Feasibility and Acceptability of a Telephone-Based Smoking Cessation Intervention for Qatari Residents
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Measures
2.3. Statistical Analysis
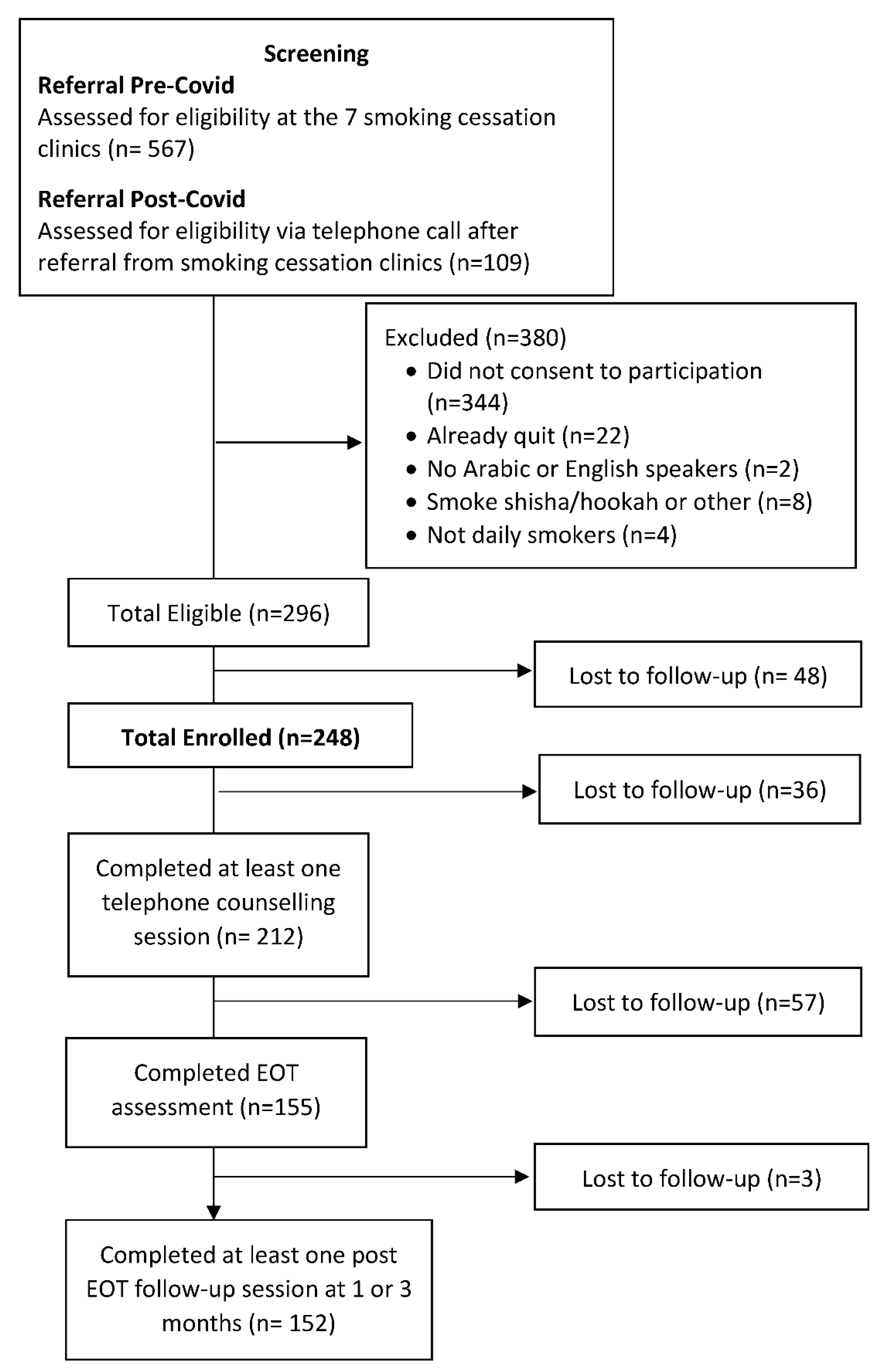
3. Results
3.1. Characteristics
3.2. Primary Outcomes
3.2.1. Feasibility
3.2.2. Acceptability
3.2.3. Quit Rates
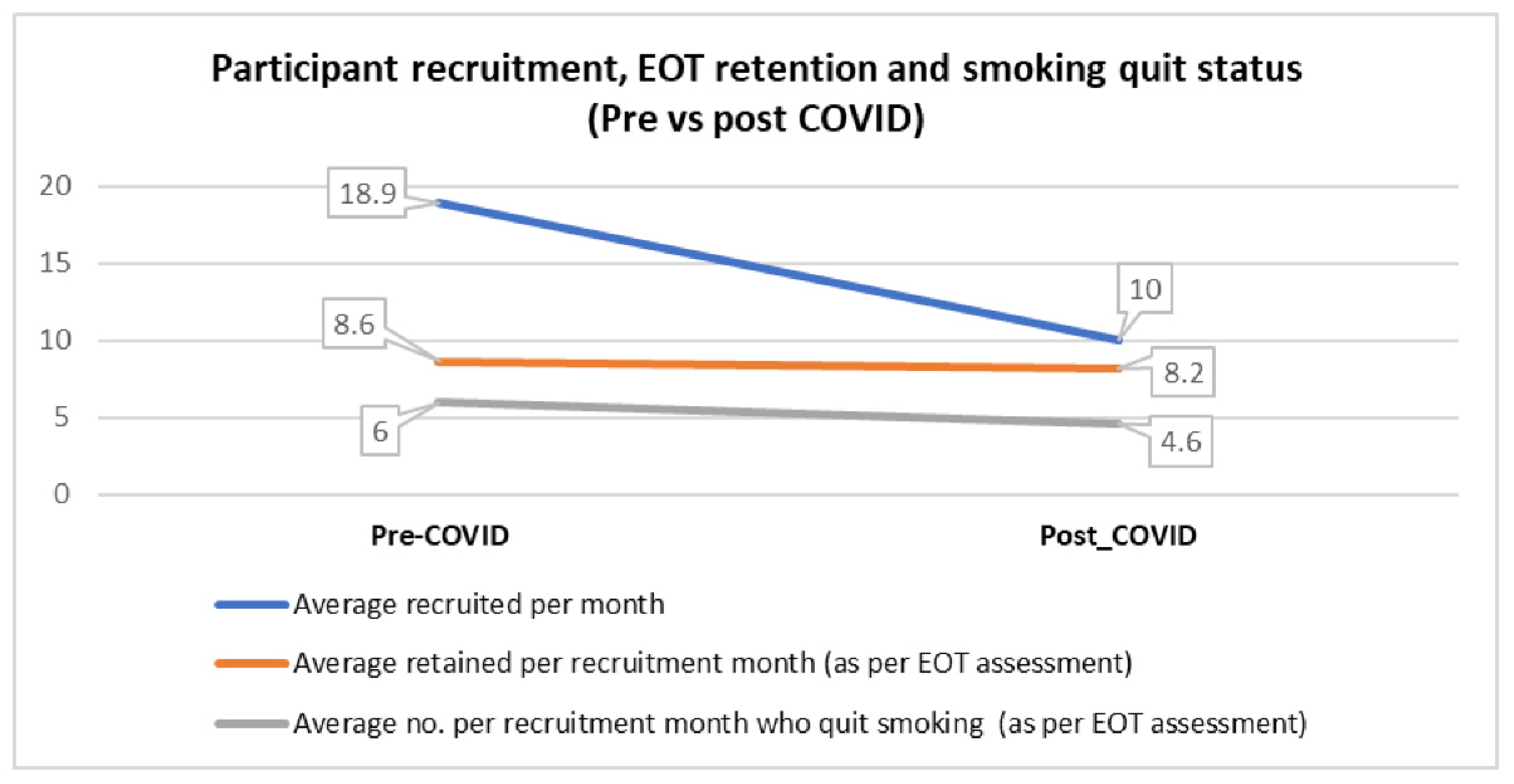
3.3. Pre- and Post-Pandemic Onset Comparison
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GT Collaborators. Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and attributable disease burden in 204 countries and territories, 1990–2019: A systematic analysis from the Global Burden of Disease Study 2019. Lancet 2021, 397, 2337–2360. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Report on the Global Tobacco Epidemic. 2017. Available online: https://apps.who.int/iris/bitstream/handle/10665/255874/9789241512824-eng.pdf?sequence=1&isAllowed=y (accessed on 11 September 2022).
- World Health Organization. WHO Report on the Global Tobacco Epidemic, 2011: Warning about the Dangers of Tobacco. 2011. Available online: https://apps.who.int/iris/handle/10665/44616 (accessed on 11 September 2022).
- Fouad, H.; Commar, A.; Hamadeh, R.R.; El-Awa, F.; Shen, Z.; Fraser, C.P. Smoking prevalence in the Eastern Mediterranean Region. East. Mediterr. Health J. 2020, 26, 94–101. [Google Scholar] [CrossRef] [PubMed]
- AlMulla, A.; Mamtani, R.; Cheema, S.; Maisonneuve, P.; Abdullah BaSuhai, J.; Mahmoud, G.; Kouyoumjian, S. Epidemiology of tobacco use in Qatar: Prevalence and its associated factors. PLoS ONE 2021, 16, e0250065. [Google Scholar] [CrossRef] [PubMed]
- Bener, A.; Zirie, M.A.; Kim, E.J.; Buz, R.; Zaza, M.; Al-Nufal, M.; Basha, B.; Hillhouse, E.W.; Riboli, E. Measuring burden of diseases in a rapidly developing economy: State of Qatar. Glob. J. Health Sci. 2013, 5, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, W.H.; Rasul, K.I.; Khinji, A.; Ahmed, M.S.; Bener, A. Clinical and epidemiological characteristics of lung cancer cases in Qatar. East. Mediterr. Health J. 2010, 16, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Monshi, S.S.; Ibrahim, J. Implementation of tobacco control measures in the Gulf Cooperation Council countries, 2008–2020. Subst. Abuse Treat. Prev. Policy 2021, 16, 57. [Google Scholar] [CrossRef] [PubMed]
- Fiore, M.C.; Baker, T.B. Ten Million Calls and Counting: Progress and Promise of Tobacco Quitlines in the U.S. Am. J. Prev. Med. 2021, 60, S103–S106. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, I.B.; Tripp, A.L.; Dean, A.K.; Mbulo, L.; Arrazola, R.A.; Twentyman, E.; King, B.A. Tobacco Smoking Cessation and Quitline Use Among Adults Aged ≥15 Years in 31 Countries: Findings from the Global Adult Tobacco Survey. Am. J. Prev. Med. 2021, 60, S128–S135. [Google Scholar] [CrossRef] [PubMed]
- Matkin, W.; Ordóñez-Mena, J.M.; Hartmann-Boyce, J. Telephone counselling for smoking cessation. Cochrane Database Syst. Rev. 2019, 2019, CD002850. [Google Scholar] [CrossRef] [PubMed]
- Al Thani, M.; Leventakou, V.; Sofroniou, A.; Eltayeb, S.M.; Sadoun, E.; Hakim, I.A.; Nair, U.; Thomson, C. A Telephone-Based Tobacco Cessation Program in the State of Qatar: Protocol of a Feasibility Study. Int. J. Environ. Res. Public Health 2021, 18, 4750. [Google Scholar] [CrossRef] [PubMed]
- Al Thani, M.; Leventakou, V.; Sofroniou, A.; Butt, H.I.; Hakim, I.A.; Thomson, C.; Nair, U.S. Factors associated with baseline smoking self-efficacy among male Qatari residents enrolled in a quit smoking study. PLoS ONE 2022, 17, e0263306. [Google Scholar] [CrossRef]
- Heatherton, T.F.; Kozlowski, L.T.; Frecker, R.C.; Fagerström, K.O. The Fagerström test for nicotine dependence: A revision of the Fagerström tolerance questionnaire. Br. J. Addict. 1991, 86, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Boccio, M.; Sanna, R.S.; Adams, S.R.; Goler, N.C.; Brown, S.D.; Neugebauer, R.S.; Schmittdiel, J.A.; Bellamy, D.J.; Wiley, D.M.; Ferrara, A. Telephone-based coaching: Comparison of tobacco cessation programs in an integrated health care system. Am. J. Health Promot. 2017, 31, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Yun, E.H.; Lim, M.K.; Oh, J.K.; Ki, I.H.; Shin, S.H.; Jeong, B.Y. Quitline activity in the republic of Korea. Asian Pac. J. Cancer Prev. 2016, 17, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Blebil, A.Q.; Sulaiman, S.A.; Hassali, M.A.; Dujaili, J.A.; Zin, A.M. Impact of additional counseling sessions through phone calls on smoking cessation outcomes among smokers in Penang State, Malaysia. BMC Public Health 2014, 14, 460. [Google Scholar] [CrossRef] [PubMed]
- Global Adult Tobacco Survey 2013. Available online: https://www.psa.gov.qa/en/statistics/Surveys/GATS-BOOK.pdf (accessed on 11 September 2022).
- World Health Organization. Global Report on Trends in Prevalence of Tobacco Use 2000–2025, Third Edition, Geneva. 2019. Available online: https://www.who.int/publications/i/item/who-global-report-on-trends-in-prevalence-of-tobacco-use-2000-2025-third-edition (accessed on 11 September 2022).
- Almulla, A.; Hassan-Yassoub, N.; Fu, D.; El-Awa, F.; Alebshehy, R.; Ismail, M.; Fraser, C.P. Smoking cessation services in the eastern mediterranean region: Highlights and findings from the WHO report on the global tobacco epidemic 2019. East. Mediterr. Health J. 2020, 26, 110–115. [Google Scholar] [CrossRef] [PubMed]
- El Hajj, M.S.; Kheir, N.; Al Mulla, A.M.; Shami, R.; Fanous, N.; Mahfoud, Z.R. Effectiveness of a pharmacist-delivered smoking cessation program in the State of Qatar: A randomized controlled trial. BMC Public Health 2017, 17, 215. [Google Scholar] [CrossRef]
- Hartmann-Boyce, J.; Hong, B.; Livingstone-Banks, J.; Wheat, H.; Fanshawe, T.R. Additional behavioural support as an adjunct to pharmacotherapy for smoking cessation. Cochrane Database Syst. Rev. 2019, 6, CD009670. [Google Scholar] [CrossRef] [PubMed]
- Pisinger, C.; Vestbo, J.; Borch-Johnsen, K.; Thomsen, T.; Jorgensen, T. Acceptance of the smoking cessation intervention in a large population-based study: The Inter99 study. Scand. J. Public Health 2005, 33, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, J.; Bauld, L.; Chesterman, J.; Judge, K. The English smoking treatment services: One-year outcomes. Addiction 2005, 100, 59–69. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Total (n = 248) | Pre-COVID (n = 203) | Post-COVID (n = 45) |
|---|---|---|---|
| Age (years), mean ± SD | 38.5 ± 9.0 | 39.1 ± 9.1 | 35.8 ± 8.4 |
| Nationality, n (%) | |||
| Qatari | 31 (12.5) | 27 (13.3) | 4 (8.9) |
| Egyptian | 74 (29.8) | 67 (33.0) | 7 (15.6) |
| Jordanian | 34 (13.7) | 22 (10.8) | 12 (26.7) |
| Syrian | 23 (9.3) | 12 (5.9) | 11 (24.4) |
| Other a | 63 (25.4) | 53 (26.1) | 10 (22.2) |
| Missing | 23 (9.3) | 22 (10.8) | 1 (2.2) |
| Marital Status b, n (%) | |||
| Married or living with partner | 201 (82.0) | 167 (83.5) | 34 (75.6) |
| Currently not married or living with partner | 44 (18.0) | 33 (16.5) | 11 (24.4) |
| Education level b, n (%) | |||
| Less than college degree | 83 (33.5) | 68 (33.5) | 15 (33.3) |
| College degree and above | 165 (66.5) | 135(66.5) | 30 (66.7) |
| Employment status b, n (%) | |||
| Employed | 231 (93.5) | 192 (95.1) | 39 (86.7) |
| Not employed | 16 (6.5) | 10 (5.0) | 6 (13.3) |
| Number of years of regular cigarette smoking, mean ± SD | 18.4 ± 8.4 | 18.8 ± 8.7 | 16.7 ± 6.7 |
| Number of daily smoked cigarettes, mean ± SD | 16.9 ± 11.0 | 17.7 ± 11.0 | 13.0 ± 10.1 |
| Nicotine dependence (FTND score), mean ± SD Number of smoking quit attempts over last year, n (%) | 4.4 ± 1.0 | 4.5 ± 1.0 | 4.2 ± 1.0 |
| None | 150 (61.7) | 120 (60.6) | 30 (66.7) |
| 1 | 56 (23.1) | 48 (24.2) | 8 (17.8) |
| ≥2 | 37 (15.2) | 30 (15.2) | 7 (15.6) |
| E-cigarette use in the past 30 days c, n (%) | |||
| Yes | 45 (18.2) | 43 (21.3) | 2 (4.4) |
| No | 197 (79.8) | 154 (76.2) | 43 (95.6) |
| Smokers in household beside yourself b, n (%) | |||
| Yes | 52 (21.1) | 44 (21.8) | 8 (17.8) |
| No | 195 (78.9) | 158 (78.2) | 37 (82.2) |
| House smoking rules c, n (%) | |||
| No ban | 59 (24.3) | 46 (23.2) | 13 (28.9) |
| Some ban | 74 (30.5) | 60 (30.3) | 14 (31.1) |
| Complete ban | 110 (45.3) | 92 (46.5) | 18 (40.0) |
| Pre-COVID (n = 246) | Post-COVID (n = 50) | Overall (n = 296) | |
|---|---|---|---|
| Recruitment rate (Avg recruited/month) | 18.9 | 10.0 | 16.4 |
| Retention a, n (%) | |||
| End of treatment | 112 (65.1) | 41 (87.2) | 153 (69.9) |
| 1-month follow-up | 100 (58.1) | 41 (87.2) | 141 (64.4) |
| 3-month follow-up | 113 (65.7) | 44 (93.6) | 157 (71.7) |
| No. of phone counselling sessions attended a, n (%) | |||
| 0 | 29 (16.9) | 2 (4.3) | 31 (14.2) |
| 1 | 29 (16.9) | 5 (10.6) | 34 (15.5) |
| 2 | 36 (20.9) | 5 (10.6) | 41 (18.7) |
| 3 | 38 (22.1) | 13 (27.7) | 51 (23.3) |
| 4 | 28 (16.3) | 17 (36.2) | 45 (20.6) |
| 5 | 12 (7.0) | 5 (10.6) | 17 (7.8) |
| Use of smoking cessation medication b, n (%) | |||
| Champix or Chantix | 98 (88.3) | 34 (82.9) | 132 (86.8) |
| Zyban (Buproprion, Wellbutrin) | 1 (0.9) | 1 (2.4) | 2 (1.3) |
| Gum | 3 (2.7) | 1 (2.4) | 4 (2.6) |
| Patch | 50 (45.1) | 25 (61.0) | 75 (49.3) |
| Lozenges | 71 (64.0) | 32 (78.1) | 103 (67.8) |
| At least one type of medication | 109 (98.2) | 36 (87.8) | 145 (95.4) |
| 7-day point prevalence smoking abstinence b, n (%) | |||
| End of treatment | 73 (65.2) | 18 (43.9) | 91 (59.5) |
| 1-month follow-up | 65 (65.0) | 19 (46.3) | 84 (59.6) |
| 3-month follow-up | 68 (61.3) | 18 (43.9) | 86 (56.7) |
| 7-day point prevalence smoking abstinence, intent to treat analysis c, n (%) | |||
| End of treatment | 73 (42.4) | 18 (38.3) | 91 (41.6) |
| 1-month follow-up | 65 (37.8) | 19 (40.4) | 84 (38.4) |
| 3-month follow-up | 68 (39.5) | 18 (38.3) | 86 (39.3) |
| 30-day point prevalence smoking abstinence b, n (%) | |||
| End of treatment | 78 (69.6) | 23 (56.1) | 101 (66.0) |
| 1-month follow-up | 67 (67.0) | 21 (51.2) | 88 (62.4) |
| 3-month follow-up | 71 (64.0) | 20 (48.8) | 91 (60.0) |
| 30-day point prevalence smoking abstinence, intent to treat analysis c, n (%) | |||
| End of treatment | 78 (45.4) | 23 (48.9) | 101 (46.1) |
| 1-month follow-up | 67 (40.0) | 21 (44.7) | 88 (40.2) |
| 3-month follow-up | 71 (41.3) | 20 (42.6) | 91 (41.6) |
| Participant satisfaction at 3-month follow-up d, n (%) | |||
| Neutral | 1 (1.7) | 0 (0.0) | 1 (1.0) |
| Satisfied | 12 (20.7) | 1 (2.4) | 13 (13.1) |
| Extremely satisfied | 45 (77.6) | 40 (97.6) | 85 (85.9) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leventakou, V.; Al Thani, M.; Sofroniou, A.; Butt, H.I.; Eltayeb, S.M.; Hakim, I.A.; Thomson, C.; Nair, U.S. Feasibility and Acceptability of a Telephone-Based Smoking Cessation Intervention for Qatari Residents. Int. J. Environ. Res. Public Health 2022, 19, 16509. https://doi.org/10.3390/ijerph192416509
Leventakou V, Al Thani M, Sofroniou A, Butt HI, Eltayeb SM, Hakim IA, Thomson C, Nair US. Feasibility and Acceptability of a Telephone-Based Smoking Cessation Intervention for Qatari Residents. International Journal of Environmental Research and Public Health. 2022; 19(24):16509. https://doi.org/10.3390/ijerph192416509
Chicago/Turabian StyleLeventakou, Vasiliki, Mohammed Al Thani, Angeliki Sofroniou, Hamza I. Butt, Safa M. Eltayeb, Iman A. Hakim, Cynthia Thomson, and Uma S. Nair. 2022. "Feasibility and Acceptability of a Telephone-Based Smoking Cessation Intervention for Qatari Residents" International Journal of Environmental Research and Public Health 19, no. 24: 16509. https://doi.org/10.3390/ijerph192416509
APA StyleLeventakou, V., Al Thani, M., Sofroniou, A., Butt, H. I., Eltayeb, S. M., Hakim, I. A., Thomson, C., & Nair, U. S. (2022). Feasibility and Acceptability of a Telephone-Based Smoking Cessation Intervention for Qatari Residents. International Journal of Environmental Research and Public Health, 19(24), 16509. https://doi.org/10.3390/ijerph192416509









