No Mitochondrial Related Transcriptional Changes in Human Skeletal Muscle after Local Heat Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Skin Temperature
2.3. Common Femoral Artery Blood Flow and Shear Rate
2.4. Intramuscular Temperature and Biopsies
2.5. Gene Expression
2.6. Statistical Analysis
3. Results
3.1. Temperature
3.2. Common Femoral Artery Blood Flow and Shear Rate
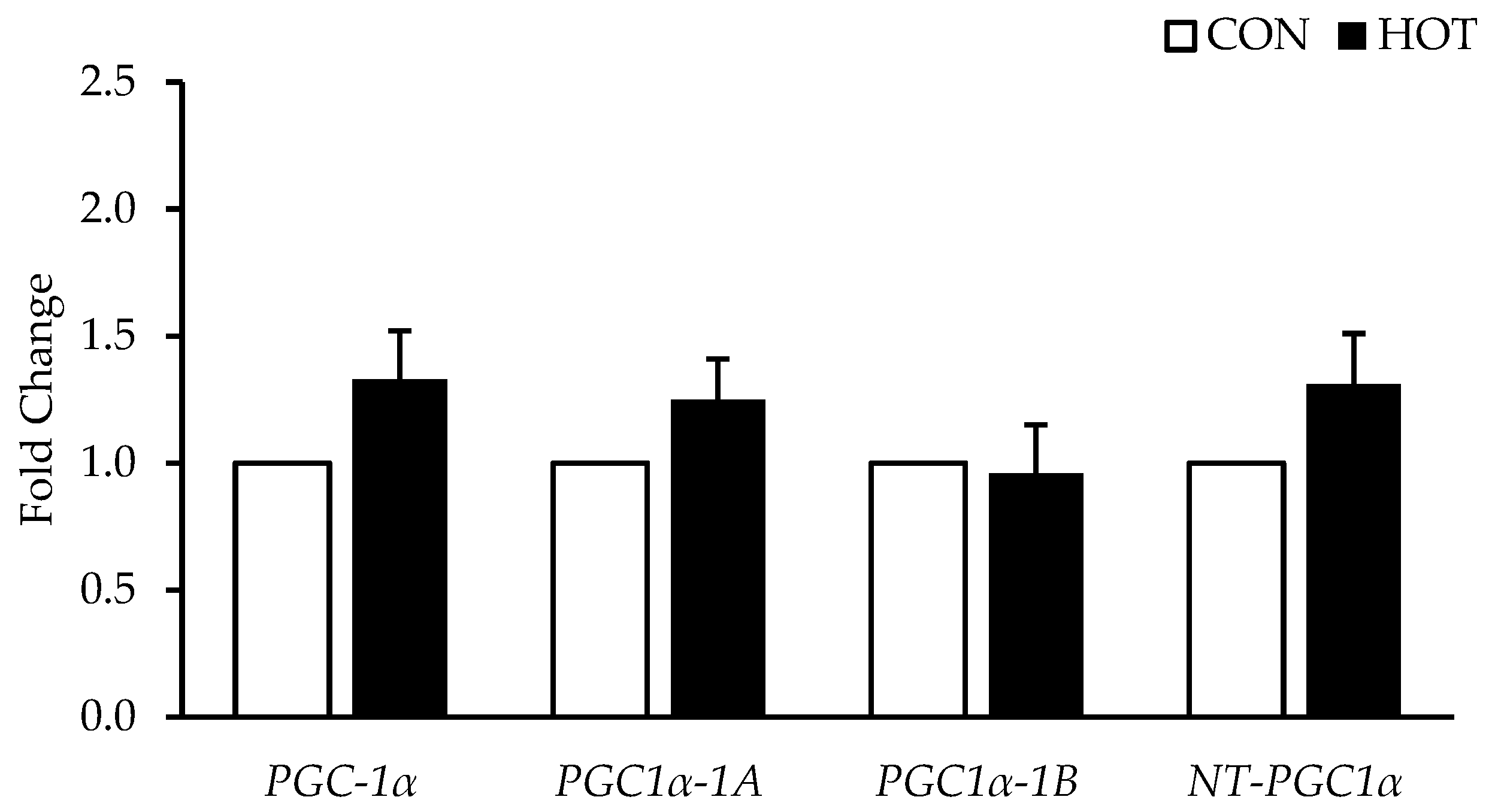
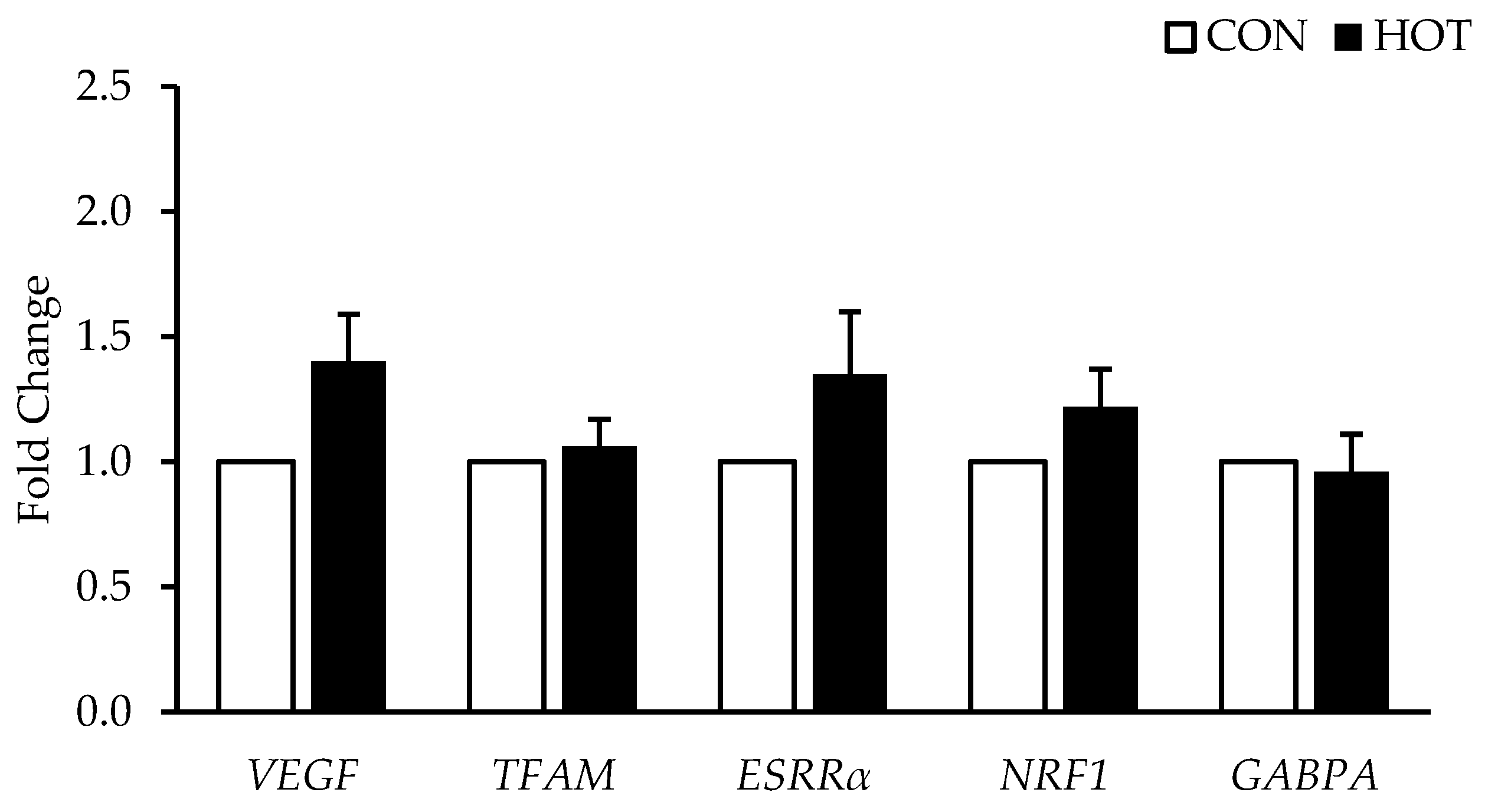
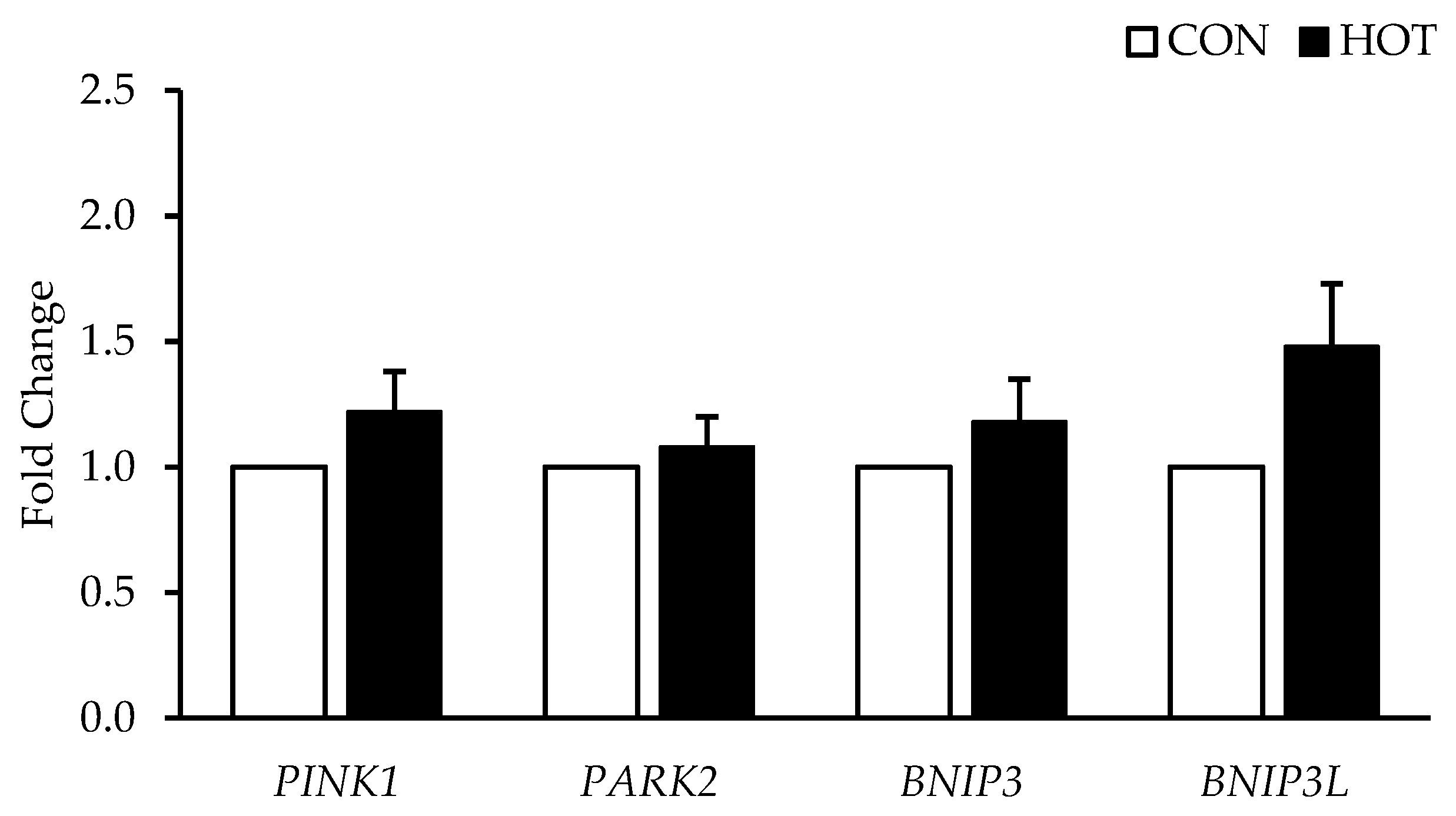
3.3. Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bournat, J.C.; Brown, C.W. Mitochondrial dysfunction in obesity. Curr. Opin. Endocrinol. Diabetes Obes. 2010, 17, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Derbre, F.; Gomez-Cabrera, M.C.; Nascimento, A.L.; Sanchis-Gomar, F.; Martinez-Bello, V.E.; Tresguerres, J.A.; Fuentes, T.; Gratas-Delamarche, A.; Monsalve, M.; Vina, J. Age associated low mitochondrial biogenesis may be explained by lack of response of PGC-1alpha to exercise training. Age 2012, 34, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Witte, M.E.; Geurts, J.J.G.; de Vries, H.E.; van der Valk, P.; van Horssen, J. Mitochondrial dysfunction: A potential link between neuroinflammation and neurodegeneration? Mitochondrion 2010, 10, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Hoppeler, H.; Lüthi, P.; Claassen, H.; Weibel, E.R.; Howald, H. The ultrastructure of the normal human skeletal muscle. A morphometric analysis on untrained men, women and well-trained orienteers. Pflügers Arch. 1973, 344, 217–232. [Google Scholar] [CrossRef]
- Hood, D.A.; Irrcher, I.; Ljubicic, V.; Joseph, A.M. Coordination of metabolic plasticity in skeletal muscle. J. Exp. Biol. 2006, 209, 2265–2275. [Google Scholar] [CrossRef]
- Garesse, R.; Vallejo, C.G. Animal mitochondrial biogenesis and function: A regulatory cross-talk between two genomes. Gene 2001, 263, 1–16. [Google Scholar] [CrossRef]
- Dominy, J.E.; Puigserver, P. Mitochondrial biogenesis through activation of nuclear signaling proteins. Cold Spring Harb. Perspect. Biol. 2013, 5, a015008. [Google Scholar] [CrossRef]
- Heil, M.; Eitenmüller, I.; Schmitz-Rixen, T.; Schaper, W. Arteriogenesis versus angiogenesis: Similarities and differences. J. Cell. Mol. Med. 2006, 10, 45–55. [Google Scholar] [CrossRef]
- Jornayvaz, F.R.; Shulman, G.I. Regulation of mitochondrial biogenesis. Essays Biochem. 2010, 47, 69–84. [Google Scholar] [CrossRef]
- McGlynn, M.L.; Schnitzler, H.; Shute, R.; Ruby, B.; Slivka, D. The Acute Effects of Exercise and Temperature on Regional mtDNA. Int. J. Environ. Res. Public Health 2021, 18, 6382. [Google Scholar] [CrossRef]
- Kubli, D.; Gustafsson, Å. Mitochondria and Mitophagy: The Yin and Yang of Cell Death Control. Circ. Res. 2012, 111, 1208–1221. [Google Scholar] [CrossRef] [PubMed]
- Puigserver, P.; Wu, Z.; Park, C.W.; Graves, R.; Wright, M.; Spiegelman, B.M. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 1998, 92, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Slivka, D.; Tucker, T.J.; Slivka, D.; Dumke, C.; Cuddy, J.; Ruby, B. Human mRNA response to exercise and temperature. Int. J. Sport. Med. 2012, 33, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Slivka, D.; Heesch, M.; Dumke, C.; Cuddy, J.; Hailes, W.; Ruby, B. Effects of post-exercise recovery in a cold environment on muscle glycogen, PGC-1α, and downstream transcription factors. Cryobiology 2013, 66, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Heesch, M.W.; Shute, R.J.; Kreiling, J.L.; Slivka, D.R. Transcriptional control, but not subcellular location, of PGC-1α is altered following exercise in a hot environment. J. Appl. Physiol. 2016, 121, 741–749. [Google Scholar] [CrossRef]
- Opichka, M.; Shute, R.; Marshall, K.; Slivka, D. Effects of exercise in a cold environment on gene expression for mitochondrial biogenesis and mitophagy. Cryobiology 2019, 90, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Zak, R.B.; Shute, R.J.; Heesch, M.W.; La Salle, D.T.; Bubak, M.P.; Dinan, N.E.; Laursen, T.L.; Slivka, D.R. Impact of hot and cold exposure on human skeletal muscle gene expression. Appl. Physiol. Nutr. Metab. 2017, 42, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.R.; Harris, M.B.; Cordaro, A.R.; Starnes, J.W. Effect of body temperature during exercise on skeletal muscle cytochrome c oxidase content. J. Appl. Physiol. 2002, 93, 526–530. [Google Scholar] [CrossRef]
- O’Reilly, N.; Collins, C.; McGlynn, M.L.; Slivka, D. Effect of local heat application during exercise on gene expression related to mitochondrial homeostasis. Appl. Physiol. Nutr. Metab. 2021, 46, 1545–1551. [Google Scholar] [CrossRef]
- Kim, K.; Reid, B.A.; Casey, C.A.; Bender, B.E.; Ro, B.; Song, Q.; Trewin, A.J.; Petersen, A.C.; Kuang, S.; Gavin, T.P.; et al. Effects of repeated local heat therapy on skeletal muscle structure and function in humans. J. Appl. Physiol. 2020, 128, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Barrett-O’Keefe, Z.; Ives, S.J.; Trinity, J.D.; Morgan, G.; Rossman, M.J.; Donato, A.J.; Runnels, S.; Morgan, D.E.; Gmelch, B.S.; Bledsoe, A.D.; et al. Endothelin-A -Mediated Vasoconstriction During Exercise with Advancing Age. J. Gerontology. Ser. A Biol. Sci. Med. Sci. 2015, 70, 554–565. [Google Scholar] [CrossRef] [PubMed]
- De Groot, P.C.E.; Poelkens, F.; Kooijman, M.; Hopman, M.T.E. Preserved flow-mediated dilation in the inactive legs of spinal cord-injured individuals. Am. J. Physiology. Heart Circ. Physiol. 2004, 287, 374. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔC(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.; Jensen, J.; Andersen, C.; Ørntoft, T. Normalization of real-time quantitative reverse transcription-PCR data: A model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004, 64, 5245–5250. [Google Scholar] [CrossRef]
- Seebacher, F.; Glanville, E. J Low levels of physical activity increase metabolic responsiveness to cold in a rat (Rattus fuscipes). PLoS ONE 2010, 5, e13022. [Google Scholar] [CrossRef] [PubMed]
- Saltin, B.; Gagge, A.P.; Stolwijk, J.A.J. Muscle temperature during submaximal exercise in man. J. Appl. Physiol. 1968, 25, 679–688. [Google Scholar] [CrossRef]
- Pearson, J.; Low, D.A.; Stöhr, E.; Kalsi, K.; Ali, L.; Barker, H.; González-Alonso, J. Hemodynamic responses to heat stress in the resting and exercising human leg: Insight into the effect of temperature on skeletal muscle blood flow. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, 663–673. [Google Scholar] [CrossRef]
- Park, S.; Wooden, T.K.; Pekas, E.J.; Anderson, C.P.; Yadav, S.K.; Slivka, D.R.; Layec, G. Effects of passive and active leg movements to interrupt sitting in mild hypercapnia on cardiovascular function in healthy adults. J. Appl. Physiol. 2022, 132, 874–887. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Pekas, E.J.; Anderson, C.P.; Kambis, T.N.; Mishra, P.K.; Schieber, M.N.; Wooden, T.K.; Thompson, J.R.; Kim, K.S.; Pipinos, I.I. Impaired microcirculatory function, mitochondrial respiration, and oxygen utilization in skeletal muscle of claudicating patients with peripheral artery disease. Am. J. Physiology. Heart Circ. Physiol. 2022, 322, H867–H879. [Google Scholar] [CrossRef]
- Pellinger, T.; Neighbors, C.; Simmons, G. Acute Lower Leg Heating Increases Exercise Capacity in Patients with Peripheral Artery Disease. J. Cardiovasc. Nurs. 2019, 34, 130–133. [Google Scholar] [CrossRef]
- Larsson, N.G.; Wang, J.; Wilhelmsson, H.; Oldfors, A.; Rustin, P.; Lewandoski, M.; Barsh, G.S.; Clayton, D.A. Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in mice. Nat. Genet. 1998, 18, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Hamasaki, N. Mitochondrial transcription factor A in the maintenance of mitochondrial DNA: Overview of its multiple roles. Ann. New York Acad. Sci. 2005, 1042, 101–108. [Google Scholar] [CrossRef]
- Kang, I.; Chu, C.T.; Kaufman, B.A. The mitochondrial transcription factor TFAM in neurodegeneration: Emerging evidence and mechanisms. FEBS Lett. 2018, 592, 793–811. [Google Scholar] [CrossRef]
- Hafen, P.S.; Preece, C.N.; Sorensen, J.R.; Hancock, C.R.; Hyldahl, R.D. Repeated exposure to heat stress induces mitochondrial adaptation in human skeletal muscle. J. Appl. Physiol. 2018, 125, 1447–1455. [Google Scholar] [CrossRef] [PubMed]
- Schwalm, C.; Deldicque, L.; Francaux, M. Lack of Activation of Mitophagy during Endurance Exercise in Human. Med. Sci. Sports Exerc. 2017, 49, 1552–1561. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, M.; Robins, L.; McGlynn, M.L.; Collins, C.; Pekas, E.J.; Park, S.-Y.; Slivka, D. No Mitochondrial Related Transcriptional Changes in Human Skeletal Muscle after Local Heat Application. Int. J. Environ. Res. Public Health 2022, 19, 17051. https://doi.org/10.3390/ijerph192417051
Kwon M, Robins L, McGlynn ML, Collins C, Pekas EJ, Park S-Y, Slivka D. No Mitochondrial Related Transcriptional Changes in Human Skeletal Muscle after Local Heat Application. International Journal of Environmental Research and Public Health. 2022; 19(24):17051. https://doi.org/10.3390/ijerph192417051
Chicago/Turabian StyleKwon, Monica, Larry Robins, Mark L. McGlynn, Christopher Collins, Elizabeth J. Pekas, Song-Young Park, and Dustin Slivka. 2022. "No Mitochondrial Related Transcriptional Changes in Human Skeletal Muscle after Local Heat Application" International Journal of Environmental Research and Public Health 19, no. 24: 17051. https://doi.org/10.3390/ijerph192417051
APA StyleKwon, M., Robins, L., McGlynn, M. L., Collins, C., Pekas, E. J., Park, S.-Y., & Slivka, D. (2022). No Mitochondrial Related Transcriptional Changes in Human Skeletal Muscle after Local Heat Application. International Journal of Environmental Research and Public Health, 19(24), 17051. https://doi.org/10.3390/ijerph192417051






