Collateral Effects of Insecticide-Treated Nets on Human and Environmental Safety in an Epidemiological Model for Malaria with Human Risk Perception
Abstract
1. Introduction
2. Materials and Methods
3. Simulations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ITN | Insecticide-treated net |
| WHO | World Health Organization |
References
- World Health Organization (WHO). Malaria. Available online: https://www.who.int/news-room/fact-sheets/detail/malaria (accessed on 3 October 2022).
- Centers for Disease Control and Prevention (CDC). About Malaria. Available online: https://www.cdc.gov/malaria/about/ (accessed on 23 August 2022).
- Almaw, A.; Yimer, M.; Alemu, M.; Tegegne, B. Prevalence of malaria and associated factors among symptomatic pregnant women attending antenatal care at three health centers in north-west Ethiopia. PLoS ONE 2022, 17, e0266477. [Google Scholar] [CrossRef]
- Uneke, C.J.; Ogbu, O.; Nwojiji, V. Potential risk of induced malaria by blood transfusion in South-eastern Nigeria. McGill J. Med. MJM 2006, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, M.; Taylor, G.; Saldaña-Núñez, V.; Córdova-Lepe, F.; Escalera-Antezana, J.P.; Alvarado-Arnez, L.E.; Rodríguez-Morales, A.J. Risk of Dengue Incidence in Children and Adolescents in Zulia, Venezuela, using a Negative Binomial Generalized Linear Mixed Model. Rev. Panam. Enferm. Infecc. 2019, 2, 39–45. [Google Scholar]
- Guyatt, H.L.; Snow, R.W. Impact of malaria during pregnancy on low birth weight in sub-Saharan Africa. Clin. Microbiol. Rev. 2004, 17, 760–769. [Google Scholar] [CrossRef]
- Roll Back Malaria. Available online: https://endmalaria.org/about-malaria/key-facts (accessed on 30 July 2022).
- World Health Organization (WHO). World Malaria Report 2021. Available online: https://www.who.int/publications/i/item/9789240040496 (accessed on 3 October 2022).
- Pryce, J.; Medley, N.; Choi, L. Indoor residual spraying for preventing malaria in communities using insecticide-treated nets. Cochrane Database Syst. Rev. 2022, 5, CD012688. [Google Scholar]
- Sherrard-Smith, E.; Winskill, P.; Hamlet, A.; Ngufor, C.; N’Guessan, R.; Guelbeogo, M.W.; Sanou, A.; Nash, R.K.; Hill, A.; Russell, E.L.; et al. Optimising the deployment of vector control tools against malaria: A data-informed modelling study. Lancet Planet. Health 2022, 6, e100–e109. [Google Scholar] [CrossRef] [PubMed]
- Pryce, J.; Richardson, M.; Lengeler, C. Insecticide-treated nets for preventing malaria. Cochrane Database Syst. Rev. 2018, 11, CD000363. [Google Scholar] [CrossRef]
- Wetzler, E.A.; Park, C.; Arroz, J.A.; Chande, M.; Mussambala, F.; Candrinho, B. Impact of mass distribution of insecticide-treated nets in Mozambique, 2012 to 2025: Estimates of child lives saved using the Lives Saved Tool. PLoS Glob. Public Health 2022, 2, e0000248. [Google Scholar] [CrossRef]
- Millat-Martínez, P.; Gabong, R.; Balanza, N.; Luana, S.; Sanz, S.; Raulo, S.; Elizah, A.; Wali, C.; Paivu, B.; Dalmas, J.; et al. Coverage, determinants of use and repurposing of long-lasting insecticidal nets two years after a mass distribution in Lihir Islands, Papua New Guinea: A cross-sectional study. Malar. J. 2021, 20, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Larsen, D.A.; Makaure, J.; Ryan, S.J.; Stewart, D.; Traub, A.; Welsh, R.; Love, D.H.; Bisesi, J.H., Jr. Implications of insecticide-treated mosquito net fishing in lower income countries. Environ. Health Perspect. 2021, 129, 015001. [Google Scholar] [CrossRef]
- McLean, K.A.; Byanaku, A.; Kubikonse, A.; Tshowe, V.; Katensi, S.; Lehman, A.G. Fishing with bed nets on Lake Tanganyika: A randomized survey. Malar. J. 2014, 13, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Short, R.; Gurung, R.; Rowcliffe, M.; Hill, N.; Milner-Gulland, E. The use of mosquito nets in fisheries: A global perspective. PLoS ONE 2018, 13, e0191519. [Google Scholar] [CrossRef]
- Berthe, S.; Harvey, S.A.; Lynch, M.; Koenker, H.; Jumbe, V.; Kaunda-Khangamwa, B.; Mathanga, D.P. Poverty and food security: Drivers of insecticide-treated mosquito net misuse in Malawi. Malar. J. 2019, 18, 1–11. [Google Scholar] [CrossRef]
- Jones, B.L.; Unsworth, R.K. The perverse fisheries consequences of mosquito net malaria prophylaxis in East Africa. Ambio 2020, 49, 1257–1267. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Guidelines for Malaria. 2022. Available online: https://apps.who.int/iris/bitstream/handle/10665/354781/WHO-UCN-GMP-2022.01-Rev.2-eng.pdf (accessed on 4 October 2022).
- RBM Partnership to End Malaria, Consensus Statement on Repurposing ITNs: Applications for BCC Messaging and Actions at the Country Level. 2018. Available online: https://pdf.usaid.gov/pdf_docs/PA00TW8X.pdf (accessed on 2 October 2022).
- USAID Programmatic Environmental Assessment: Integrated Vector Management Programs for Malaria Vector Control (version 2017). Available online: https://d1u4sg1s9ptc4z.cloudfront.net/uploads/2021/03/integrated-vector-management-programs-for-malaria-vector-control-programmatic-environmental-assessment-2017-2.pdf (accessed on 4 October 2022).
- Hu, R.; Huang, X.; Huang, J.; Li, Y.; Zhang, C.; Yin, Y.; Chen, Z.; Jin, Y.; Cai, J.; Cui, F. Long- and Short-Term Health Effects of Pesticide Exposure: A Cohort Study from China. PLoS ONE 2015, 10, 128766. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Park, S.; Kim, S.K.; Kim, C.S.; Kim, T.H.; Min, S.H.; Oh, S.S.; Koh, S.B. Correction: Pesticide exposure and cognitive decline in a rural South Korean population. PLoS ONE 2019, 14, 216310. [Google Scholar] [CrossRef]
- Liu, J.; Schelar, E. Pesticide Exposure and Child Neurodevelopment: Summary and Implications. Workplace Health Saf. 2012, 60, 235–242. [Google Scholar] [CrossRef]
- Muñoz-Quezada, M.T.; Lucero, B.A.; Gutiérrez-Jara, J.P.; Buralli, R.J.; Zúñiga-Venegas, L.; Muñoz, M.P.; Ponce, K.V.; Iglesias, V. Longitudinal exposure to pyrethroids (3-PBA and trans-DCCA) and 2,4-D herbicide in rural schoolchildren of Maule region, Chile. Sci. Total Environ. 2020, 749, 141512. [Google Scholar] [CrossRef]
- Zúñiga-Venegas, L.A.; Hyland, C.; Muñoz-Quezada, M.T.; Quirós-Alcalá, L.; Butinof, M.; Buralli, R.; Cardenas, A.; Fernandez, R.A.; Foerster, C.; Gouveia, N.; et al. Health Effects of Pesticide Exposure in Latin American and the Caribbean Populations: A Scoping Review. Environ. Health Perspect. 2022, 130, 096002. [Google Scholar] [CrossRef]
- Anyanwu, E.C.; Ehiri, J.E.; Kanu, I.; Merrick, J. Health effects of long-term exposure to insecticide-treated mosquito nets in the control of malaria in endemic regions, revised. Sci. World J. 2006, 6, 1631–1641. [Google Scholar] [CrossRef] [PubMed]
- Kolaczinski, J.; Curtis, C. Chronic illness as a result of low-level exposure to synthetic pyrethroid insecticides: A review of the debate. Food Chem. Toxicol. 2004, 42, 697–706. [Google Scholar] [CrossRef]
- Bomann, W. How safe are pyrethroid-treated mosquito nets? An evaluation based on the example of Solfac EW 050. Public Health 1995, 12, 30–35. [Google Scholar]
- Lu, G.; Traoré, C.; Meissner, P.; Kouyaté, B.; Kynast-Wolf, G.; Beiersmann, C.; Coulibaly, B.; Becher, H.; Müller, O. Safety of insecticide-treated mosquito nets for infants and their mothers: Randomized controlled community trial in Burkina Faso. Malar. J. 2015, 14, 1–7. [Google Scholar] [CrossRef]
- Brauer, F.; Castillo-Chavez, C.; Mubayi, A.; Towers, S. Some models for epidemics of vector-transmitted diseases. Infect. Dis. Model. 2016, 1, 79–87. [Google Scholar] [CrossRef]
- Simoy, M.I.; Aparicio, J.P. Ross-Macdonald models: Which one should we use? Acta Trop. 2020, 207, 105452. [Google Scholar] [CrossRef]
- Gutiérrez-Jara, J.P.; Salazar-Viedma, M.; González, C.R.; Cancino-Faure, B. The emergence of Dirofilaria repens in a non-endemic area influenced by climate change: Dynamics of transmission using a mathematical model. Acta Trop. 2022, 226, 106230. [Google Scholar] [CrossRef] [PubMed]
- Ross, R. Some a priori pathometric equations. Br. Med. J. 1915, 1, 546. [Google Scholar] [CrossRef] [PubMed]
- Ngonghala, C.N. Assessing the impact of insecticide-treated nets in the face of insecticide resistance on malaria control. J. Theor. Biol. 2022, 555, 111281. [Google Scholar] [CrossRef]
- Ndamuzi, E.; Gahungu, P. Mathematical Modeling of Malaria Transmission Dynamics: Case of Burundi. J. Appl. Math. Phys. 2021, 9, 2447–2460. [Google Scholar] [CrossRef]
- Witbooi, P.; Abiodun, G.; Nsuami, M. A model of malaria population dynamics with migrants. Math. Biosci. Eng. 2021, 18, 7301–7317. [Google Scholar] [CrossRef] [PubMed]
- Vogt-Geisse, K.; Ngonghala, C.N.; Feng, Z. The impact of vaccination on malaria prevalence: A vaccine-age-structured modeling approach. J. Biol. Syst. 2020, 28, 475–513. [Google Scholar] [CrossRef]
- Vogt-Geisse, K.; Lorenzo, C.; Feng, Z. Impact of age-dependent relapse and immunity on malaria dynamics. J. Biol. Syst. 2013, 21, 1340001. [Google Scholar] [CrossRef]
- Mandal, S.; Sarkar, R.R.; Sinha, S. Mathematical models of malaria-a review. Malar. J. 2011, 10, 1–19. [Google Scholar] [CrossRef]
- Lindsay, S.W.; Thomas, M.B.; Kleinschmidt, I. Threats to the effectiveness of insecticide-treated bednets for malaria control: Thinking beyond insecticide resistance. Lancet Glob. Health 2021, 9, e1325–e1331. [Google Scholar] [CrossRef] [PubMed]
- White, L.J.; Maude, R.J.; Pongtavornpinyo, W.; Saralamba, S.; Aguas, R.; Van Effelterre, T.; Day, N.P.; White, N.J. The role of simple mathematical models in malaria elimination strategy design. Malar. J. 2009, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chitnis, N.; Schapira, A.; Smith, T.; Steketee, R. Comparing the effectiveness of malaria vector-control interventions through a mathematical model. Am. J. Trop. Med. Hyg. 2010, 83, 230. [Google Scholar] [CrossRef] [PubMed]
- Agusto, F.B.; Del Valle, S.Y.; Blayneh, K.W.; Ngonghala, C.N.; Goncalves, M.J.; Li, N.; Zhao, R.; Gong, H. The impact of bed-net use on malaria prevalence. J. Theor. Biol. 2013, 320, 58–65. [Google Scholar] [CrossRef]
- Chinebu, T.I.; Ezennorom, E.O.; Okwor, J.U. Simulation of a Mathematical Model of Malaria Transmission Dynamics in the Presence of Mosquito Net, Fumigation And Treatment. Int. J. Trend Sci. Res. Dev. 2018, 2, 339–360. [Google Scholar] [CrossRef][Green Version]
- Aldila, D. Dynamical Analysis on a Malaria Model with Relapse Preventive Treatment and Saturated Fumigation. Comput. Math. Methods Med. 2022, 2022, 1135452. [Google Scholar] [CrossRef]
- Córdova-Lepe, F.; Pinto, M.; González-Olivares, E. A new class of differential equations with impulses at instants dependent on preceding pulses. Applications to management of renewable resources. Nonlinear Anal. Real World Appl. 2012, 13, 2313–2322. [Google Scholar] [CrossRef]
- Liu, H.; Yu, J.; Zhu, G. Analysis of a vector-host malaria model with impulsive effect and infection-age. Adv. Complex Syst. 2006, 9, 237–248. [Google Scholar] [CrossRef]
- Huang, M.; Song, X.; Li, J. Modelling and analysis of impulsive releases of sterile mosquitoes. J. Biol. Dyn. 2017, 11, 147–171. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Jara, J.P.; Córdova-Lepe, F.; Muñoz-Quezada, M.T.; Chowell, G. Pesticide application, educational treatment and infectious respiratory diseases: A mechanistic model with two impulsive controls. PLoS ONE 2020, 15, 243048. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, M.; Cordova-Lepe, F.; Gutierrez-Jara, J.P.; Vogt Geisse, K. An SIR type epidemiological model that integrates social distancing as a dynamic law based on point prevalence and socio-behavioral factors. Sci. Rep. 2021, 11, 10170. [Google Scholar] [CrossRef]
- Gutiérrez-Jara, J.P.; Vogt Geisse, K.; Cabrera, M.; Cordova-Lepe, F.; Muñoz-Quezada, M.T. Effects of human mobility and behavior on disease transmission in a COVID-19 mathematical model. Sci. Rep. 2022, 12, 10840. [Google Scholar] [CrossRef]
- Gutiérrez-Jara, J.P.; Saracini, C. Risk Perception Influence on Vaccination Program on COVID-19 in Chile: A Mathematical Model. Int. J. Environ. Res. Public Health 2022, 19, 2022. [Google Scholar] [CrossRef]
- Chitnis, N.; Hyman, M.J.; Cushing, J.M. Determining Important Parameters in the Spread of Malaria Through the Sensitivity Analysis of a Mathematical Model. Bull. Math. Biol. 2008, 70, 1272–1296. [Google Scholar] [CrossRef]
- Nah, K.; Kim, Y.; Lee, J.M. The dilution effect of the domestic animal population on the transmission of P. vivax malaria. J. Theor. Biol. 2010, 266, 299–306. [Google Scholar] [CrossRef]
- de Alencar, F.E.; Malafronte, R.D.S.; Cerutti Junior, C.; Natal Fernandes, L.; Buery, J.C.; Fux, B.; Rezende, H.R.; Duarte, A.M.R.D.C.; Medeiros-Sousa, A.R.; Miranda, A.E. Assessment of asymptomatic Plasmodium spp. infection by detection of parasite DNA in residents of an extra-Amazonian. Malar. J. 2021, 17, 1475–2875. [Google Scholar] [CrossRef]
- Medeiros-Sousa, A.R.; Laporta, G.Z.; Coutinho, R.M.; Mucci, L.F.; Marrelli, M.T. A mathematical model for zoonotic transmission of malaria in the Atlantic Forest: Exploring the effects of variations in vector abundance and acrodendrophily. PLoS Negl. Trop. Dis. 2021, 15, 1–21. [Google Scholar] [CrossRef]
- Kim, S.; Byun, J.H.; Park, A.; Jung, I.H. A mathematical model for assessing the effectiveness of controlling relapse in Plasmodium vivax malaria endemic in the Republic of Korea. PLoS ONE 2020, 15, 227919. [Google Scholar] [CrossRef] [PubMed]
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxical Profile for Pyrethrins and Pyrethroids. Available online: https://www.atsdr.cdc.gov/toxprofiledocs/index.html?id=787&tid=153 (accessed on 5 August 2022).
- Rodríguez, J.L.; Ares, I.; Martínez, M.; Martínez-Larrañaga, M.R.; Anadón, A.; Martínez, M.A. Bioavailability and nervous tissue distribution of pyrethroid insecticide cyfluthrin in rats. Food Chem. Toxicol. 2018, 118, 220–226. [Google Scholar] [CrossRef] [PubMed]
- World Bank Data, Life Expectancy at Birth, Total (Years)—Sub-Saharan Africa. Available online: https://data.worldbank.org/indicator/SP.DYN.LE00.IN?locations=ZG (accessed on 15 August 2022).
- The World Factbook, Central Intelligence Agency. Life Expectancy at Birth. Available online: https://www.cia.gov/the-world-factbook/field/life-expectancy-at-birth/country-comparison (accessed on 14 August 2022).
- Insecticide-Treated Mosquito Net Interventions: A Manual for National Control Programme Managers. Edited by Roll Back Malaria. Available online: https://apps.who.int/iris/handle/10665/42685 (accessed on 15 August 2022).
- Laporta, G.Z.; Prado, P.I.K.L.d.; Kraenkel, R.A.; Coutinho, R.M.; Sallum, M.A.M. Biodiversity Can Help Prevent Malaria Outbreaks in Tropical Forests. PLoS Negl. Trop. Dis. 2013, 7, e2139. [Google Scholar] [CrossRef]
- Kouassi, B.L.; Edi, C.; Tia, E.; Konan, L.Y.; Akré, M.A.; Koffi, A.A.; Ouattara, A.F.; Tanoh, A.M.; Zinzindohoue, P.; Kouadio, B.; et al. Susceptibility of Anopheles gambiae from Côte d’Ivoire to insecticides used on insecticide-treated nets: Evaluating the additional entomological impact of piperonyl butoxide and chlorfenapyr. Malar. J. 2020, 19, 454. [Google Scholar] [CrossRef] [PubMed]
- Mwandya, A.W.; Gullström, M.; Öhman, M.C.; Andersson, M.H.; Mgaya, Y.D. Fish assemblages in Tanzanian mangrove creek systems influenced by solar salt farm constructions. Estuar. Coast. Shelf Sci. 2009, 82, 193–200. [Google Scholar] [CrossRef]
- El Ganainy, A.; Khouraiba, H.; Aly, M.; Osman, M.A. Fishery assessment of the common silver biddy gerres oyena from the gulf of Suez, Red Sea, Egypt. Egypt. J. Aquat. Biol. Fish. 2020, 24, 437–447. [Google Scholar] [CrossRef]
- Yongo, E.; Agembe, S.; Outa, N.; Owili, M. Growth, mortality and recruitment of Nile perch (Lates niloticus) in Lake Victoria, Kenya. Lakes Reserv. Res. Manag. 2018, 23, 17–23. [Google Scholar] [CrossRef]
- Owusu, V.; Andriesse, E. From open access regime to closed fishing season: Lessons from small-scale coastal fisheries in the Western Region of Ghana. Mar. Policy 2020, 121, 104162. [Google Scholar] [CrossRef]
- Cabrera, M.; Taylor, G. Modelling spatio-temporal data of dengue fever using generalized additive mixed models. Spat. Spatio-Temporal Epidemiol. 2019, 28, 1–13. [Google Scholar] [CrossRef]
- Córdova-Lepe, F.; Vogt-Geisse, K. Adding a reaction-restoration type transmission rate dynamic-law to the basic SEIR COVID-19 model. PLoS ONE 2022, 17, e0269843. [Google Scholar] [CrossRef]
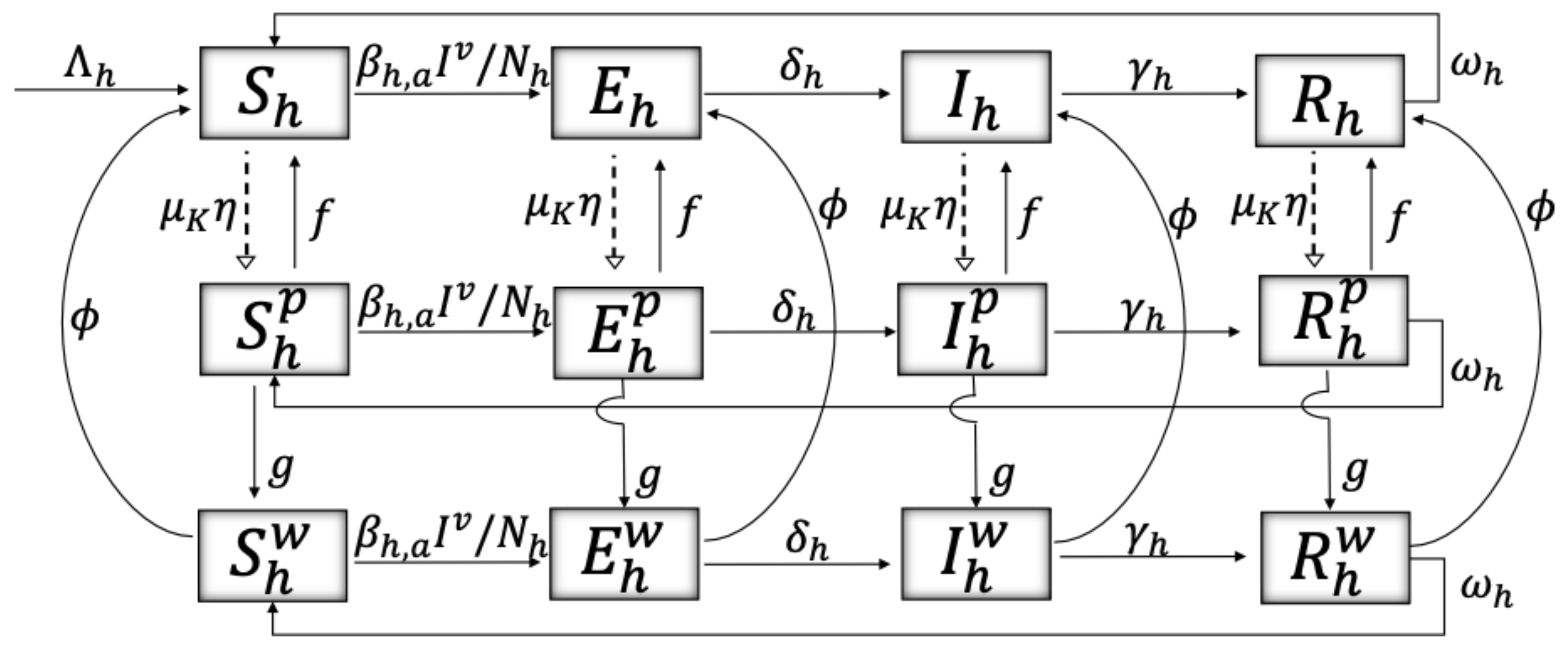

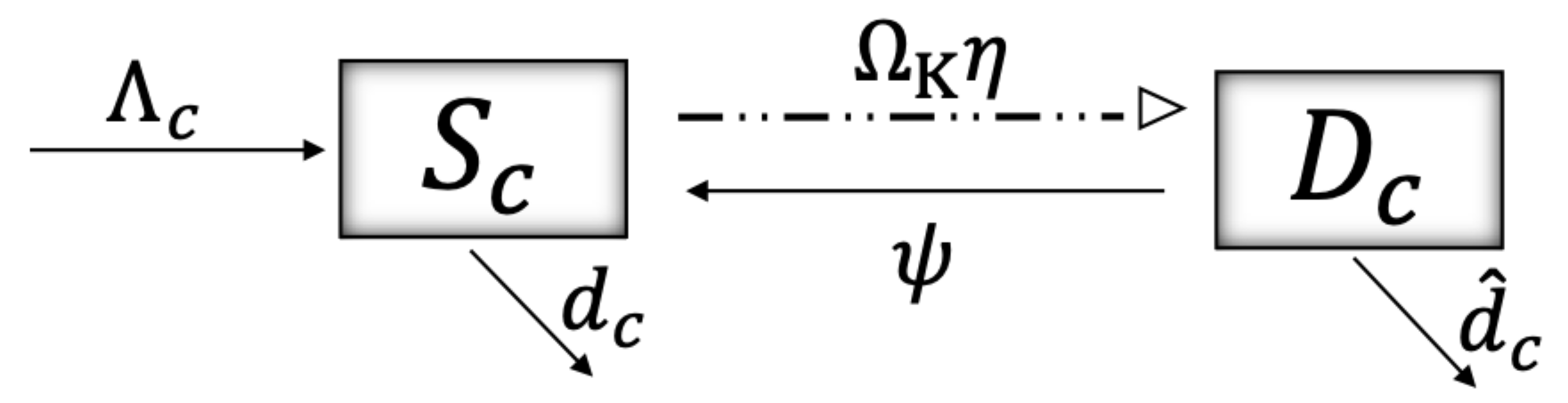
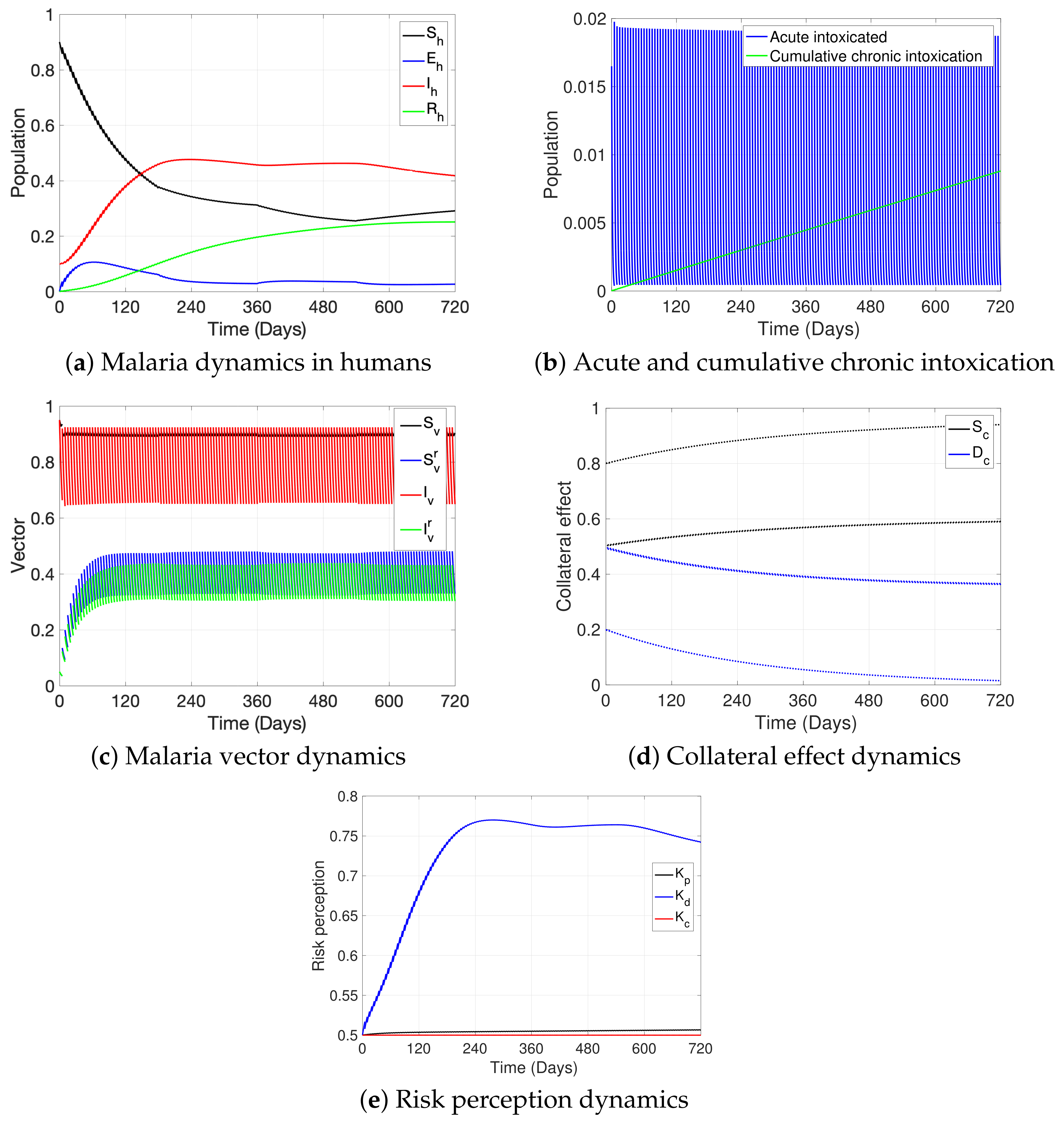
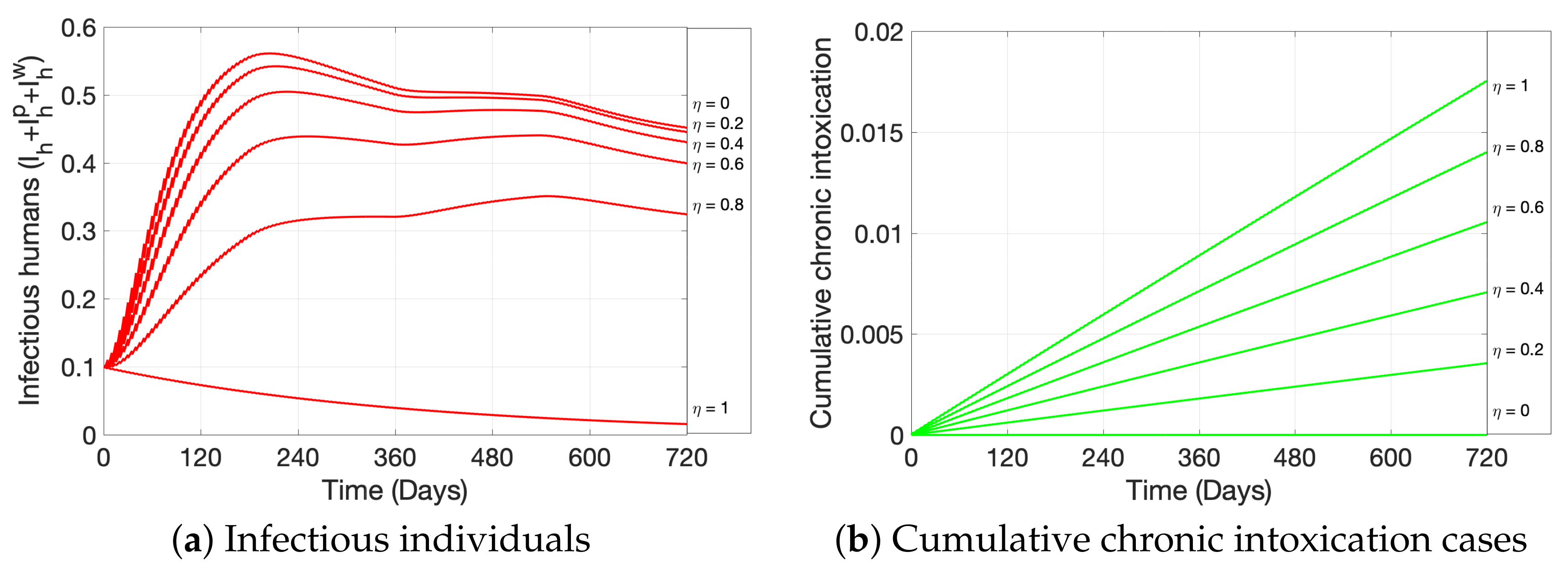
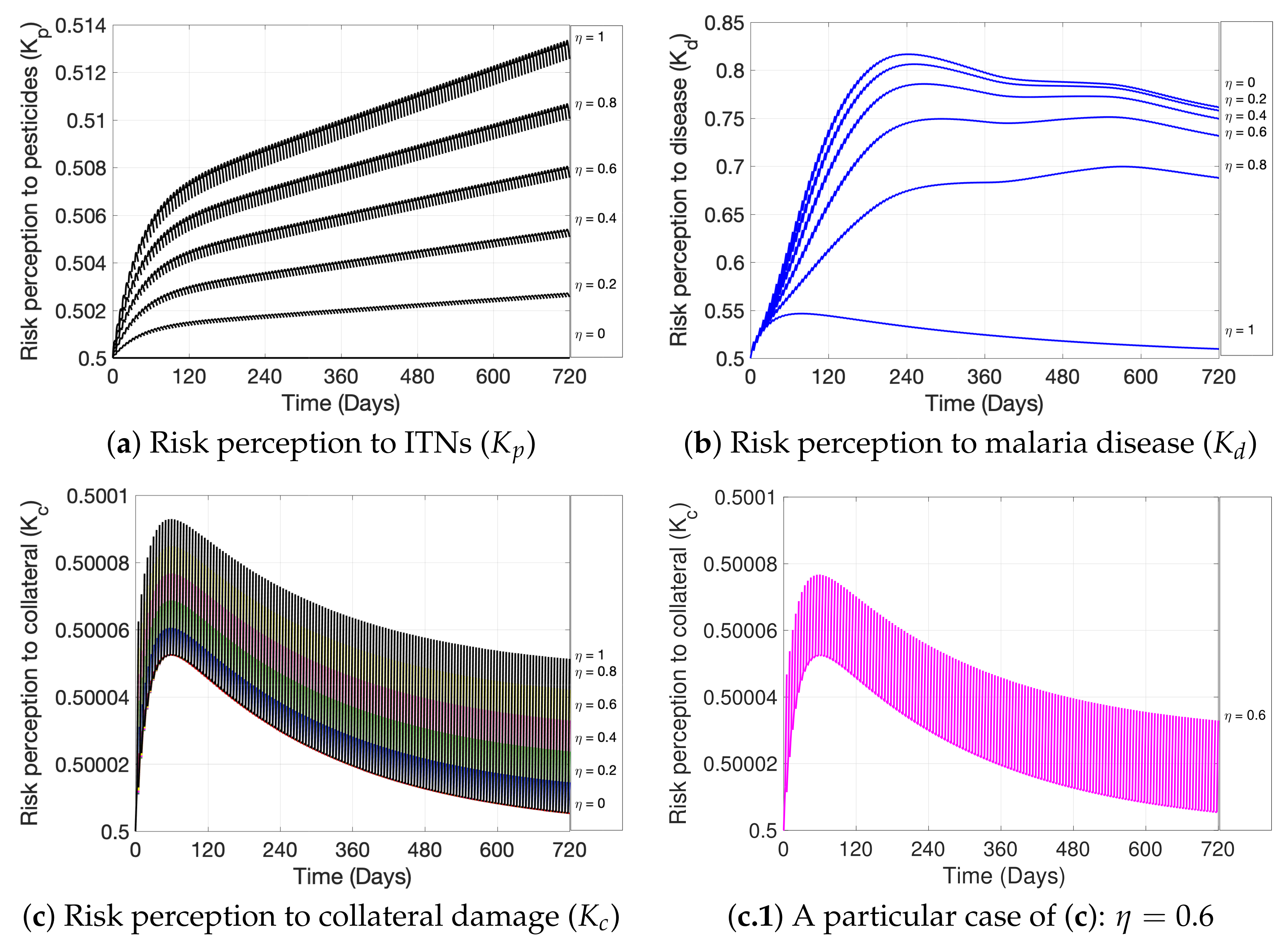
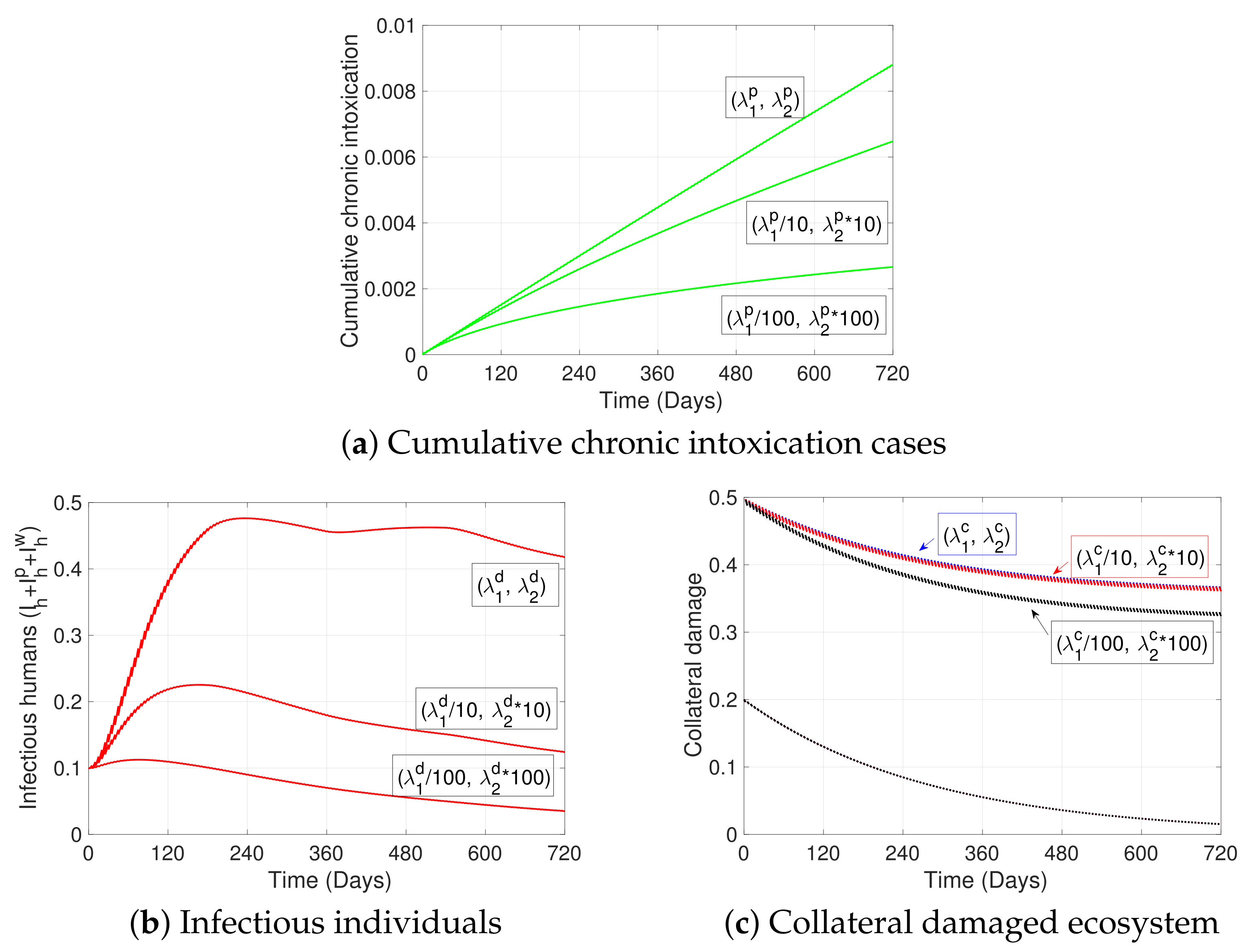
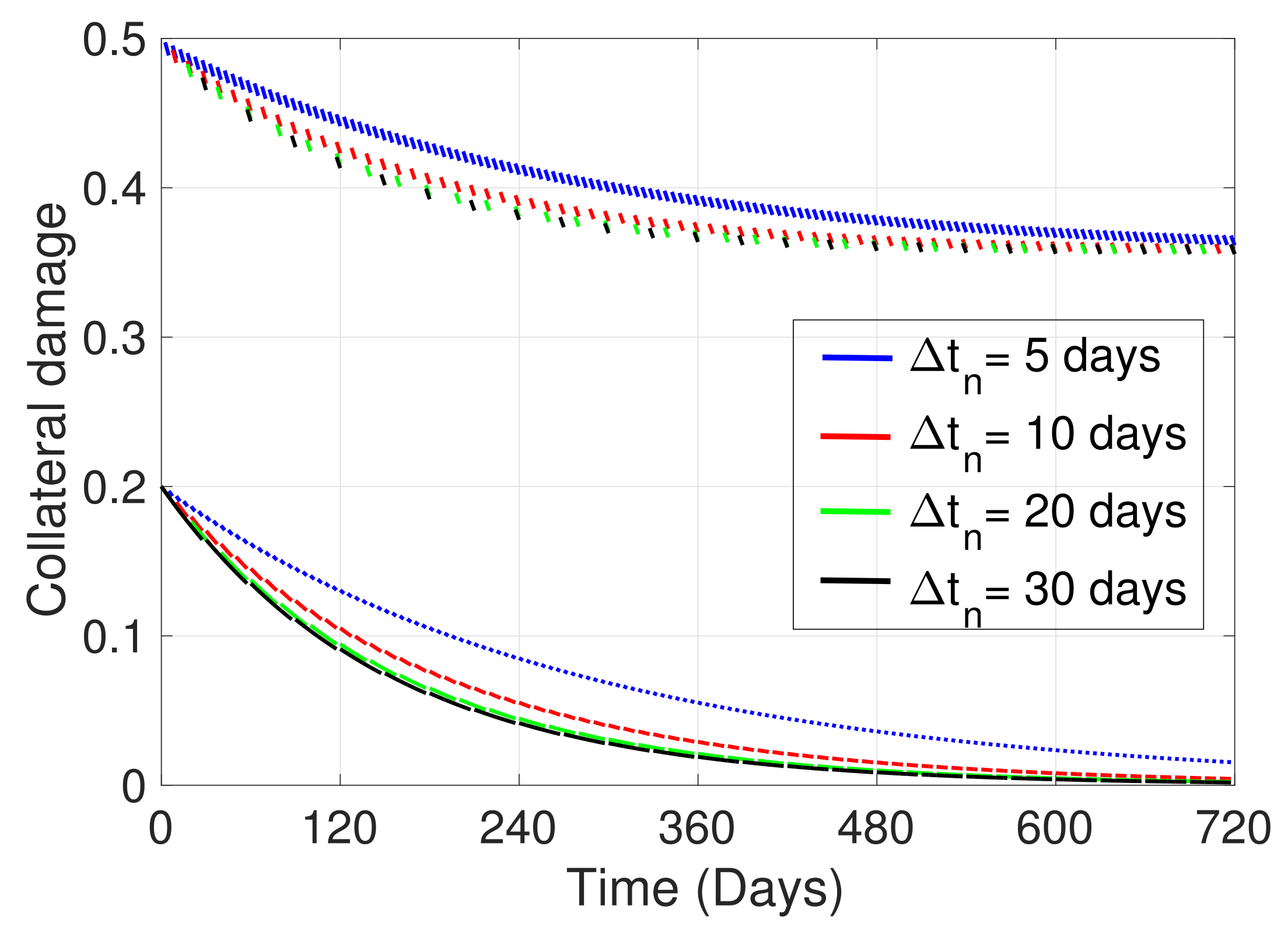
| Variable | Definition | Units * |
|---|---|---|
| Humans | ||
| Malaria-susceptible individuals w/o, w/ acute, w/ chronic intoxication | H | |
| Malaria-exposed individuals w/o, w/ acute, w/ chronic intoxication | H | |
| Malaria-infected individuals w/o, w/ acute, w/ chronic intoxication | H | |
| Malaria-recovered individuals w/o, w/ acute, w/ chronic intoxication | H | |
| Risk perception to ITNs toxicity | R | |
| Risk perception to malaria disease | R | |
| Risk perception to collateral ecosystem damage from misuse of ITNs | R | |
| Vector | ||
| Malaria susceptible mosquito w/o, w/ insecticide resistance | M | |
| Malaria infected mosquito w/o, w/ insecticide resistance | M | |
| Collateral effect—aquatic ecosystem | ||
| Ecosystem susceptible to damage | C | |
| Damaged ecosystem | C | |
| Parameter | Definition | Baseline | Units * | Reference |
|---|---|---|---|---|
| Humans | ||||
| Malaria transmission rate to human | [0.015, 0.22] | D | [54,55,56,57,58] | |
| Average incubation time | [12, 30] | D | [55,58] | |
| Mean infectious period | [180, 720] | D | [54,55,56,57,58] | |
| Loss of immunity rate | D | [54] | ||
| Intoxication proportion | [0, 0.3] | U | [26,30] | |
| Mean detoxification period | [1/6, 2] | D | [59,60] | |
| g | Health effects after prolonged exposure | 0.00289 | D | [25,26] |
| Recovery rate from chronic toxicity | [0, 0.5] | D | Author chosen | |
| Recruitment rate | HD | Author chosen | ||
| Natural mortality rate | D | [61,62] | ||
| Disease-induced mortality rate | D | [54] | ||
| Rate of resistance to change risk perception | [0, 1] | D | [25,51,52,53] | |
| Per capita reaction to change risk perception | [0, 1] | RD, R | [25,51,52,53] | |
| Mosquito net coverage | [0, 1] | U | [7] | |
| Vector | ||||
| Malaria transmission rate to mosquito | [0.015, 0.24] | D | [54,55,56,57,58] | |
| Proportion of mosquitoes becoming resistant | [0, 1] | U | [1,7,63] | |
| Recruitment rate | MD | Author chosen | ||
| Natural mortality rate | [1/30, 1] | D | [57,64] | |
| Increase in the mortality rate | [4.4, 48.9]% | U | [65] | |
| Collateral effect—aquatic ecosystem | ||||
| Percentage of species susceptible to ITN fishing | U | [66] | ||
| Recruitment rate | CD | Author chosen | ||
| Natural mortality rate | D | [67,68] | ||
| Damage-induced mortality rate | D | [67,68] | ||
| Loss of damage rate | D | [67,68] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez-Jara, J.P.; Vogt-Geisse, K.; Cabrera, M. Collateral Effects of Insecticide-Treated Nets on Human and Environmental Safety in an Epidemiological Model for Malaria with Human Risk Perception. Int. J. Environ. Res. Public Health 2022, 19, 16327. https://doi.org/10.3390/ijerph192316327
Gutiérrez-Jara JP, Vogt-Geisse K, Cabrera M. Collateral Effects of Insecticide-Treated Nets on Human and Environmental Safety in an Epidemiological Model for Malaria with Human Risk Perception. International Journal of Environmental Research and Public Health. 2022; 19(23):16327. https://doi.org/10.3390/ijerph192316327
Chicago/Turabian StyleGutiérrez-Jara, Juan Pablo, Katia Vogt-Geisse, and Maritza Cabrera. 2022. "Collateral Effects of Insecticide-Treated Nets on Human and Environmental Safety in an Epidemiological Model for Malaria with Human Risk Perception" International Journal of Environmental Research and Public Health 19, no. 23: 16327. https://doi.org/10.3390/ijerph192316327
APA StyleGutiérrez-Jara, J. P., Vogt-Geisse, K., & Cabrera, M. (2022). Collateral Effects of Insecticide-Treated Nets on Human and Environmental Safety in an Epidemiological Model for Malaria with Human Risk Perception. International Journal of Environmental Research and Public Health, 19(23), 16327. https://doi.org/10.3390/ijerph192316327






