Microbial Contamination of Bedding Material: One Health in Poultry Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Registration
2.2. Search Strategy and Inclusion and Exclusion Criteria
2.3. Studies’ Selection and Data Extraction
2.4. Quality Assessment
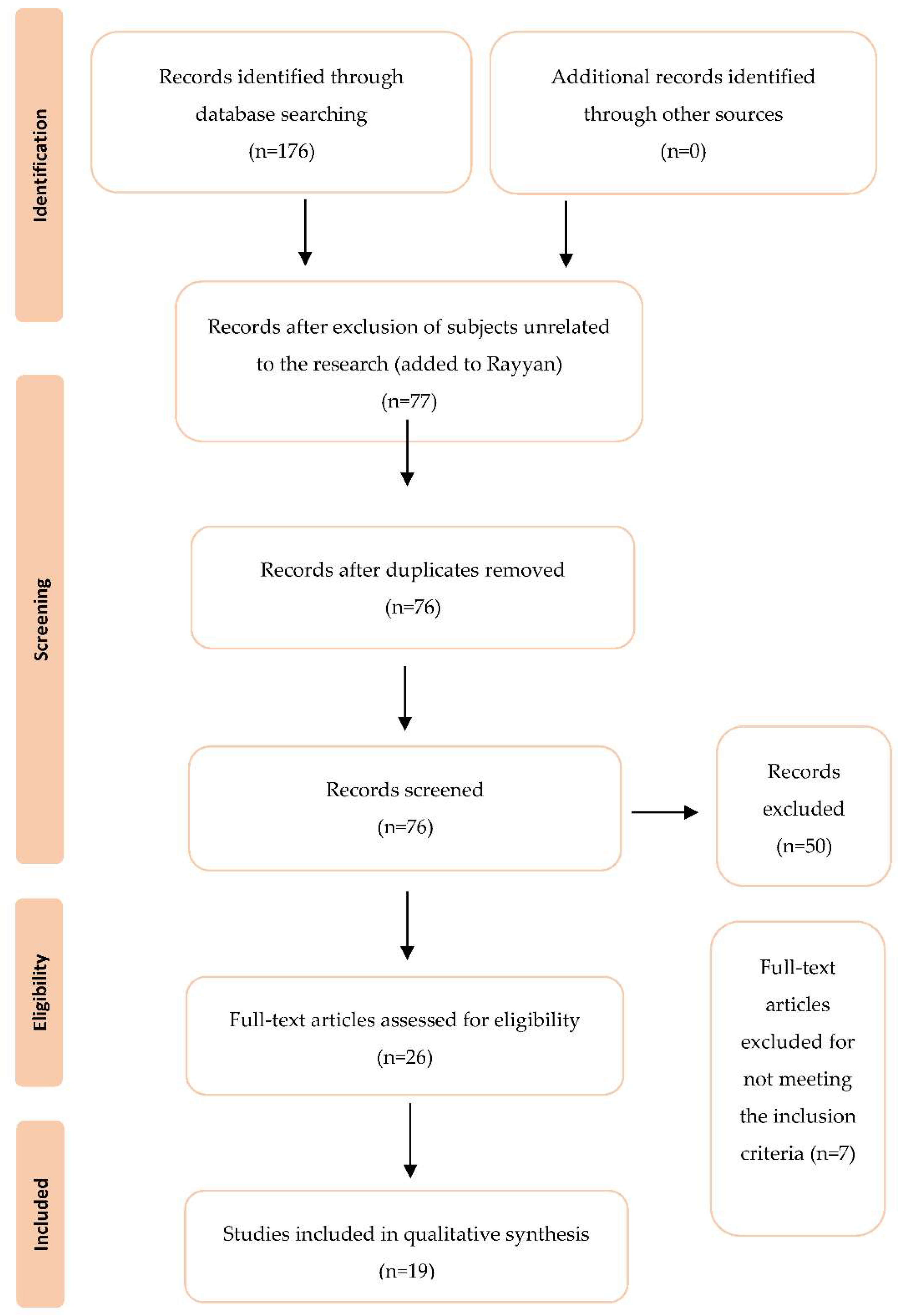
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rushton, J.; Bruce, M. Using a One Health approach to assess the impact of parasitic disease in livestock: How does it add value? Parasitology 2017, 144, 15–25. [Google Scholar] [CrossRef] [Green Version]
- Fulleringer, S.; Seguin, D.; Warin, S.; Bezille, A.; Desterque, C.; Arné, P.; Chermette, R.; Bretagne, S.; Guillot, J. Evolution of the Environmental Contamination by Thermophilic Fungi in a Turkey Confinement House in France. Poult. Sci. 2006, 85, 1875–1880. [Google Scholar] [CrossRef]
- Kostadinova, G.; Petkov, G.; Denev, S.; Stefanova, R.; Penev, T. Microbial pollution of manure, litter, air and soil in a poultry farm. Bulg. J. Agric. Sci. 2014, 20, 56–65. [Google Scholar]
- Ngogang, M.P.; Ernest, T.; Kariuki, J.; Mouiche, M.M.M.; Ngogang, J.; Wade, A.; Van Der Sande, M.A.B. Microbial Contamination of Chicken Litter Manure and Antimicrobial Resistance Threat in an Urban Area Setting in Cameroon. Antibiotics 2020, 10, 20. [Google Scholar] [CrossRef]
- Pujiastuti, E.S.; Tarigan, J.R.; Sianturi, E.; Ginting, B.B. The effect of chicken manure and beneficial microorganisms of EM-4 on growth and yield of kale (Brassica oleraceae acephala) grown on Andisol. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2018; Volume 205, p. 012020. [Google Scholar] [CrossRef]
- Khosravinia, H.; Mohammad, H.G.; Darvishnia, M. Litter mycology and the impacts of litter type and preslaughter feed withdrawal on crop bacterial community in broiler chicken. Afr. J. Microbiol. Res. 2009, 3, 844–850. [Google Scholar]
- Williams, C. Poultry waste management in developing countries. Food and Agriculture Organization of the United Nations. Poult. Dev. Rev. 2008, 5, 3–9. [Google Scholar]
- Skóra, J.; Matusiak, K.; Wojewódzki, P.; Nowak, A.; Sulyok, M.; Ligocka, A.; Okrasa, M.; Hermann, J.; Gutarowska, B. Evaluation of Microbiological and Chemical Contaminants in Poultry Farms. Int. J. Environ. Res. Public Health 2016, 13, 192. [Google Scholar] [CrossRef]
- Mesquita, M.S.S.; Lim, K.; Monte, D.F.M.; Givisiez, P.E.N.; Alves, L.B.R.; Freitas, N.O.C.; Kariuki, S.; Júnior, A.B.; Oliveira, C.J.B.; Gebreyes, W.A. Antimicrobial resistance in the globalized food chain: A One Health perspective applied to the poultry industry. Braz. J. Microbiol. 2022, 53, 465–486. [Google Scholar]
- Viegas, C.; Carolino, E.; Malta-Vacas, J.; Sabino, R.; Viegas, S.; Veríssimo, C. Fungal Contamination of Poultry Litter: A Public Health Problem. J. Toxicol. Environ. Heal. Part A 2012, 75, 1341–1350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutierrez, A.; Jaysankar, D.; Schneider, K.R. Prevalence, Concentration, and Antimicrobial Resistance Profiles of Salmonella Isolated from Florida Poultry Litter. J. Food Prot. 2020, 83, 2179–2186. [Google Scholar] [CrossRef] [PubMed]
- Munir, M.; Belloncle, C.; Irle, M.; Federighi, M. Wood-based litter in poultry production: A review. World Poult. Sci. J. 2019, 75, 5–16. [Google Scholar] [CrossRef]
- Roth, N.; Käsbohrer, A.; Mayrhofer, S.; Zitz, U.; Hofacre, C.; Domig, K.J. The application of antibiotics in broiler production and the resulting antibiotic resistance in Escherichia coli: A global overview. Poult. Sci. 2019, 98, 1791–1804. [Google Scholar] [CrossRef]
- Dahshan, H.; Abd-Elall, A.M.M.; Megahed, A.M.; Abd-El-Kader, M.A.; Nabawy, E.E. Veterinary antibiotic resistance, residues, and ecological risks in environmental samples obtained from poultry farms, Egypt. Environ. Monit. Assess. 2015, 187, 2. [Google Scholar] [CrossRef]
- Panyako, P.M.; Lichoti, J.K.; Ommeh, S.C. Review Article: Antimicrobial drug resistance in poultry pathogens: Challenges and opportunities. J. Agric. Sci. Technol. 2022, 21, 62–82. [Google Scholar] [CrossRef]
- Whyte, R. Occupational exposure of poultry stockmen in current barn systems for egg production in the United Kingdom. Br. Poult. Sci. 2010, 43, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Milner, P. Bioaerosols associated with animal production operations. Bioresour. Technol. 2009, 100, 5379–5385. [Google Scholar] [CrossRef]
- Tsapko, V.; Chudnovets, A.; Sterenbogen, M.; Papach, V.; Dutkiewicz, J.; Skórska, C.; Krysi´nska-Traczyk, E.; Golec, M. Exposure to bioaerosols in the selected agricultural facilities of the Ukraine and Poland—A review. Ann. Agric. Environ. Med. 2011, 18, 19–27. [Google Scholar] [PubMed]
- Anbu, P.; Hilda, A.; Gopinath, S.C.B. Keratinophilic fungi of poultry farm and feather dumping soil in Tamil Nadu, India. Mycopathologia 2004, 158, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Douphrate, D. Animal Agriculture and the One Health Approach. J. Agromedicine 2021, 26, 85–87. [Google Scholar] [CrossRef]
- Dahshan, H.; Merwad, A.M.A.; Mohamed, T.S. Listeria Species in Broiler Poultry Farms: Potential Public Health Hazards. J. Microbiol. Biotechnol. 2016, 26, 1551–1556. [Google Scholar] [CrossRef]
- Al-Zenki, S.; Al-Nasser, A.; Al-Safar, A.; Alomirah, H.; Al-Haddad, A.; Hendriksen, R.S.; Aarestrup, F.M. Prevalence and Antibiotic Resistance of Salmonella Isolated from a Poultry Farm and Processing Plant Environment in the State of Kuwait. Foodborne Pathog. Dis. 2007, 4, 367–373. [Google Scholar] [CrossRef]
- Elsayed, M.; El-Gohary, F.; Zakaria, A.; Gwida, M. Tracing of salmonella contaminations throughout an integrated broiler production chain in Dakahlia Governorate, Egypt. Pak. Vet. J. 2019, 39, 558–562. [Google Scholar] [CrossRef]
- Viegas, C.; Aranha Caetano, L.; Viegas, S. Occupational exposure to Aspergillus section Fumigati: Tackling the knowledge gap in Portugal. Environ. Res. 2021, 194, 110674. [Google Scholar] [CrossRef]
- Viegas, C.; Cervantes, R.; Dias, M.; Gomes, B.; Pena, P.; Carolino, E.; Twarużek, M.; Kosicki, R.; Soszczyńska, E.; Viegas, S.; et al. Unveiling the Occupational Exposure to Microbial Contamination in Conservation–Restoration Settings. Microorganisms 2022, 10, 1595. [Google Scholar] [CrossRef]
- Viegas, C.; Cervantes, R.; Dias, M.; Gomes, B.; Pena, P.; Carolino, E.; Twarużek, M.; Kosicki, R.; Soszczyńska, E.; Viegas, S.; et al. Six Feet under Microbiota: Microbiologic Contamination and Toxicity Profile in Three Urban Cemeteries from Lisbon, Portugal. Toxins 2022, 14, 348. [Google Scholar] [CrossRef]
- Burks, C.; Darby, A.; Londoño, L.G.; Momany, M.; Brewer, M.T. Azole-resistant Aspergillus fumigatus in the environment: Identifying key reservoirs and hotspots of antifungal resistance. PLOS Pathog. 2021, 17, e1009711. [Google Scholar] [CrossRef] [PubMed]
- Bart, F.; Sarah, A.; Steve, H.; Andy, M.; John, L. The Multi-Fungicide Resistance Status of Aspergillus fumigatus Populations in Arable Soils and the Wider European Environment. Front. Microbiol. 2020, 11, 599233. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nayak, R.; Stewart-King, T. Molecular Epidemiological Analysis and Microbial Source Tracking of Salmonella enterica Serovars in a Preharvest Turkey Production Environment. Foodborne Pathog. Dis. 2008, 5, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, L.; Givens, C.; Griffin, D.; Iwanowicz, L.; Meyer, M.; Kolpin, D. Poultry litter as potential source of pathogens and other contaminants in groundwater and surface water proximal to large-scale confined poultry feeding operations. Sci. Total. Environ. 2020, 735, 139459. [Google Scholar] [CrossRef]
- Schroeder, M.W.; Eifert, J.D.; Ponder, M.A.; Schmale, D. Association of Campylobacter spp. levels between chicken grow-out environmental samples and processed carcasses. Poult. Sci. 2014, 93, 734–741. [Google Scholar] [CrossRef] [PubMed]
- Brooks, J.; McLaughlin, M.; Scheffler, B.; Miles, D. Microbial and antibiotic resistant constituents associated with biological aerosols and poultry litter within a commercial poultry house. Sci. Total Environ. 2010, 408, 4770–4777. [Google Scholar] [CrossRef] [PubMed]
- Brooks, J.P.; McLaughlin, M.R.; Adeli, A.; Miles, D.M. Cultivation and qPCR Detection of Pathogenic and Antibiotic-Resistant Bacterial Establishment in Naive Broiler Houses. J. Environ. Qual. 2016, 45, 958–966. [Google Scholar] [CrossRef]
- Roberts, B.N.; Bailey, R.H.; McLaughlin, M.R.; Miles, D.M.; Brooks, J.P. Spatial and temporal analysis of microbial populations in production broiler house litter in the southeastern United States. J. Appl. Poult. Res. 2013, 22, 759–770. [Google Scholar] [CrossRef]
- Lydekaitiene, V.L.; Kudirkiene, E. Prevalence and genetic diversity of C. jejuni isolated from broilers and their environment using flaA-RFLP typing and MLST analysis. Ann. Anim. Sci. 2020, 20, 485–501. [Google Scholar] [CrossRef]
- Khireddine, G.; Amira, L.D.; Nedjoua, L.; Melisa, L.; Sameh, B.; Abdennour, A.; Rayane, M.; Ahmed, Z.; Daoud, C.; Messaoud, T.; et al. Risk factors related to bacterial contamination by Enterobacteriaceae and fecal coliforms and the prevalence of Salmonella spp. in Algerian farms, slaughterhouses and butcheries: A two-year follow-up study. AIMS Agric. Food 2021, 6, 768–785. [Google Scholar]
- Al-Naamani, L.; Dobretsov, S.; Dutta, J.; Burgess, J.G. Chitosan-ZnO nanocomposite coatings for the prevention of marine biofouling. Chemosphere 2017, 168, 408–417. [Google Scholar] [CrossRef]
- Kim, T.-S.; Kim, G.-S.; Son, J.-S.; Lai, V.D.; Mo, I.-P.; Jang, H. Prevalence, biosecurity factor, and antimicrobial susceptibility analysis of Salmonella species isolated from commercial duck farms in Korea. Poult. Sci. 2021, 100, 100893. [Google Scholar] [CrossRef]
- Fries, R.; Akcan, M.; Bandick, N.; Kobe, A. Microflora of two different types of poultry litter. Br. Poult. Sci. 2005, 46, 668–672. [Google Scholar] [CrossRef]
- Dyar, P.M.; Fletcher, O.J.R.K. Turkeys Associated with Use of Contaminated Litter. Avian Dis. Am. Assoc. Avian Pathol. 1984, 28, 250–255. [Google Scholar]
- Nayak, R.; Kenney, P.B.; Keswani, J.; Ritz, C. Isolation and characterisation of Salmonella in a turkey production facility. Br. Poult. Sci. 2003, 44, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Oppliger, A.; Charrière, N.; Droz, P.-O.; Rinsoz, T. Exposure to Bioaerosols in Poultry Houses at Different Stages of Fattening; Use of Real-time PCR for Airborne Bacterial Quantification. Ann. Occup. Hyg. 2008, 52, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Nevalainen, A.; Pastuszka, J.; Liebhaber, F.; Willeke, K. Performance of bioaerosol samplers: Collection characteristics and sampler design considerations. Atmospheric Environ. Part A. Gen. Top. 1992, 26, 531–540. [Google Scholar] [CrossRef]
- Sastry, A.S.; Bhat, S. Essentials of Medical Microbiology. Health Sci. 2016, 37, 43–52. [Google Scholar] [CrossRef]
- Viegas, C.; Almeida, B.; Monteiro, A.; Caetano, L.A.; Carolino, E.; Gomes, A.Q.; Twarużek, M.; Kosicki, R.; Marchand, G.; Viegas, S. Bioburden in health care centers: Is the compliance with Portuguese legislation enough to prevent and control infection? Build. Environ. 2019, 160, 106226. [Google Scholar] [CrossRef]
- Jürgensen, C.W.; Madsen, A.M. Influence of everyday activities and presence of people in common indoor environments on exposure to airborne fungi. AIMS Environ. Sci. 2016, 3, 77–95. [Google Scholar] [CrossRef]
- Dias, M.; Gomes, B.; Cervantes, R.; Pena, P.; Viegas, S.; Viegas, C. Microbial Occupational Exposure Assessments in Sawmills—A Review. Atmosphere 2022, 13, 266. [Google Scholar] [CrossRef]
- Ribeiro, E.; Faria, I. Analyses Approaches for Bacteria. In Exposure to Microbiological Agents in Indoor and Occupational Environments; Viegas, C., Viegas, S., Gomes, A.Q., Täubel, M., Sabino, R., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 97–108. [Google Scholar]
- Viegas, C.; Faria, T.; de Oliveira, A.C.; Caetano, L.A.; Carolino, E.; Quintal-Gomes, A.; Twarużek, M.; Kosicki, R.; Soszczyńska, E.; Viegas, S. A new approach to assess occupational exposure to airborne fungal contamination and mycotoxins of forklift drivers in waste sorting facilities. Mycotoxin Res. 2017, 33, 285–295. [Google Scholar] [CrossRef]
- Cox, J.; Mbareche, H.; Lindsley, W.G.; Duchaine, C. Field sampling of indoor bioaerosols. Aerosol Sci. Technol. 2020, 54, 572–584. [Google Scholar] [CrossRef]
- Viegas, C.; Gomes, B.; Pimenta, R.; Dias, M.; Cervantes, R.; Caetano, L.A.; Carolino, E.; Twarużek, M.; Soszczyńska, E.; Kosicki, R.; et al. Microbial contamination in firefighter Headquarters’: A neglected occupational exposure scenario. Build. Environ. 2022, 213, 108862. [Google Scholar] [CrossRef]
- Cervantes, R.; Dias, M.; Gomes, B.; Carolino, E.; Viegas, C. Development of an Indexed Score to Identify the Most Suitable Sampling Method to Assess Occupational Exposure to Fungi. Atmosphere 2022, 13, 1123. [Google Scholar] [CrossRef]
- Madsen, A.M.; Frederiksen, M.W.; Jacobsen, M.H.; Tendal, K. Towards a risk evaluation of workers’ exposure to handborne and airborne microbial species as exemplified with waste collection workers. Environ. Res. 2020, 183, 109177. [Google Scholar] [CrossRef] [PubMed]
- Reponen, T. Sampling for Microbial Determinations. In Exposure to Microbiological Agents in Indoor and Occupational Environments; Viegas, C., Viegas, S., Gomes, A.Q., Täubel, M., Sabino, R., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 85–96. [Google Scholar]
- Pitkäranta, M.; Meklin, T.; Hyvärinen, A.; Paulin, L.; Auvinen, P.; Nevalainen, A.; Rintala, H. Analysis of Fungal Flora in Indoor Dust by Ribosomal DNA Sequence Analysis, Quantitative PCR, and Culture. Appl. Environ. Microbiol. 2008, 74, 233–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franchitti, E.; Pascale, E.; Fea, E.; Anedda, E.; Traversi, D. Methods for bioaerosol characterization: Limits and perspectives for human health risk assessment in organicwaste treatment. Atmosphere 2020, 11, 452. [Google Scholar] [CrossRef]
- Douwes, J.; Thorne, P.; Pearce, N.; Heederik, D. Bioaerosol Health Effects and Exposure Assessment: Progress and Prospects. Ann. Occup. Hyg. 2003, 47, 187–200. [Google Scholar] [CrossRef] [Green Version]
- Viegas, C.; Gomes, B.; Dias, M.; Carolino, E.; Aranha Caetano, L. Aspergillus Section Fumigati in Firefighter Headquarters. Microorganisms 2021, 9, 2112. [Google Scholar] [CrossRef]
- Sabino, R.; Faísca, V.; Carolino, E.; Veríssimo, C.; Viegas, C. Occupational Exposure to Aspergillus by Swine and Poultry Farm Workers in Portugal. J. Toxicol. Environ. Heal. Part A 2012, 75, 1381–1391. [Google Scholar] [CrossRef] [Green Version]
- Viegas, S.; Veiga, L.; Almeida, A.; dos Santos, M.; Carolino, E.; Viegas, C. Occupational Exposure to Aflatoxin B1 in a Portuguese Poultry Slaughterhouse. Ann. Occup. Hyg. 2015, 60, 176–183. [Google Scholar] [CrossRef] [Green Version]
- Schoustra, S.E.; Debets, A.J.M.; Rijs, A.J.M.M.; Zhang, J.; Snelders, E.; Leendertse, P.C.; Melchers, W.J.G.; Rietveld, A.G.; Zwaan, B.J.; Verweij, P.E. Environmental Hotspots for Azole Resistance Selection of Aspergillus fumigatus, the Netherlands. Emerg. Infect. Dis. 2019, 25, 1347–1353. [Google Scholar] [CrossRef] [Green Version]
- Verweij, P.E.; Van De Sande-Bruisma, N.; Kema, G.H.J.; Melchers, W.J.G. Azole resistance in Aspergillus fumigatus in the Netherlands—Increase due to environmental fungicides? Ned. Tijdschr. Voor Geneeskd. 2012, 156, A4458. [Google Scholar]
- Viegas, C.; Almeida, B.; Aranha Caetano, L.; Afanou, A.; Straumfors, A.; Veríssimo, C.; Gonçalves, P.; Sabino, R. Algorithm to assess the presence of Aspergillusfumigatus resistant strains: The case of Norwegian sawmills. Int. J. Environ. Health Res. 2020, 32, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Jeanvoine, A.; Rocchi, S.; Reboux, G.; Crini, N.; Crini, G.; Millon, L. Azole-resistant Aspergillus fumigatus in sawmills of Eastern France. J. Appl. Microbiol. 2017, 123, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Määttä, J.; Lehto, M.; Leino, M.; Tillander, S.; Haapakoski, R.; Majuri, M.-L.; Wolff, H.; Rautio, S.; Welling, I.; Husgafvel-Pursiainen, K.; et al. Mechanisms of Particle-Induced Pulmonary Inflammation in a Mouse Model: Exposure to Wood Dust. Toxicol. Sci. 2006, 93, 96–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velasquez, C.G.; Macklinc, K.S.; Kumar, M.; Bailey, P.E.; Ebner, H.F.; Oliver, F.S.; Martin-Gonzalez, F.S.; Singh, M. Prevalence and antimicrobial resistance patterns of Salmonella isolated from poultry farms in southeastern United States. Poult. Sci. 2018, 97, 2144–2152. [Google Scholar] [CrossRef]
- Chai, S.J.; Cole, D.; Nisler, A.; Mahon, B.E. Poultry: The most common food in outbreaks with known pathogens, United States, 1998–2012. Epidemiol. Infect. 2016, 145, 316–325. [Google Scholar] [CrossRef] [Green Version]
- Nichita, I.; Tirziu, E. Investigations on airborne fungi in poultry houses. Lucrari Stiintifice—Universitatea de Stiinte Agricole a Banatului Timisoara. Med. Vet. 2008, 41, 932–935. [Google Scholar]
- Wan-Kuen, J.; Jung-Hwan, K. Exposure Levels of Airborne Bacteria and Fungi in Korean Swine and Poultry Sheds. Arch. Environ. Occup. Health 2005, 60, 140–146. [Google Scholar]
- Viegas, C.; Veríssimo, C.; Rosado, L.; Santos, C.S. Poultry fungal contamination as a public health problem. Ecol. Environ. 2010, 132, 45–53. [Google Scholar] [CrossRef] [Green Version]
- Viegas, C.; Viegas, S.; Monteiro, A.; Carolino, E.; Sabino, R.; Veríssimo, C. Comparison of indoor and outdoor fungi and particles in poultry units. Environ. Impact Assess. Rev. 2012, 162, 589–596. [Google Scholar]
- Teixeira, A.S.; Goiano, I.F.; De Oliveira, M.C.; Menezes, J.F.S.; Gouvea, B.M.; Teixeira, S.R.; Gomes, A.R. Poultry litter of wood shavings and/or sugarcane bagasse: Animal performance and bed quality. Rev. Colomb. Cienc. Pecu. 2015, 28, 238–246. [Google Scholar] [CrossRef]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Articles published in the English language | Articles published in other languages |
| Articles published from 1 January 2000 to 1 January 2022 | Articles published prior to 2000 |
| Articles published in any country | |
| Articles related to microbial contamination of litter in poultry production | Articles related exclusively to litter abiotic conditions, without mentioning the microbial contamination |
| Original scientific articles on the topic | Abstracts of congress, reports, reviews/state-of-the-art articles |
| Database | Title | Country | Occupational Environments | Sampling Methods | Other Analysed Matrixes | Analytical Methods | Main Findings | References |
|---|---|---|---|---|---|---|---|---|
| PUBMED | The prevalence of Campylobacter species in broiler flocks and their environment: assessing the efficiency of chitosan/zinc oxide nanocomposite for adopting control strategy | Egypt | Poultry farms (n = 4) | Bedding material samples (n = 15) | Cloacal swabs (n = 100) Water (n = 15) Attendants’ hand swabs (n = 30) | Culture-based methods; morphologic identification (bacteria); biochemical tests; molecular tools (PCR). | The prevalence rate of Campylobacter coli in broiler poultry farms was (27.3%; 60/220). C. coli was detected in the highest percentage in the manure storage area and bedding material samples (66.7%; 10/15 and 53.3%; 8/15, respectively) followed by feeders, attendants’ hands, and drinkers (40.0%; 6/15, 33.3%; 5/15, 16.7%; 5/30, and 13.3%; 2/15, respectively). | [38] |
| Prevalence, Concentration, and Antimicrobial Resistance Profiles of Salmonella Isolated fromFlorida Poultry Litter | USA | Poultry farms (n = 18) | Bedding material samples (n = 54) Days = 45 days Material = pine shavings | nr | Culture-based methods; morphologic identification (bacteria—Salmonella sp.); molecular tools/PCR; serotyping antibiotic resistance; physical and chemical parameters. | Salmonella was recovered from all farms (n = 18) with a sample prevalence of 61.1% (33/54). The prevalence and concentration of Salmonella recovered from animals’ bedding did not significantly differ between seasons. Overall, no correlation was found between the concentration of Salmonella and any of the chemical, physical, or microbial properties measured. Salmonella isolates (n = 47) tested for antimicrobial susceptibility were observed to be resistant to tetracycline (29.8%), sulfisoxazole (23.4%), and streptomycin (14.9%). | [11] | |
| Listeria Species in Broiler Poultry Farms: Potential Public Health Hazards | Cameroon | Poultry farms (n = 10) | Bedding material samples (n = 8) | Chicken meat carcass (n = 8) Feed (n = 2) Water (n = 2) | Culture-based methods; morphologic identification (bacteria—Listeria sp.); biochemical tests; antibiotic resistance. | Listeria sp. was found in 95 of 200 tested samples (47.5%), of which 42 were from animals’ bedding, 37 from raw meat, 14 from feed, and 2 from water L. innocua was the predominant Listeria sp. L. ivanovii was also isolated from broiler poultry farm samples and was the second most predominant (12.5%). The presence of L. ivanovii might be due to the nature of animals’ bedding used that is mainly from pasture-raised animals (such as wood shavings, hay, or chopped rice straw). There was a Listeria sp. prevalence in animals’ bedding of 52.5% (42/80). The results indicated high levels of resistance to amoxicillin/clavulanate (40%), followed by norfloxacin (38%), amoxicillin/flucloxacillin (35%), ofloxacin (32%), and ciprofloxacin (25%). | [21] | |
| Fungal Contamination of Poultry Litter: A Public Health Problem | Portugal | Poultry farms (n = 7) | Bedding material samples (n = 21) Weight = 10 g Days = fresh (7) and aged (14) Material = pine shavings; straw; wood shavings; rice bulls | Air samples (n = 27 impaction) | Culture-based methods; morphologic identification (fungi). | Twelve different fungal species were detected in fresh animal-bedding material. Penicillium sp. was the most frequent genus found (59.9%), followed by Alternaria sp. (17.8%), Cladosporium sp. (7.1%), and Aspergillus sp. (5.7%), while on the used bedding material, 19 different fungal species were detected. Penicillium sp. was the most frequently isolated (42.3%), followed by Scopulariopsis sp. (38.3%), Trichosporon sp. (8.8%), and Aspergillus sp. (5.5%). In the new bedding material, Aspergillus fumigatus was the most frequent species identified (32.6%) from Aspergillus genus, and A. flavus was also isolated in 9.9% of the samples. In the aged bedding material, Aspergillus nidulantes was the most frequent (73.4%) among the Aspergillus genus, but A. fumigatus, A. flavus, and A. niveus were also identified. | [10] | |
| Molecular Epidemiological Analysis and Microbial Source Tracking of Salmonella enterica Serovarsin a Preharvest Turkey Production Environment | USA | Big poultry farm (n = 1) | Bedding material samples (n = 36) Days = 2/10 and 18 weeks Material = fresh pine shavings Collection = 5 cm | Feed = 6 Drinker swabs (n = 36) Turkey caeca swab (n = 72) Air (n = 26, impaction) Environmental swabs (n = 42, walls, ventilation fans, feathers, employee shoes, feed storage, and door handles) | Culture-based methods (bacteria—Salmonella sp.); morphologic identification; biochemical tests. | From the 991 samples, 6% were positive for Salmonella. 42/145 of these were positive from animals’ bedding samples, 4/145 from feed, 24/145 from drinkers, 3/145 from leftover feed, and 12/145 from environmental swabs. The frequency of Salmonella detected in flocks 1, 2, and 4 was 83%, 11%, and 6%, respectively. Salmonella heidelberg was the most prevalent Salmonella serovar isolated. Overall, 79% of Salmonella strains were resistant to one or more antimicrobials. | [30] | |
| Prevalence and Antibiotic Resistance of Salmonella Isolated from a Poultry Farm and Processing Plant Environment in the State of Kuwait | Kuwait | Big poultry farm (n = 1) | Bedding material samples (n = 550) Weight = 10 g | Feed (n = 550/10 g) Water (n = 546/10 mL) Air (n = 72, impaction) Drinker swabs (n = 5) Paper tray liners (n = 24) | Culture-based methods (bacteria—Salmonella sp.); morphologic identification; serotyping. | Out of 2882 samples collected, 156 samples (5.4%) were positive for Salmonella sp. Contamination was 1.5% (8/550) from animals’ bedding, 0.7% (4/550) from feed, 0% (0/30) in water, 0.2% (1/546) in drinkers’ swabs, and 0% in (0/24) in paper trays. Salmonella was not detected in any of the paper liner, air, or water samples. | [22] | |
| Evolution of the Environmental Contamination by Thermophilic Fungi in a Turkey Confinement House in France | France | Small poultry farm (n = 1) | Bedding material samples (n = 124)Weight = 1 g Days = over 16 weeks Material = Fresh straw | Air (n = 112, Impaction) Feed (n = 48/1 g) | Culture-based methods (fungi); morphologic identification; molecular tools (A. fumigatus). | The three species that were most frequently identified were: Absidia corymbifera (114 samples; 40.1%), Aspergillus fumigatus (114 samples; 40.1%), and A. flavus (67 samples; 23.6%). Scopulariopsis sp. and Penicillium sp. were also regularly encountered, in addition to yeasts of the genus Candida. The opportunistic species C. albicans was detected from 195 environmental samples (68.7%). Samples obtained during the 16-week study period yielded A. fumigatus at 0.3 CFU/g (from 0.0 to 1.5) in animals’ bedding. After new bedding material was added at week 10, there was no isolation of fungi for 2 weeks. However, during week 14, the number of A. fumigatus colonies increased (1.5 CFU/g). | [2] | |
| SCOPUS | Prevalence, biosecurity factor, and antimicrobial susceptibility analysis of Salmonella species isolated from commercial duck farms in Korea | Korea | Big duck farms (n = 31) | Bedding material samples (n = 465) Weight = 10 g | Wall swab (n = 186) Nipple swab (n = 186) Feed pan swab (n = 279) Dust sample (n = 31; 10 gr) | Culture-based methods (bacteria—E. coli); morphologic identification; molecular tools (PCR); serotyping; susceptibility test. | Salmonella-positivity rate increased up to 35.9% after the introduction of ducklings. From 4 week the detection rate decreased by 11.4%. Similarly, the actual number of Salmonella-positive samples was highest when the ducklings were 1–3 weeks of age, followed by when they were 4–6 weeks of age. The contamination rate was 7.5% for animals’ bedding, 3.2% for wall swabs, 3.2% for feed pan swabs, and 1.6% for dust samples. All isolates were resistant to erythromycin (194 isolates; 100%) and 122 isolates (62.9%) were resistant to nalidixic acid, followed by ampicillin (85 isolates; 43.8%), trimethoprim/sulfamethoxazole (77 isolates; 39.7%), tetracycline (74 isolates; 38.1%), cefazolin (39 isolates; 36.6%), streptomycin (39 isolates; 20.1%), and ciprofloxacin (23 isolates; 11.9%). | [39] |
| Microbial Contamination of Chicken Litter Manure and Antimicrobial Resistance Threat in an Urban Area Setting in Cameroon | Cameroon | Big poultry farm (n = 26) | Bedding material samples (n = 71) Days = 26 new (in store bags) + 45 aged Material = wood shavings | nr | Culture-based methods (bacteria); morphologic identification; biochemical tests; antibiotic resistance. | E. coli sp. and Salmonella sp. were isolated in 80.8% and 36.8% of farms, respectively. 59.2% of animals’ bedding samples tested positive for E. coli, and 15.5% of wood shaving samples were positive for Salmonella sp. 28% of E. coli isolates were resistant to five antibiotics or more. For Salmonella sp., 36% were multidrug-resistant while 27% of isolates were found to be sensitive to all antibiotics tested. | [4] | |
| Poultry litter as potential source of pathogens and other contaminants in groundwater and surface water proximal to large-scale confined poultry feeding operations | USA | Poultry farm (n = 9) | Bedding material samples (n = 4) | nr | Culture-based methods (bacteria); morphologic identification; biochemical tests; molecular tools; mycotoxins. | Trace organic contaminants were most frequently detected in animals’ bedding. Mycotoxin compound zearalenone was detected in all animals’ bedding samples. Animals’ bedding had the largest number of microbial detections and all samples (100%) were positive for presumptive Campylobacter sp., Enterococci, Staphylococci, and Lactobacilli growth. | [31] | |
| Prevalence and genetic diversity of C. jejuni isolated from broilers and their environment using flaA-RFLP typing and MLST analysis | Lithuania | Poultry farms (n = 4) | Bedding material samples (n = 310) Days = 1/week over 2 years | Cloacae swabs (n = 402) Drinker swab (n = 50) | Culture-based methods (bacteria—Campylobacter); morphologic identification; molecular tools (PCR). | Campylobacter sp. was detected in 12 out of 13 broiler flocks (92.3%). From 1479 samples, 315 (21.3%) samples were positive for Campylobacter sp. C. jejuni was identified in 269 (85.4%) samples and C. coli in 26 (8.3%) samples. The highest positive samples of Campylobacter sp. were found in broiler cloacae, puddle water, and in animals’ bedding of additional houses. | [36] | |
| Tracing of Salmonella Contaminations Throughout an Integrated Broiler Production Chain in Dakahlia Governorate, Egypt | Egypt | Poultry farms (n = 3) | Bedding material samples (n = 15) | Cloacae swabs (n = 145) Feed (n = 15) Water (n = 15) Workers’ hand swabs (n = 15) Slaughterhouses’ environmental samples (n = 15) Samples from chicken carcasses (n = 15) | Culture-based methods (bacteria—Salmonella); morphologic identification; biochemical tests; molecular tools; serotyping. | The overall frequency of Salmonella contamination in the live broiler flocks was 40.9% (90/220) with a prevalence of 60% (9/15) from animals’ bedding samples, 37.9% (55/145) from cloaca swabs, 40% (6/15) in feed, 53.3% (8/15%), 20% (13/15) on workers’ hands, 60% from slaughterhouses (6/10), and 25.6% (120/30) from chicken carcasses. The isolated serovars from broiler farms were distributed as follows: S. enteritidis 38.8% (35/90), S. kentucky 23.3% (21/90), S. typhimurium 11.1% (10/90), S. molade 7.8% (7/90), S.takoradi 6.7% (6/90), S. bargny 2.2% (2/90) and 3.3% (3/90) for each of S. papuana, S. tamale, and S. infantis. | [23] | |
| MICROBIAL POLLUTION OF MANURE, LITTER, AIR AND SOIL IN A POULTRY FARM | Bulgaria | Big poultry farm (n = 1) | Bedding material samples (n = 8) Weight = 200 g Days = 40 days (first and last week) | Air samples (n = 4, sedimentation method Matusevich) | Culture-based methods (bacteria); morphologic identification (E. coli). | The number of cultivable microorganisms in animals’ bedding (logCFU/kg−3) varied between 6.08 and 6.92, 3.92, and 5.28 in air log CFU/m−3. Fresh bedding material is a source of inside and outside air and soil pollution with saprophytic microorganisms including coliform bacteria, subject to sanitary control. | [3] | |
| Association of Campylobacter spp. levels between chicken grow-outenvironmental samples and processed carcasses | USA | Small poultry farm (n = 4) | Bedding material samples | Air samples (n = 10, filtration), shoe coverings (n = 10), feed and drinker swabs (n = 10) | Culture-based methods (bacteria—Campylobacter sp.); morphologic identification; biochemical tests. | Campylobacter sp. was discovered in 27% (32/120) of all house samples (air, fecal/bedding material, and sponge). | [32] | |
| Microbial and antibiotic resistant constituents associated with biological aerosols and poultry litter within a commercial poultry house | USA | Poultry farms (n = 8) | Bedding material samples (n = 17) Weight = 10 g Collection of surface litter (0–7.5 cm) | Air (n = 89, impinger) | Culture-based methods (bacteria); morphologic identification; biochemical tests; molecular tools (PCR); antibiotic resistance. | Bacteria contamination was found in animals’ bedding samples with Staphylococci most likely accounting for approximately 90% of all culturable bacteria. House aerosol levels significantly increased from outside to inside the house, from approximately 6.7 × 103 to 4.0 × 106 CFU/m−3 for aerosolized heterotrophic plate count bacteria. Approximately 80% of animals’ bedding isolates were resistant to at least one antibiotic class regardless of broiler presence. However, poultry aerosol isolates’ antibiotic resistance was directly influenced by the presence of the flock. Approximately 66% (244/367) of aerosol isolates were resistant to at least one antibiotic class. | [33] | |
| Web of Science | Risk factors related to bacterial contamination by Enterobacteriaceae and fecal coliforms and the prevalence of Salmonella spp. in Algerian farms, slaughterhouses and butcheries: a two-year follow-up study | Algeria | Poultry farms (n = 10) | Bedding material samples (n = 10) Weight = 5 g Days = aged | Floor and wall swabs (n = 20), feed (n = 10), water (n = 10). | Culture-based methods (bacteria); morphologic identification. | The highest presence of E. coli was observed at the poultry farms, mainly on the floors and feed (100%), bedding material (80%), floor/walls (50%), and water (20%). The contamination by E. coli was found in walls (100%), floors (60%), water (40%), liver and neck skin (6.66%) samples, respectively. Salmonella sp. were mainly isolated from neck skin (60%), liver (33.33%), walls, water, and floors (40%). The presence of E. coli in chicken meat was 46.66%. In addition, 28% of the chicken meat samples were contaminated with Salmonella sp. E. coli was isolated from the majority of poultry farms (70%) and Salmonella sp. in 22% of the poultry farms. | [37] |
| Cultivation and qPCR Detection of Pathogenic and Antibiotic- Resistant Bacterial Establishment in Naive Broiler Houses | USA | Small poultry farm (n = 3) | Bedding material samples (n = 11) Weight = 100 g Days = 3 weeks Material = rice hull Collection = 10–15 cm | nr | Culture-based methods (bacteria) Morphologic identification; Molecular tools (PCR); Antibiotic resistance. | Soil levels for HPC, Staphylococci, and fecal indicators (E. coli, C. perfringens, and enterococci) were 4 × 106 CFU/g−1, 2 × 104 CFU/g−1, and below detection, respectively. 100% of bedding material samples were positive for Salmonella sp. and Listeria sp. The microbial levels in the preflock were lower than typical in-use animal-bedding levels. Salmonella sp., Campylobacter sp., and Listeria sp. were not detected in animals’ bedding from the preflock. Surprisingly, given the overall low moisture of fresh bedding material (~90% solid content), E. coli and Enterococci were detected. Most bacterial isolates were resistant to at least one antibiotic class, with most isolates resistant to more than one antibiotic class. | [34] | |
| Spatial and temporal analysis of microbial populations in production broiler house litter in the southeastern United States | USA | Poultry farms (n = 8) | Bedding material samples (n = 16) Weight = 100 g Days = fresh and aged (0.2.4 and 6 wk) Material = fresh pine shavings | nr | Culture-based methods (Bacteria) Morphologic identification Molecular tools (PCR) Antibiotic resistance | Staphylococcus sp., Enterococcus sp., and C. perfringens were isolated from all locations. Campylobacter sp. was not detected from any sample collected. Broiler age had a significant effect on nearly all studied microbes (p < 0.05). Salmonella enterica, L. monocytogenes, Enterococcus sp., Staphylococcus sp., and C. perfringens levels were all associated with broiler age. Of the 192 samples analyzed, 28 Salmonella-positive and 47 Listeria-positive samples were identified from three flocks. Staphylococcus sp. were present at greater levels than any other bacteria in animal bedding samples. Most Staphylococcus sp. isolates (29/48) were predominantly susceptible to all tested antibiotics. Salmonella enterica, Enterococcus sp., C. perfringens, and L. monocytogenes isolates possessed multiple antibiotic resistance profiles. | [35] | |
| Litter mycology and the impacts of litter type and preslaughter feed withdrawal on crop bacterial community in broiler chicken | Iran | Small poultry farm (n = 1) | Bedding material samples (n = 273) Weight = 100 gr Days = 56 days Material = wood shavings (WS), cow dung (CD), shredded paper (SP), barley stalks (BS), rice husks (RH) and a mixture of identical proportionfrom all materials (Mix) Collection = 5 cm | nr | Culture-based methods (fungi); Morphologic identification | There was a significant frequency of Mucor sp. (14/41 rice husk), Pencillium sp. (17/62 cow dung), Aspergillus sp. (20/64 mixture of all bedding materials) Geothrichum sp. (18/53 shredded paper), Monobelpharios sp. (5/12 barley stalks), Alternaria sp. (1/3 rice husk, cow dung and mix) Rhizopus sp. (15/37 barley stalks) and Fusarium sp. (1/1 barley stalks) in animals’ bedding material compared to other fungi species (p < 0.05). The frequency of occurrence for Aspergillus sp. was significantly greater in the mix when compared with other materials used (p < 0.01). | [6] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, B.; Pena, P.; Cervantes, R.; Dias, M.; Viegas, C. Microbial Contamination of Bedding Material: One Health in Poultry Production. Int. J. Environ. Res. Public Health 2022, 19, 16508. https://doi.org/10.3390/ijerph192416508
Gomes B, Pena P, Cervantes R, Dias M, Viegas C. Microbial Contamination of Bedding Material: One Health in Poultry Production. International Journal of Environmental Research and Public Health. 2022; 19(24):16508. https://doi.org/10.3390/ijerph192416508
Chicago/Turabian StyleGomes, Bianca, Pedro Pena, Renata Cervantes, Marta Dias, and Carla Viegas. 2022. "Microbial Contamination of Bedding Material: One Health in Poultry Production" International Journal of Environmental Research and Public Health 19, no. 24: 16508. https://doi.org/10.3390/ijerph192416508
APA StyleGomes, B., Pena, P., Cervantes, R., Dias, M., & Viegas, C. (2022). Microbial Contamination of Bedding Material: One Health in Poultry Production. International Journal of Environmental Research and Public Health, 19(24), 16508. https://doi.org/10.3390/ijerph192416508








