Mas-Coma, S. (2022). One Health Action against Human Fascioliasis in the Bolivian Altiplano: Food, Water, Housing, Behavioural Traditions, Social Aspects, and Livestock Management Linked to Disease Transmission and Infection Sources. International Journal of Environmental Research and Public Health, 19(3), 1120. https://doi.org/10.3390/ijerph19031120