Characterization of Metallic Off-Flavors in Drinking Water: Health, Consumption, and Sensory Perception
Abstract
1. Introduction
2. Methods and Materials
2.1. Artificial Saliva Mixture
2.2. Metal Salts Stock Solutions
2.3. Salivary Lipid Oxidation Experiments with Artificial Saliva
2.4. Study Participants
2.5. Beverage Intake Questionnaire
2.6. Metallic Flavor Sensory Threshold Determination
2.7. Data Analyses
3. Results
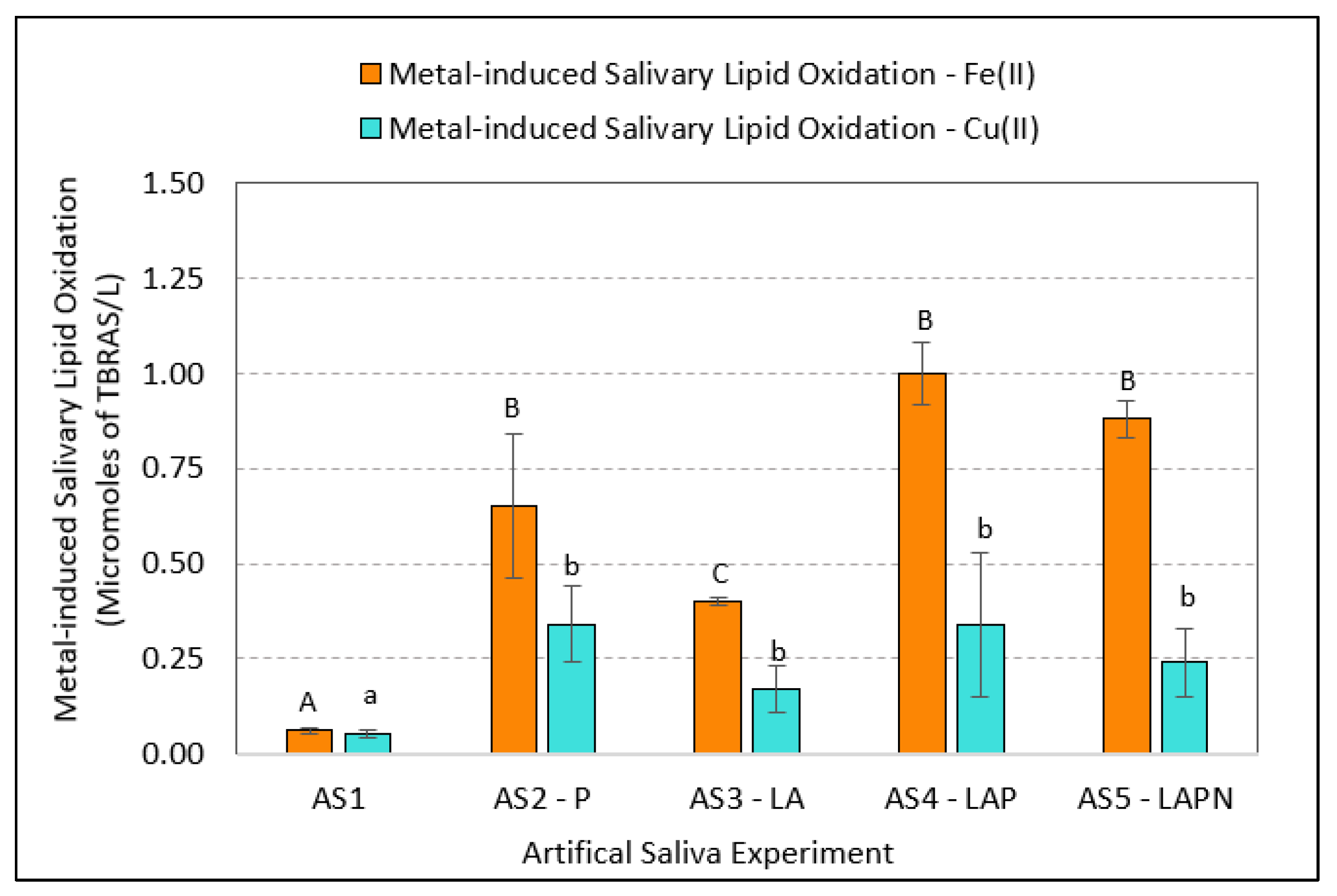
3.1. Variations in Metal-Induced Lipid Oxidation in Artificial Saliva
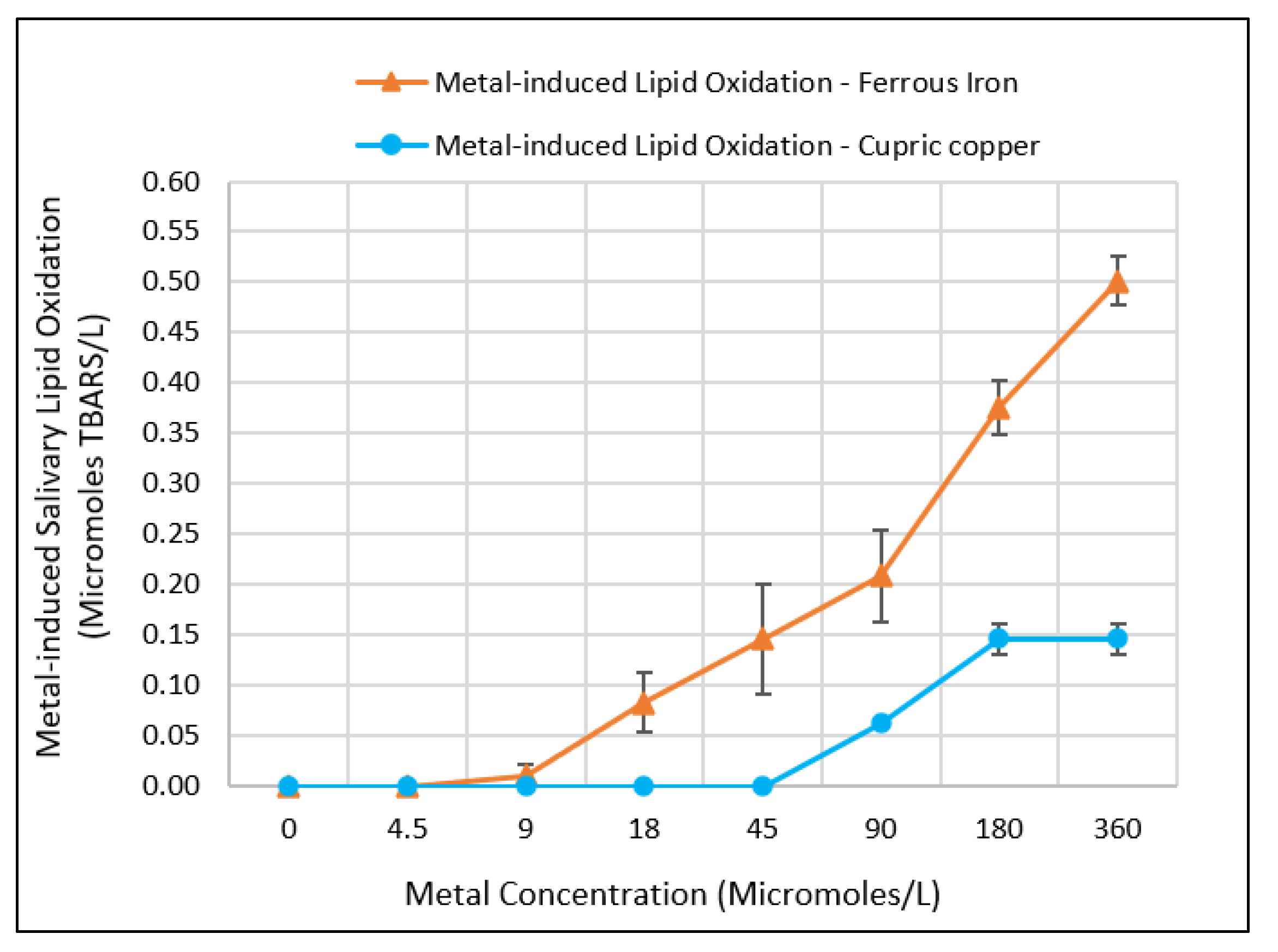
3.2. Effect of Metal Concentration on Inducing Salivary Lipid Oxidation
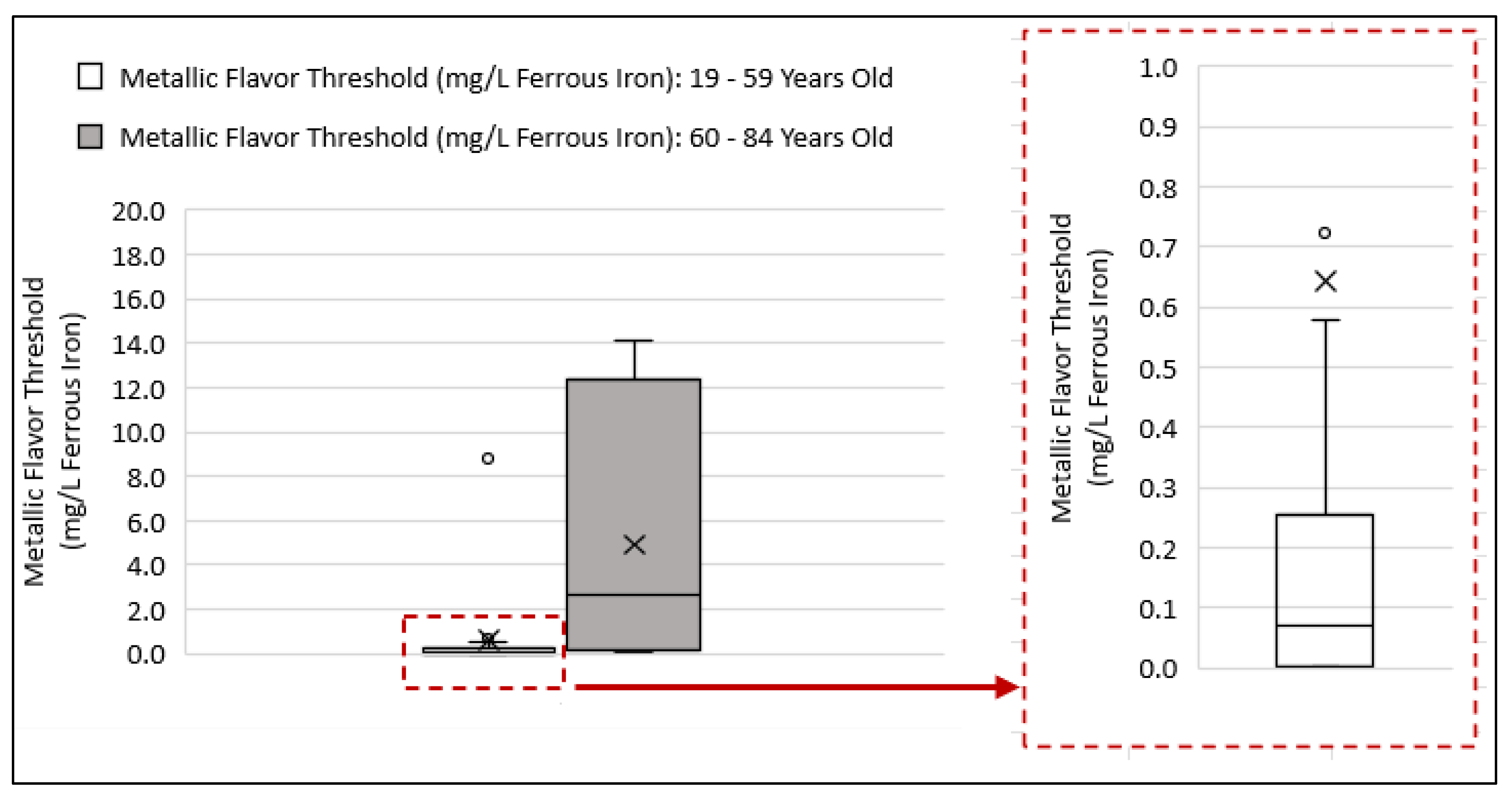
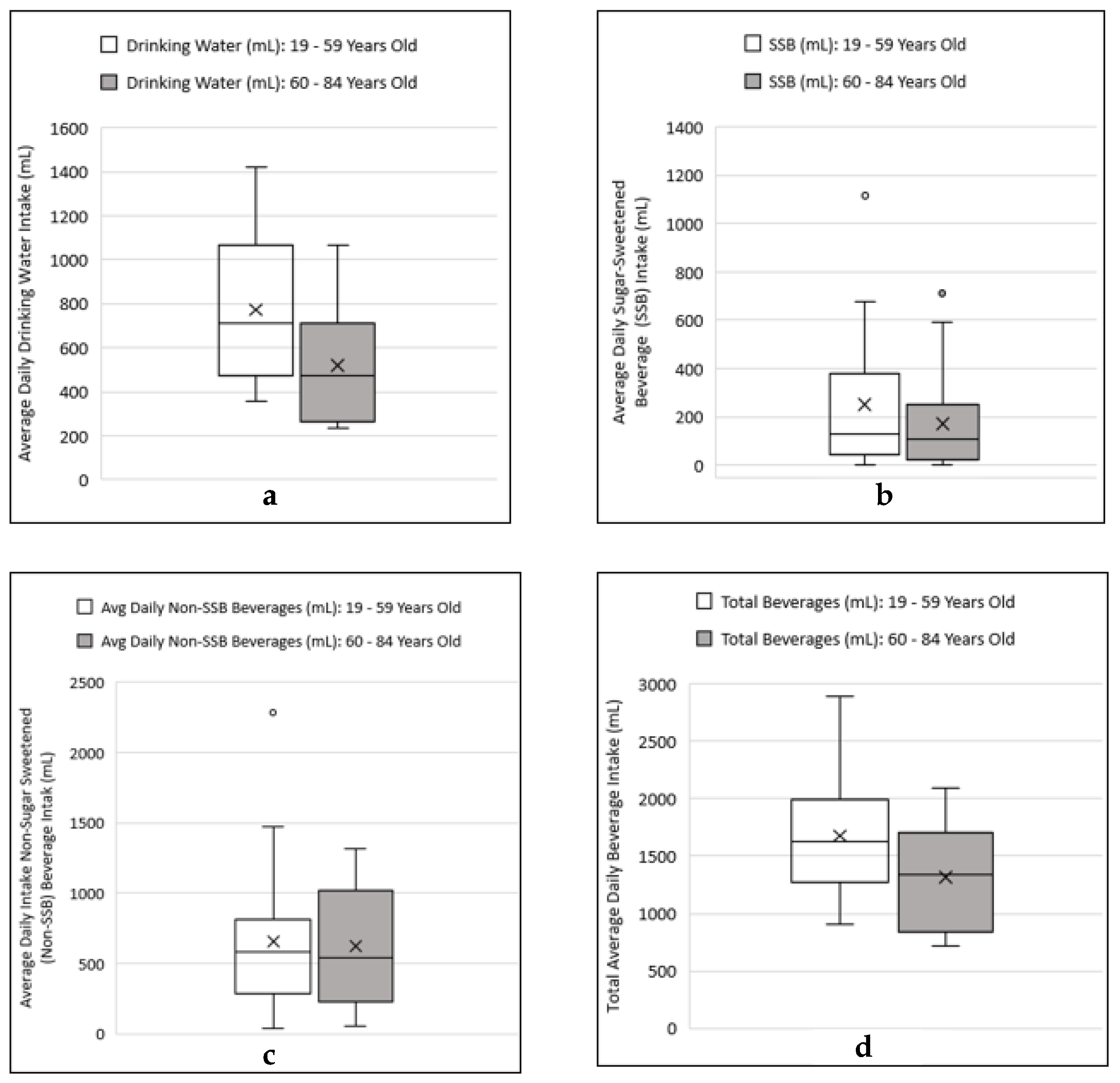
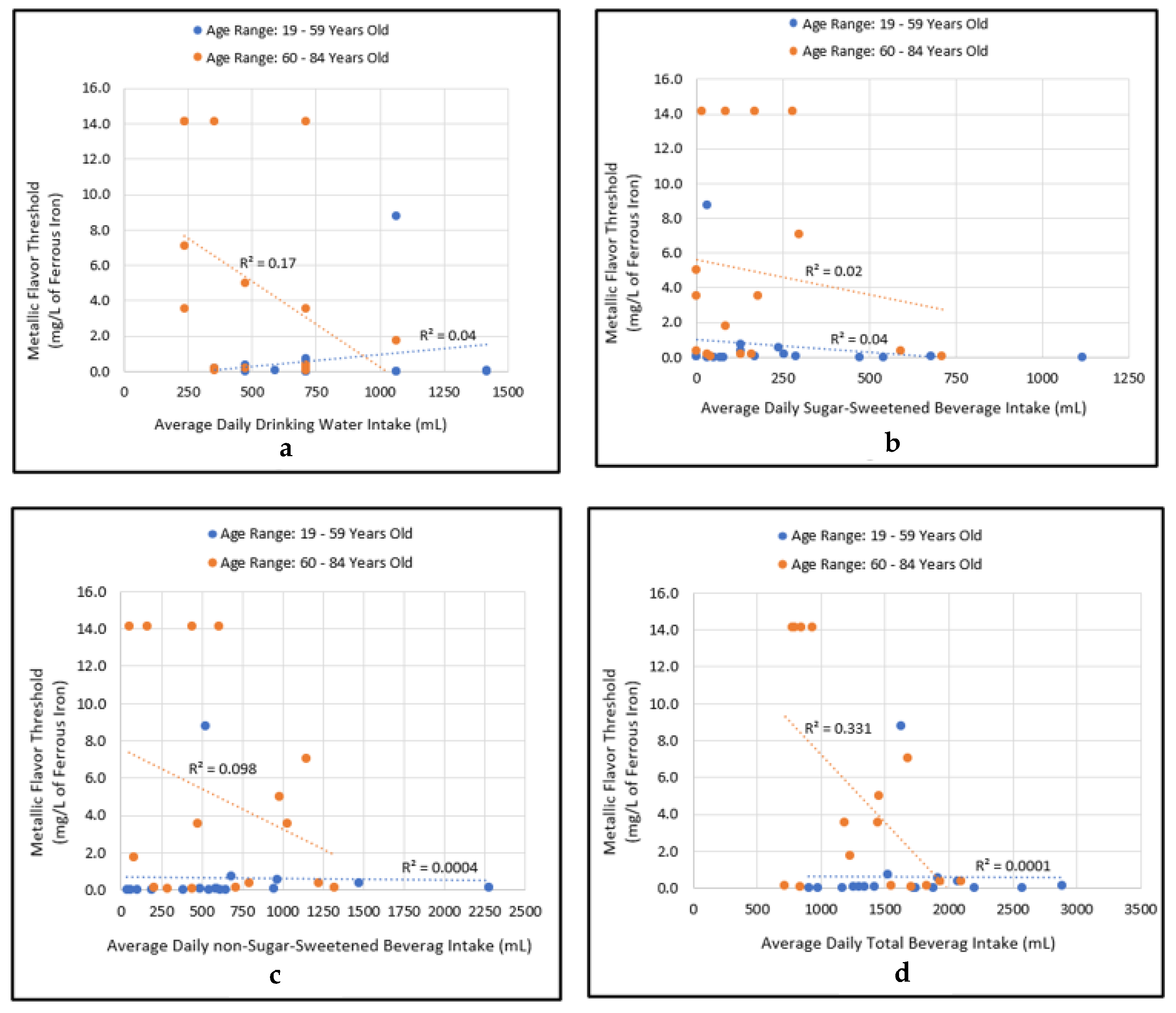
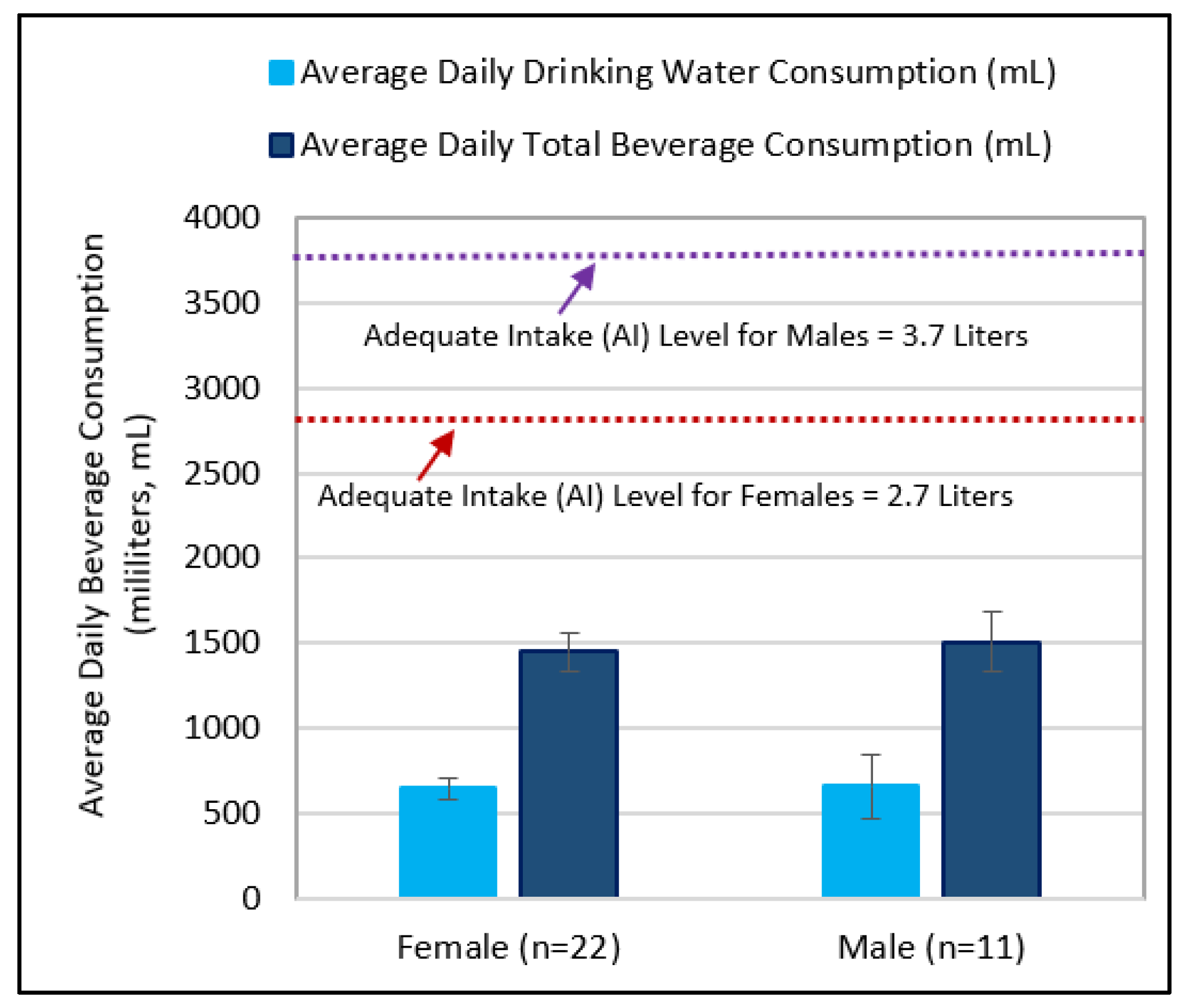
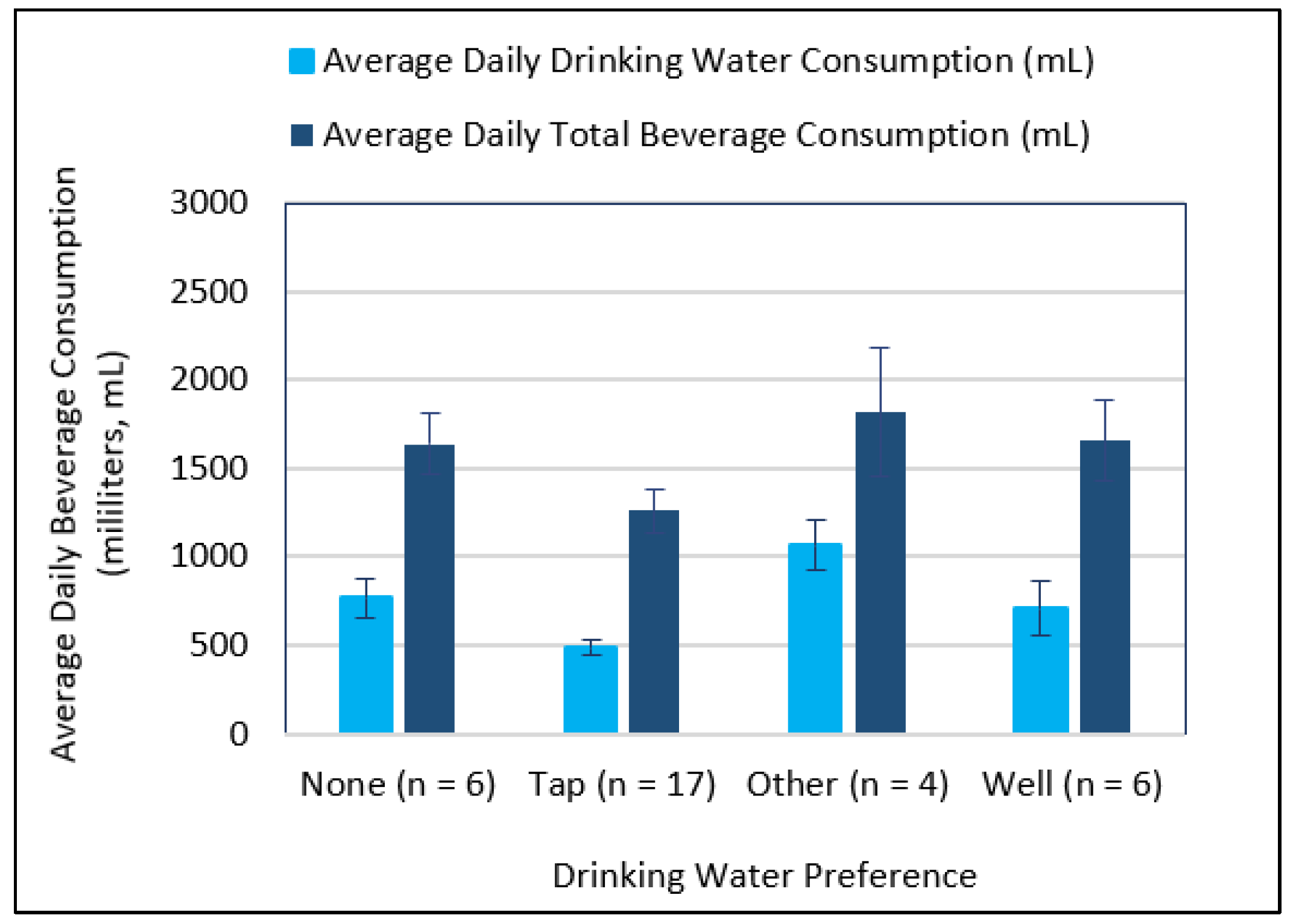
3.3. Comparing Beverage Consumptions and Metallic Flavor Sensitivity Levels between Younger and Older Age Groups
3.4. Correlational Relationship between Beverage Consumptions and Metallic Flavor Sensitivity Levels
4. Discussion
4.1. Assessing the Influence of Salivary Constituents on Metallic Flavor Production Measured by the Thiobarbituric Acid Reactive Substances (TBARS)
4.2. Comparing the Fe(II)- and Cu(II)-Induced Salivary Lipid Oxidation by Metal Concentration as Measures of Metallic Flavor Intensity
4.3. Examining Beverage Consumptions and Metallic Flavor Sensitivity Levels between Younger and Older Age Groups
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United States Environmental Protection Agency (USEPA). Drinking Water Standards and Health Advisories Tables; EPA-822-F-18-001; Office of Water, U.S. Environmental Protection Agency: Washington, DC, USA, 2018.
- WHO. Guidelines for Drinking Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2017.
- Ömür-Özbek, P. Global taste and odor survey of water utilities: Final report to the American Water Works Association from the Taste and Odor Committee; AWWA: Denver, CO, USA, 2012. [Google Scholar]
- Tong, H.; Li, Z.; Hu, X.; Xu, W.; Li, Z. Metals in Occluded Water: A New Perspective for Pollution in Drinking Water Distribution Systems. Int. J. Environ. Res. Public Health 2019, 16, 2849. [Google Scholar] [CrossRef] [PubMed]
- Kirmeyer, G.J. Guidance Manual for Maintaining Distribution System Water Quality; AWWA Research Foundation and American Water Works Association: Denver, CO, USA, 2000. [Google Scholar]
- Dietrich, A.; Glindemann, D.; Pizarro, F.; Gidi, V.; Olivares, M.; Araya, M.; Camper, A.; Duncan, S.; Dwyer, S.; Whelton, A.; et al. Health and aesthetic impacts of copper corrosion on drinking water. Water Sci. Technol. 2004, 49, 55–62. [Google Scholar] [CrossRef] [PubMed]
- DeSimones, L.A.; Hamilton, P.A.; Gilliom, R.J. The Quality of Our Nation’s Waters-Quality of Water from Domestic Wells in Principal Aquifers of the United States, 1991–2004-Overview of Major Findings: U.S. Geological Survey Circular 1332 U.S.; Department of Interior: Washington, DC, USA, 2009.
- Epke, E.M.; Lawless, H.T. Retronasal smell and detection thresholds of iron and copper salts. Physiol. Behav. 2007, 92, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.H.; Duncan, S.E.; Dietrich, A.M.; O’Keefe, S.F. Evaluation of the operationally defined soluble, insoluble, and complexing copper consumed through drinking water in human saliva. Eur. Food Res. Technol. 2010, 231, 977–984. [Google Scholar] [CrossRef]
- Ömur-Özbek, P.; Dietrich, A.M. Retronasal perception and flavour thresholds of iron and copper in drinking water. J. Water Health 2011, 9, 1–9. [Google Scholar] [CrossRef]
- Zacarías, I.; Yáñez, C.G.; Araya, M.; Oraka, C.; Olivares, M.; Uauy, R. Determination of the taste threshold of copper in water. Chem. Senses 2001, 26, 85–89. [Google Scholar] [CrossRef]
- Ömür-Özbek, P.; Dietrich, A.M.; Duncan, S.E.; Lee, Y. Role of Lipid Oxidation, Chelating Agents, and Antioxidants in Metallic Flavor Development in the Oral Cavity. J. Agric. Food Chem. 2012, 60, 2274–2280. [Google Scholar] [CrossRef]
- Ecarma, M.J.Y.; Nolden, A.A. A review of the flavor profile of metal salts: Understanding the complexity of metallic sensation. Chem. Senses 2021, 46, bjab043. [Google Scholar] [CrossRef]
- Mirlohi, S. In Vitro Evaluation of Iron-Induced Salivary Lipid Oxidation Associated with Exposure to Iron Nanoparticles: Application Possibilities and Limitations for Food and Exposure Sciences. Int. J. Environ. Res. Public Healh 2020, 17, 3622. [Google Scholar] [CrossRef]
- Dietrich, A.M. The sense of smell: Contributions of orthonasal and retronasal perception applied to metallic flavor of drinking water. J. Water Supply: Res. Technol. 2009, 58, 562–570. [Google Scholar] [CrossRef]
- Glindemann, D.; Dietrich, A.M.; Staerk, H.J.; Kuschk, P. The two smells of touched or pickled iron (skin) carbonyl-hydrocarbons and organophosphines. Angew. Chem. Int. Ed. 2006, 45, 7006–7009. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Taniguchi, K.; Suzuki, Y.; Okubo, T.; Takata, R.; Konno, T. Iron Is an Essential Cause of Fishy Aftertaste Formation in Wine and Seafood Pairing. J. Agric. Food Chem. 2009, 57, 8550–8556. [Google Scholar] [CrossRef] [PubMed]
- Larsson, B.; Olivecrona, G.; Ericson, T. Lipids in human saliva. Arch. Oral. Biol. 1996, 41, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Repetto, M.G.; Ferrarotti, N.F.; Boveris, A. The involvement of transition metal ions on iron-dependent lipid peroxidation. Arch. Toxicol. 2010, 84, 255–262. [Google Scholar] [CrossRef]
- Gorelik, S.; Kohen, R.; Ligumsky, M.; Kanner, J. Saliva plays a dual role in oxidation process in stomach medium. Arch. Biochem. Biophys. 2007, 458, 236–243. [Google Scholar] [CrossRef]
- Hong, J.H.; Duncan, S.E.; Dietrich, A.M.; O’Keefe, S.F.; Eigel, W.N.; Mallikarjunan, K. Interaction of Copper and Human Salivary Proteins. J. Agric. Food Chem. 2009, 57, 6967–6975. [Google Scholar] [CrossRef]
- Tang, J. Modeling Contaminant Transport in Polyethylene and Metal Speciation in Saliva. Master’s Thesis, Virginia Tech, Blacksburg, Virginia, 2010. [Google Scholar]
- Shacter, E. Quantification and significance of protein oxidation in biological samples. Drug Metab. Rev. 2000, 32, 307–326. [Google Scholar] [CrossRef]
- Kehm, R.; Baldensperger, T.; Raupbach, J.; Höhn, A. Protein oxidation—Formation mechanisms, detection and relevance as biomarkers in human diseases. Redox Biol. 2021, 42, 101901. [Google Scholar] [CrossRef]
- Elias, R.J.; Kellerby, S.S.; Decker, E.A. Antioxidant Activity of Proteins and Peptides. Crit. Rev. Food Sci. Nutr. 2008, 48, 430–441. [Google Scholar] [CrossRef]
- Delompré, T.; Guichard, E.; Briand, L.; Salles, C. Taste Perception of Nutrients Found in Nutritional Supplements: A Review. Nutrients 2019, 11, 2050. [Google Scholar] [CrossRef]
- Schwartz, M.; Neiers, F.; Feron, G.; Canon, F. The Relationship Between Salivary Redox, Diet, and Food Flavor Perception. Front. Nutr. 2021, 7, 612735. [Google Scholar] [CrossRef] [PubMed]
- Mennella, I.; Fogliano, V.; Vitaglione, P. Salivary lipase and α-amylase activities are higher in overweight than in normal weight subjects: Influences on dietary behavior. Food Res. Int. 2014, 66, 463–468. [Google Scholar] [CrossRef]
- Dsamou, M.; Palicki, O.; Septier, C.; Chabanet, C.; Lucchi, G.; Ducoroy, P.; Chagnon, M.-C.; Morzel, M. Salivary Protein Profiles and Sensitivity to the Bitter Taste of Caffeine. Chem. Senses 2011, 37, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kleczyk, E.; Bosch, D.; Tanellari, E.; Dwyer, S.; Dietrich, A. Case Study: Preference Trade-Offs toward Home Plumbing Attributes and Materials. J. Water Resour. Plan. Manag. 2009, 135, 237–243. [Google Scholar] [CrossRef]
- Whelton, A.; Dietrich, A.; Burlingame, G.; Schechs, M.; Duncan, S. Minerals in drinking water: Impacts on taste and importance to consumer health. Water Sci. Technol. 2007, 55, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Delpla, I.; Legay, C.; Proulx, F.; Rodriguez, M.J. Perception of tap water quality: Assessment of the factors modifying the links between satisfaction and water consumption behavior. Sci. Total Environ. 2020, 722, 137786. [Google Scholar] [CrossRef]
- Benameur, T.; Benameur, N.; Saidi, N.; Tartag, S.; Sayad, H.; Agouni, A. Predicting factors of public awareness and perception about the quality, safety of drinking water, and pollution incidents. Environ. Monit. Assess. 2021, 194, 22. [Google Scholar] [CrossRef]
- Park, S.; Onufrak, S.J.; Cradock, A.L.; Hecht, C.; Patel, A.; Chevinsky, J.R.; Blanck, H.M. Factors Related to Water Filter Use for Drinking Tap Water at Home and Its Association With Consuming Plain Water and Sugar-Sweetened Beverages Among U.S. Adults. Am. J. Health Promot. 2022, 36, 813–822. [Google Scholar] [CrossRef]
- Hong, J.H.; Kim, K.-O. Operationally defined solubilization of copper and iron in human saliva and implications for metallic flavor perception. Eur. Food Res. Technol. 2011, 233, 973–983. [Google Scholar] [CrossRef]
- Craun, G.F.; Brunkard, J.M.; Yoder, J.S.; Roberts, V.A.; Carpenter, J.; Wade, T.; Calderon, R.L.; Roberts, J.M.; Beach, M.J.; Roy, S.L. Causes of Outbreaks Associated with Drinking Water in the United States from 1971 to 2006. Clin. Microbiol. Rev. 2010, 23, 507–528. [Google Scholar] [CrossRef]
- Benham, B.; Ling, E.J.; Scott, J.P.; Lutz, J.; Thompson, J.; Thompson, J. Evaluation of Household Water Quality in Fluvanna and Louisa Counties, Virginia. October 2010, Virginia Household Water Quality Program; Virginia Cooperative Extension: Blacksburg, VA, USA, 2010. [Google Scholar]
- Dietrich, A.M.; Burlingame, G.A. Critical Review and Rethinking of USEPA Secondary Standards for Maintaining Organoleptic Quality of Drinking Water. Environ. Sci. Technol. 2015, 49, 708–720. [Google Scholar] [CrossRef] [PubMed]
- Jequier, E.; Constant, F. Water as an essential nutrient: The physiological basis of hydration. Eur. J. Clin. Nutr. 2010, 64, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Popkin, B.M.; D’Anci, K.E.; Rosenberg, I.H. Water, hydration, and health. Nutr. Rev. 2010, 68, 439–458. [Google Scholar] [CrossRef]
- Aznar, L.M.; Salvadó, J.S.; Eizaguirre, F.M.; Rodríguez-Mañas, L.; de Pipaón, M.S.; Miñana, I.V. Importancia del consumo de agua en la salud y la prevención de la enfermedad: Situación actual The importance of water consumption in health and disease prevention: The current situation. Nutr. Hosp. 2020, 37, 1072–1086. [Google Scholar] [CrossRef]
- Ritz, P.; Berrut, G. The Importance of Good Hydration for Day-to-Day Health. Nutr. Rev. 2005, 63, 6–13. [Google Scholar] [CrossRef]
- Institute of Medicine (IOM). Dietary reference intakes for water, potassium, sodium, chloride, and sulfate/Panel on Dietary Reference Intakes for Electrolytes and Water, Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Food and Nutrition Board. Institute of Medicine (U.S.); Panel on Dietary Reference Intakes for Electrolytes and Water: Washington, DC, USA, 2004. [Google Scholar]
- Asai, J.L. Nutrition and the geriatric rehabilitation patient—Challenges and solutions. Top Geriatr. Rehabil. 2004, 20, 34–45. [Google Scholar] [CrossRef]
- Bratlund, C.V.; O’Donoghue, C.R.; Rocchiccioli, J.T. Dehydration and Dysphagia: Challenges in the Older Adult. J. Med. SpeechLang Pathol. 2020, 18, 1–10. [Google Scholar]
- Kant, A.K.; Graubard, B.I.; Atchison, E.A. Intakes of plain water, moisture in foods and beverages, and total water in the adult US population-nutritional, meal pattern, and body weight correlates: National Health and Nutrition Examination Surveys 1999-2006. Am. J. Clin. Nutr. 2009, 90, 655–663. [Google Scholar] [CrossRef]
- De França Doria, M. Factors influencing public perception of drinking water quality. Water Policy 2010, 12, 1–19. [Google Scholar] [CrossRef]
- de França Doria, M.; Pidgeon, N.; Hunter, P.R. Perceptions of drinking water quality and risk and its effect on behaviour: A cross-national study. Sci. Total. Environ. 2009, 407, 5455–5464. [Google Scholar] [CrossRef]
- Dupont, D.; Adamowicz, W.L.; Krupnick, A. Differences in water consumption choices in Canada: The role of socio-demographics, experiences, and perceptions of health risks. J. Water Health 2010, 8, 671–686. [Google Scholar] [CrossRef] [PubMed]
- Puputti, S.; Hoppu, U.; Sandell, M. Taste Sensitivity Is Associated with Food Consumption Behavior but not with Recalled Pleasantness. Foods 2019, 8, 444. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.-Y.; Tucker, R.M. Sweet Taste as a Predictor of Dietary Intake: A Systematic Review. Nutrients 2019, 11, 94. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Duncan, S.E.; Dietrich, A.M. Effect of iron on taste perception and emotional response of sweetened beverage under different water conditions. Food Qual. Preference 2016, 54, 58–66. [Google Scholar] [CrossRef]
- Risso, D.; Drayna, D.; Morini, G. Alteration, Reduction and Taste Loss: Main Causes and Potential Implications on Dietary Habits. Nutrients 2020, 12, 3284. [Google Scholar] [CrossRef] [PubMed]
- Mirlohi, S.; Dietrich, A.M.; Duncan, S.E. Age-Associated Variation in Sensory Perception of Iron in Drinking Water and the Potential for Overexposure in the Human Population. Environ. Sci. Technol. 2011, 45, 6575–6583. [Google Scholar] [CrossRef]
- Actisa, B.; Perovicb, N.; Defago, D.; Beccaceceb, C.; Eynarda, A. Fatty acid profile of human saliva: A possible indicator of dietary fat intake. Arch. Oral. Biol. 2005, 50, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S. A comparison of ghrelin, glucose, alpha-amylase and protein levels in saliva from diabetics. BMB Rep. 2007, 40, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Slomiany, B.; Zdebska, E.; Murty, V.; Slomiany, A.; Petropoulou, K.; Mandel, I. Lipid composition of human labial salivary gland secretions. Arch. Oral Biol. 1983, 28, 711–714. [Google Scholar] [CrossRef]
- Ben-Aryeh, H.; Shalev, A.; Szargel, R.; Laor, A.; Laufer, D.; Gutman, D. The salivary flow rate and composition of whole and parotid resting and stimulated saliva in young and old healthy subjects. Biochem. Med. Metab. Biol. 1986, 36, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Spanier, A.M. A rapid, direct chemical assay for the quantitive determination of thiobarbituric acid reactive substances in raw, cooked, and cooked/stored muscle foods. J. Muscle Foods 1991, 2, 165–176. [Google Scholar] [CrossRef]
- Wang, H. Determination of Thiobarbituric Acid Reactive Substances (TBARS) for Water Base Liquid Food with Low Oxidation; Virginia Tech: Blacksburg, Virginia, 2009. [Google Scholar]
- Janero, D.R. Malondialdehyde and Thiobarbituric acid reactivity as diagnostic indexes of lipid-peroxidation and peroxidative tissue injury. Free Radic. Biol. Med. 1990, 9, 515–540. [Google Scholar] [CrossRef] [PubMed]
- Hedrick, V.E.; Comber, D.L.; Estabrooks, P.A.; Savla, J.; Davy, B.M. The Beverage Intake Questionnaire: Determining Initial Validity and Reliability. J. Am. Diet. Assoc. 2010, 110, 1227–1232. [Google Scholar] [CrossRef] [PubMed]
- American Standards for Testing Materials (ASTM). Standard Practice for Determination of Odor and Taste Thresholds by a Forced Choice Ascending Concentration series Method of Limits; E-679-79. (Annual Book of Standards); American Society for Testing of Materials (ASTM): Philadelphia, PA, USA, 1997. [Google Scholar]
- Lawless, H.; Heymann, H. Sensory Evaluation of Food: Principles and Practices; Aspen Publishers: New York, NY, USA, 1999. [Google Scholar]
- American Public Health Association (APHA); The American Water Works Association (AWWA); The Water Environment Federation (WEF) (Eds.) Standard Methods for Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Rosinger, A.; Herrick, K. Daily Water Intake among U.S. Men and Women, 2009–2012; NCHS data brief, no 242.; National Center for Health Statistics: Hyattsville, MD, USA, 2016.
- Hezel, M.; Weitzberg, E. The oral microbiome and nitric oxide homoeostasis. Oral Dis. 2015, 21, 7–16. [Google Scholar] [CrossRef]
- Méjean, C.; Morzel, M.; Neyraud, E.; Issanchou, S.; Martin, C.; Bozonnet, S.; Urbano, C.; Schlich, P.; Hercberg, S.; Péneau, S.; et al. Salivary Composition Is Associated with Liking and Usual Nutrient Intake. PLoS ONE 2015, 10, e0137473. [Google Scholar] [CrossRef]
- Murr, A.; Pink, C.; Hammer, E.; Michalik, S.; Dhople, V.M.; Holtfreter, B.; Völker, U.; Kocher, T.; Salazar, M.G. Cross-Sectional Association of Salivary Proteins with Age, Sex, Body Mass Index, Smoking, and Education. J. Proteome Res. 2017, 16, 2273–2281. [Google Scholar] [CrossRef]
- Suzuki, T.; Fujiwara, S.; Kinoshita, S.; Butovich, I.A. Cyclic Change of Fatty Acid Composition in Meibum During the Menstrual Cycle. Investig. Opthalmology Vis. Sci. 2019, 60, 1724–1733. [Google Scholar] [CrossRef]
- Valko, M.; Morris, H.; Cronin, M.T.D. Metals, Toxicity and Oxidative Stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef] [PubMed]
- Bossingham, M.J.; Carnell, N.S.; Campbell, W.W. Water balance, hydration status, and fat-free mass hydration in younger and older adults. Am. J. Clin. Nutr. 2005, 81, 1342–1350. [Google Scholar] [CrossRef]
- Kennedy, O.; Law, C.; Methven, L.; Mottram, D.; Gosney, M. Investigating age-related changes in taste and affects on sensory perceptions of oral nutritional supplements. Age Ageing 2010, 39, 733–738. [Google Scholar] [CrossRef]
- Mojet, J.; Christ-Hazelhof, E.; Heidema, J. Taste perception with age: Pleasantness and its relationships with threshold sensitivity and supra-threshold intensity of five taste qualities. Food Qual. Preference 2005, 16, 413–423. [Google Scholar] [CrossRef]
- Xue, Y.; Wen, Q.; Xu, C.; Zhang, X.; Zeng, J.; Sha, A.M.; Lan, C.; Li, L.; Wang, H.; Yang, X.; et al. Elevated Salt Taste Threshold Is Associated with Increased Risk of Coronary Heart Disease. J. Cardiovasc. Transl. Res. 2020, 13, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Wotton, K.; Crannitch, K.; Munt, R. Prevalence, risk factors and strategies to prevent dehydration in older adults. Contemp. Nurse 2008, 31, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Vieux, F.; Maillot, M.; Rehm, C.D.; Barrios, P.; Drewnowski, A. Opposing Consumption Trends for Sugar-Sweetened Beverages and Plain Drinking Water: Analyses of NHANES 2011–16 Data. Front. Nutr. 2020, 7, 587123. [Google Scholar] [CrossRef]
- Liska, D.; Mah, E.; Brisbois, T.; Barrios, P.L.; Baker, L.B.; Spriet, L.L. Narrative Review of Hydration and Selected Health Outcomes in the General Population. Nutrients 2019, 11, 70. [Google Scholar] [CrossRef] [PubMed]
- Davy, B.M.; Dennis, E.A.; Dengo, A.L.; Wilson, K.L.; Davy, K.P. Water Consumption Reduces Energy Intake at a Breakfast Meal in Obese Older Adults. J. Am. Diet. Assoc. 2008, 108, 1236–1239. [Google Scholar] [CrossRef]
- Dennis, E.A.; Flack, K.D.; Davy, B.M. Beverage consumption and adult weight management: A review. Eat. Behav. 2009, 10, 237–246. [Google Scholar] [CrossRef]
- Cruz, A.J.; Bracamontes-Castelo, G.; Bacardí-Gascón, M. Effect of water consumption on weight loss: A systematic review. Efecto del consumo de agua sobre la pérdida de peso: Revisión sistemática. Nutr. Hosp. 2019, 36, 1424–1429. [Google Scholar] [CrossRef]
- Imgrund, K.; Kreutzwiser, R.; De Loë, R. Influences on the water testing behaviors of private well owners. J. Water Health 2011, 9, 241–252. [Google Scholar] [CrossRef]
- Jones, A.Q.; Dewey, C.E.; Doré, K.; Majowicz, S.E.; McEwen, S.A.; David, W.-T.; Eric, M.; Carr, D.J.; Henson, S.J. Public perceptions of drinking water: A postal survey of residents with private water supplies. BMC Public Health 2006, 6, 94. [Google Scholar] [CrossRef]
- Colburn, A.T.; Kavouras, S.A. Tap Water Consumption and Perceptions in United States Latinx Adults. Nutrients 2021, 13, 2999. [Google Scholar] [CrossRef] [PubMed]
- Puget, S.; Beno, N.; Chabanet, C.; Guichard, E.; Thomas-Danguin, T. Tap water consumers differ from non-consumers in chlorine flavor acceptability but not sensitivity. Water Res. 2010, 44, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Teillet, E.; Urbano, C.; Cordelle, S.; Schlich, P. Consumer perception and preference of bottled and tap water. J. Sens. Stud. 2010, 25, 463–480. [Google Scholar] [CrossRef]
- Pirkwieser, P.; Behrens, M.; Somoza, V. Metallic Sensation—Just an Off-Flavor or a Biologically Relevant Sensing Pathway? J. Agric. Food Chem. 2021, 69, 1775–1780. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Yoshimura, K.; Toya, S.; Tsuchimochi, M. Pathogenesis of taste impairment and salivary dysfunction in COVID-19 patients. Jpn. Dent. Sci. Rev. 2021, 57, 111–122. [Google Scholar] [CrossRef]
- Thomas, D.C.; Chablani, D.; Parekh, S.; Pichammal, R.C.; Shanmugasundaram, K.; Pitchumani, P.K. Dysgeusia: A review in the context of COVID-19. J. Am. Dent. Assoc. 2022, 153, 251–264. [Google Scholar] [CrossRef] [PubMed]
| Abbreviation | Artificial Saliva (AS) Sample Description | Fatty Acid: Linoleic Acid, LA (g/L) | Proteins: Alpha-Amylase + Mucin (g/L) | Metal Concentration (µM) Fe(II) or Cu(II) |
|---|---|---|---|---|
| AS1 | AS | 0 | 0 | 0, 180 |
| AS2—P | AS + Protein | 0 | 3.78 | 0, 180 |
| AS3—LA | AS + LA | 0.03 | 0 | 0, 4.5, 9, 18, 45, 90, 180, 360 |
| AS4—LAP | AS + LA + Protein | 0.03 | 3.78 | 0, 180 |
| AS5—LAPN | AS + Protein + LA + Nitrite | 0.03 | 3.78 | 0, 180 |
| Artificial Saliva Sample (1) | No. of Replicates (N) | Metal-Induced SLO (Micromoles/L) | SEM (2) | 95% Confidence Interval |
|---|---|---|---|---|
| Lipid Oxidation Experiments with Fe(II) | ||||
| AS1 | 4 | 0.06 | 0.01 | [0.03–0.09] |
| AS2—P | 4 | 0.65 | 0.19 | [0.04–1.23] |
| AS3—LA | 4 | 0.40 | 0.01 | [0.36–0.44] |
| AS4—LAP | 4 | 1.00 | 0.08 | [0.74–1.26] |
| AS5—LAPN | 4 | 0.88 | 0.05 | [0.71–1.04] |
| Lipid Oxidation Experiments with Cu(II) | ||||
| AS1 | 4 | 0.05 | 0.01 | [0.04–0.06] |
| AS2—P | 4 | 0.34 | 0.10 | [0.19–0.50] |
| AS3—LA | 4 | 0.17 | 0.06 | [0.06–0.27] |
| AS4—LAP | 4 | 0.34 | 0.19 | [0.04–0.64] |
| AS5—LAPN | 4 | 0.24 | 0.09 | [0.10–0.38] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirlohi, S. Characterization of Metallic Off-Flavors in Drinking Water: Health, Consumption, and Sensory Perception. Int. J. Environ. Res. Public Health 2022, 19, 16829. https://doi.org/10.3390/ijerph192416829
Mirlohi S. Characterization of Metallic Off-Flavors in Drinking Water: Health, Consumption, and Sensory Perception. International Journal of Environmental Research and Public Health. 2022; 19(24):16829. https://doi.org/10.3390/ijerph192416829
Chicago/Turabian StyleMirlohi, Susan. 2022. "Characterization of Metallic Off-Flavors in Drinking Water: Health, Consumption, and Sensory Perception" International Journal of Environmental Research and Public Health 19, no. 24: 16829. https://doi.org/10.3390/ijerph192416829
APA StyleMirlohi, S. (2022). Characterization of Metallic Off-Flavors in Drinking Water: Health, Consumption, and Sensory Perception. International Journal of Environmental Research and Public Health, 19(24), 16829. https://doi.org/10.3390/ijerph192416829







