Are We Prepared for the Next Pandemic? Management, Systematic Evaluation and Lessons Learned from an In-Hospital COVID-19 Vaccination Centre for Healthcare Workers
Abstract
1. Introduction
2. Materials and Methods
2.1. Organisation and Programme Monitoring of the In-Hospital Vaccination Centre
2.2. Satisfaction with the Vaccination Process
3. Results
3.1. Programme Monitoring of the In-Hospital Vaccination Centre
3.1.1. Vaccinations, Personnel and No-Show Rates
3.1.2. Administrative Organisation
3.2. Satisfaction with the Vaccination Centre and Process by Vaccinees
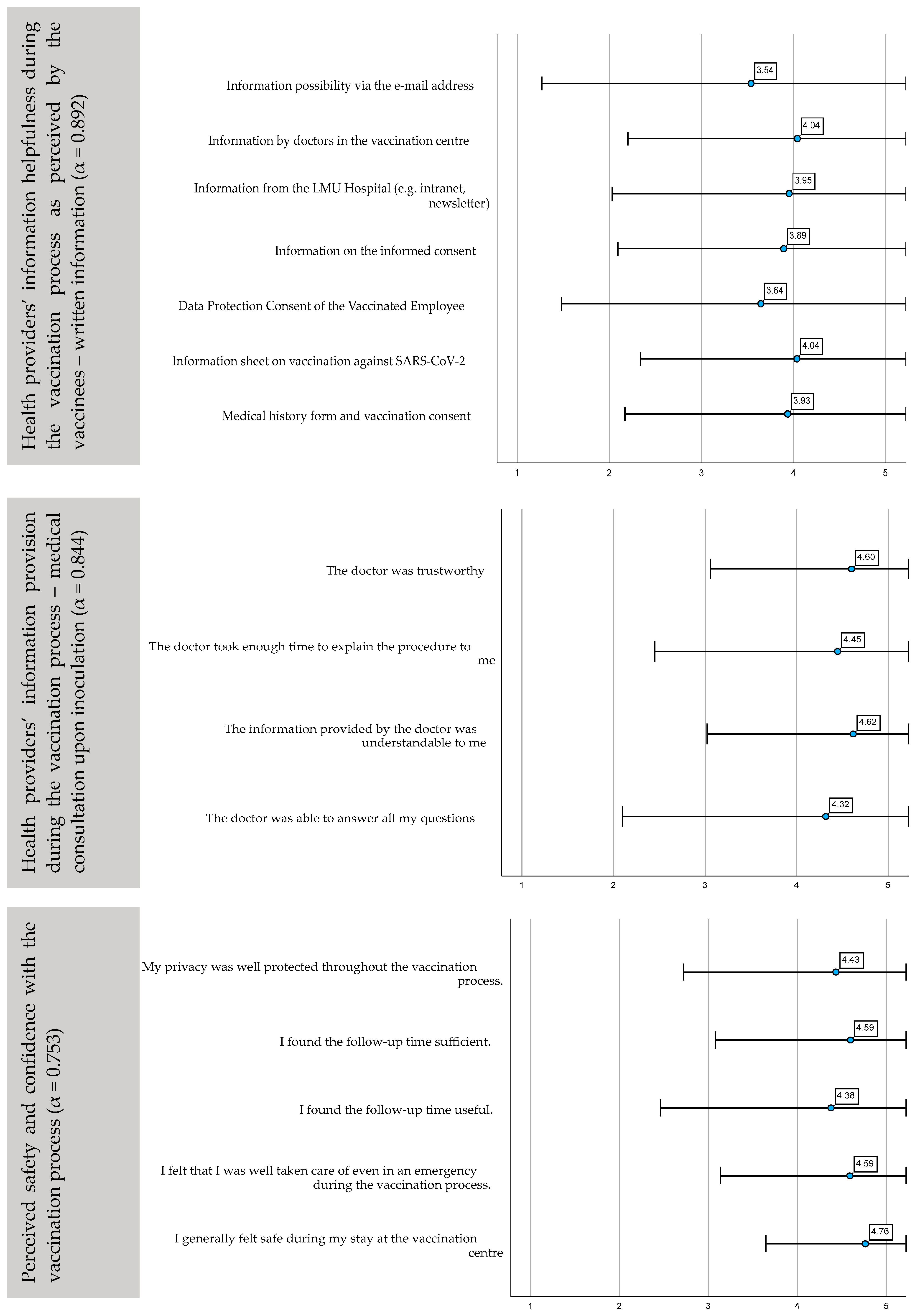
3.2.1. Satisfaction with the Process and Vaccine-Specific Issues
3.2.2. Satisfaction with the Provided Information Sources Prior to Inoculation
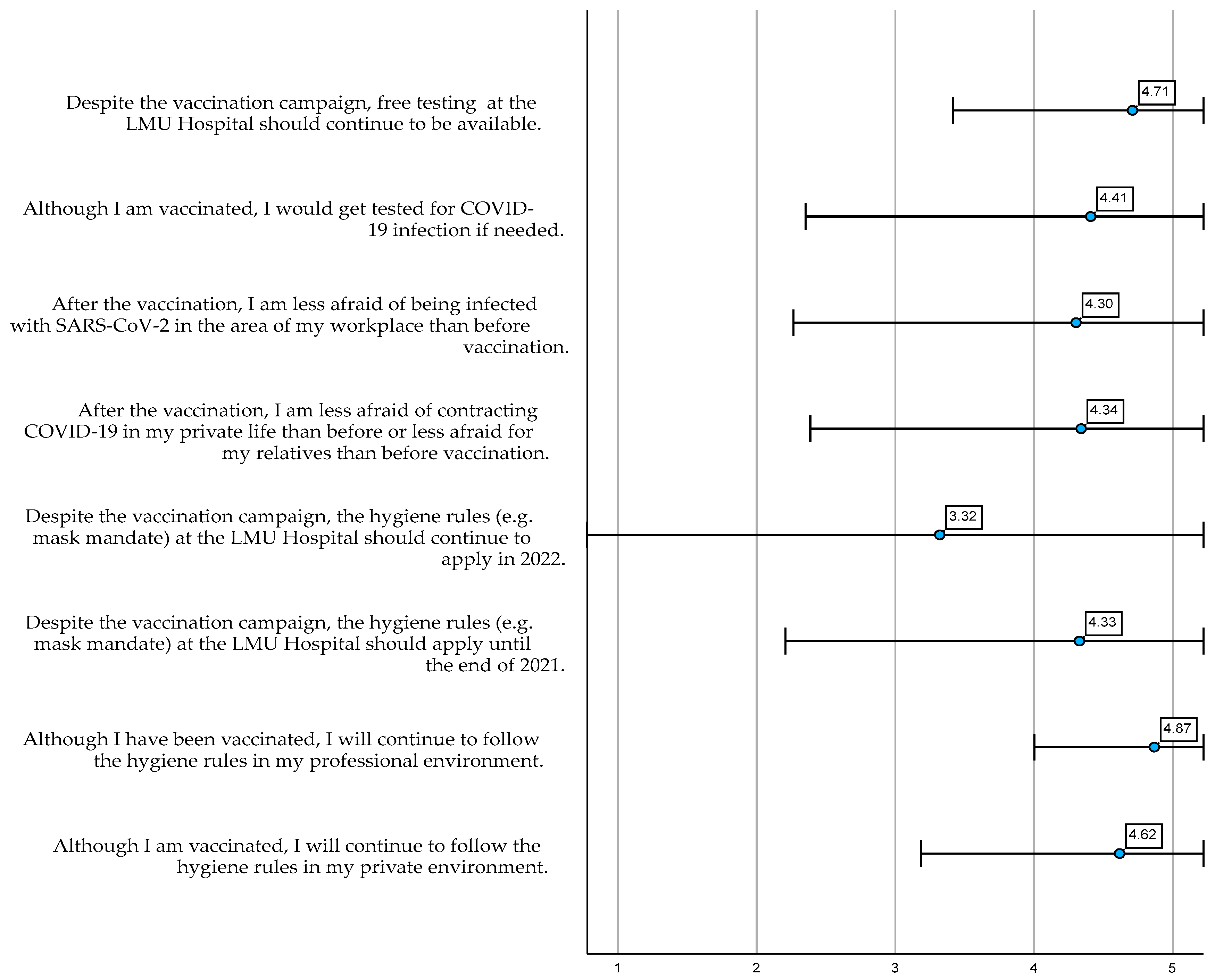
3.2.3. COVID-19 Health Behaviour following COVID-19 Vaccination
3.2.4. Observed Adverse Events following Immunization (AEFIs)
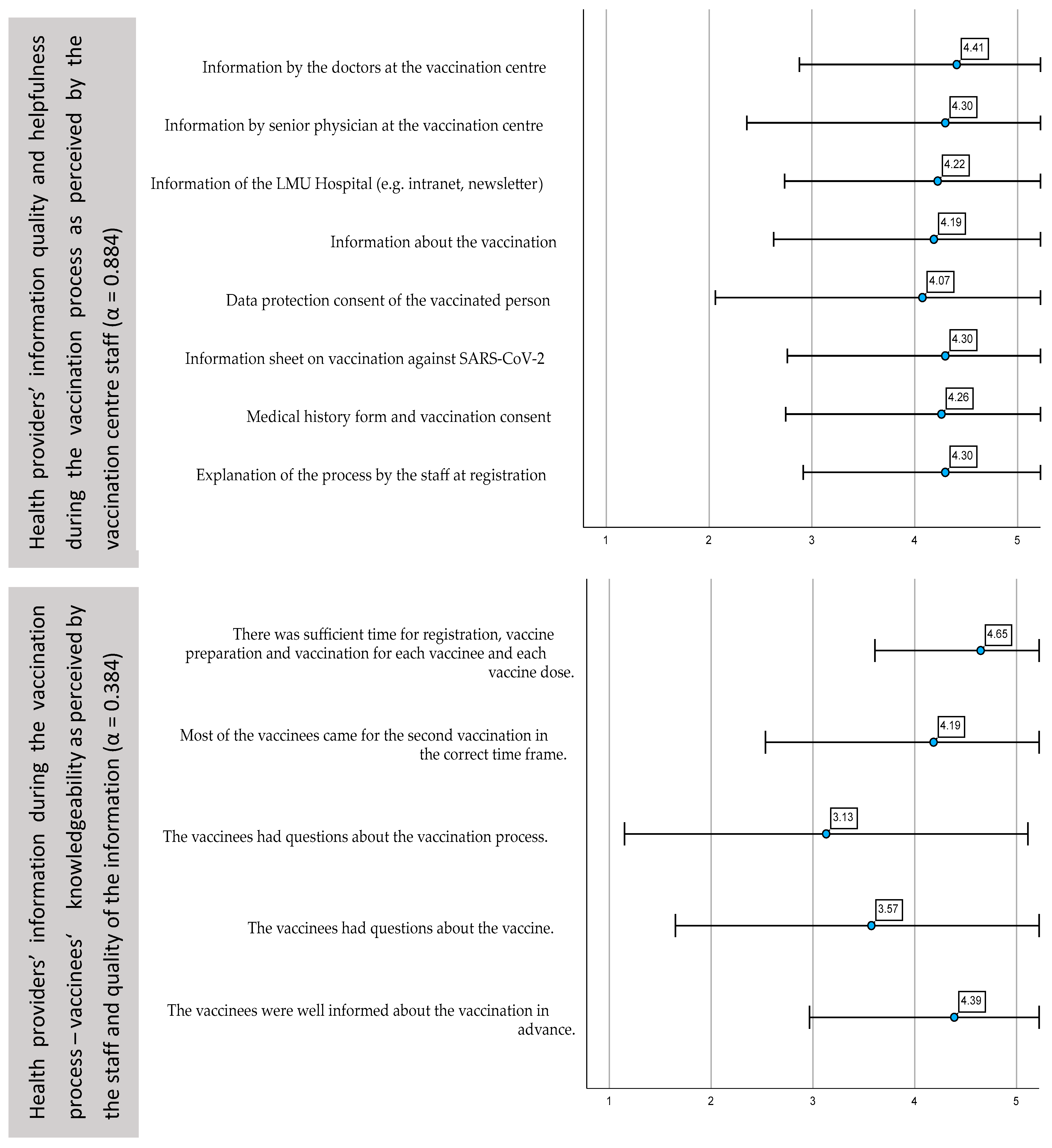
3.3. Satisfaction of the Vaccination Centre Staff with the Process and Organisation of the Vaccination Campaign
4. Discussion
4.1. Organisation of the Vaccination Centre—Implementation Considerations
4.2. Satisfaction with the Vaccination Process
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suwono, B.; Steffen, A.; Schweickert, B.; Schonfeld, V.; Brandl, M.; Sandfort, M.; Willrich, N.; Eckmanns, T.; Haller, S. SARS-CoV-2 outbreaks in hospitals and long-term care facilities in Germany: A national observational study. Lancet Reg. Health Eur. 2022, 14, 100303. [Google Scholar] [CrossRef] [PubMed]
- Labetoulle, R.; Detoc, M.; Gagnaire, J.; Berthelot, P.; Pelissier, C.; Fontana, L.; Botelho-Nevers, E.; Gagneux-Brunon, A. COVID-19 in health-care workers: Lessons from SARS and MERS epidemics and perspectives for chemoprophylaxis and vaccines. Expert Rev. Vaccines 2020, 19, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Bundesministerium für Gesundheit. Verordnung zum Anspruch auf Schutzimpfung Gegen das Coronavirus SARS-CoV-2. (Coronavirus-Impfverordnung–CoronaImpfV); BAnz AT 08.02.2021 V1; Bundesministerium der Justiz und für Verbraucherschutz: Bonn, Germany, 2021; p. 8. [Google Scholar]
- European Medicines Agency. EMA Recommends First COVID-19 Vaccine for Authorisation in the EU. Available online: https://www.ema.europa.eu/en/news/ema-recommends-first-covid-19-vaccine-authorisation-eu (accessed on 10 May 2022).
- Bayerische Krankenhausgesellschaft; Freistaat Bayern. Rahmenvereinbarung Zwischen der Bayerische Krankenhausgesellschaft e.V. (BKG) und dem Freistaat Bayern; Bayerisches Staatsministerium für Gesundheit und Pflege: Munich, Germany, 2020; Available online: https://www.bkg-online.de/media/mediapool_BKG/02_infos-services/BIK-Impfportal/2021.01.28_Rahmenvereinbarung_BKG.pdf (accessed on 13 June 2022).
- Zhelyazkova, A.; Kim, S.; Klein, M.; Prueckner, S.; Horster, S.; Kressirer, P.; Choukér, A.; Coenen, M.; Adorjan, K. COVID-19 Vaccination Intent, Barriers and Facilitators in Healthcare Workers: Insights from a Cross-Sectional Study on 2500 Employees at LMU University Hospital in Munich, Germany. Vaccines 2022, 10, 1231. [Google Scholar] [CrossRef] [PubMed]
- Musterhygieneplan Impfzentren Stand 23.03.2021. Bayerisches Landesamt für Gesundheit und Lebensmittelsicherheit. Bayerisches Landesamt für Gesundheit und Lebensmittelsicherheit: Munich, Germany, 2021.
- Bayerisches Staatsministerium für Gesundheit und Pflege. Leitfaden COVID-19 Impfstellen. Grundbausteine zur Organisation von COVID-19 Impfungen in zentralen Impfstellen; Bayerisches Staatsministerium für Gesundheit und Pflege: Munich, Germany, 2020. [Google Scholar]
- European Medicines Agency; Comirnaty. Tozinameran / COVID-19 mRNA Vaccine (Nucleoside Modified). Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/comirnaty#product-information-section (accessed on 13 June 2022).
- Mayflower GmbH. Public-Health-Informatics-Munich/COVID19-Teststation-Termine. Available online: https://github.com/Public-Health-Informatics-Munich/covid19-teststation-termine (accessed on 14 July 2022).
- Horster, S.; Andraschko, M.; Ostermann, H. Organisation Eines Innerklinischen Impfzentrums: Minutiöse Planung. Available online: https://www.aerzteblatt.de/archiv/218037/Organisation-eines-innerklinischen-Impfzentrums-Minutioese-Planung (accessed on 24 March 2022).
- Larson, H.J.; Jarrett, C.; Schulz, W.S.; Chaudhuri, M.; Zhou, Y.; Dube, E.; Schuster, M.; MacDonald, N.E.; Wilson, R.; Hesitancy, S.W.G.o.V. Measuring vaccine hesitancy: The development of a survey tool. Vaccine 2015, 33, 4165–4175. [Google Scholar] [CrossRef] [PubMed]
- SAGE Working Group on Vaccine Hesitancy. Report of the SAGE Working Group on Vaccine Hesitancy; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Robert Koch-Institut. Ständige Impfkommission (STIKO). Available online: https://www.rki.de/DE/Content/Kommissionen/STIKO/stiko_node.html (accessed on 8 April 2022).
- Paul-Ehrlich-Institut. Coronavirus and COVID-19. Available online: https://www.pei.de/EN/newsroom/dossier/coronavirus/coronavirus-content.html;jsessionid=A2894E806B1009528A4440E64130C611.intranet231 (accessed on 7 May 2022).
- Gianfredi, V.; Moretti, M.; Lopalco, P.L. Countering vaccine hesitancy through immunization information systems, a narrative review. Hum. Vaccines Immunother. 2019, 15, 2508–2526. [Google Scholar] [CrossRef]
- Brambilla, A.; Mangili, S.; Macchi, M.; Trucco, P.; Perego, A.; Capolongo, S. COVID-19 Massive Vaccination Center Layouts. Acta Bio-Med. Atenei Parm. 2021, 92, e2021446. [Google Scholar] [CrossRef]
- Goralnick, E.; Kaufmann, C.; Gawande, A.A. Mass-Vaccination Sites—An Essential Innovation to Curb the COVID-19 Pandemic. N. Engl. J. Med. 2021, 384, e67. [Google Scholar] [CrossRef]
- De Micco, F.; De Benedictis, A.; Sommella, L.; Di Mattia, A.; Campanozzi, L.L.; Alloni, R.; Tambone, V. Vaccines Administration in the Perspective of Patient Safety and Quality of Healthcare: Lesson from the Experience of an Italian Teaching Hospital for Pandemic Preparedness. Vaccines 2022, 10, 1495. [Google Scholar] [CrossRef]
- Hsiao, S.H.; Huang, S.J.; Huang, C.Y. Vaccination Strategies at a COVID-19 Mass Vaccination Site. Int. J. Health Policy Manag. 2022, 11, 1981–1982. [Google Scholar] [CrossRef]
- Robert Koch-Institut. Robert Koch-Institut: COVID-19-Dashboard. Auswertungen Basierend auf den aus den Gesundheitsämtern Gemäß IfSG übermittelten Meldedaten. Available online: https://experience.arcgis.com/experience/478220a4c454480e823b17327b2bf1d4 (accessed on 7 April 2022).
- Ständige Impfkommission (STIKO). STIKO-Empfehlung zur COVID-19-Impfung. Available online: https://www.rki.de/DE/Content/Infekt/Impfen/ImpfungenAZ/COVID-19/Impfempfehlung-Zusfassung.html (accessed on 30 March 2022).
- Bundesministerium für Gesundheit. Impf-Priorisierung Aufgehoben. Available online: https://www.bundesregierung.de/breg-de/themen/coronavirus/corona-impfung-priorisierung-entfaellt-1914756 (accessed on 14 November 2022).
- Thomson, A.; Robinson, K.; Vallee-Tourangeau, G. The 5As: A practical taxonomy for the determinants of vaccine uptake. Vaccine 2016, 34, 1018–1024. [Google Scholar] [CrossRef]
- Halliday, L.; Thomson, J.A.; Roberts, L.; Bowen, S.; Mead, C. Influenza vaccination of staff in aged care facilities in the ACT: How can we improve the uptake of influenza vaccine? Aust. N. Z. J. Public Health 2003, 27, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Bedford, H.; Attwell, K.; Danchin, M.; Marshall, H.; Corben, P.; Leask, J. Vaccine hesitancy, refusal and access barriers: The need for clarity in terminology. Vaccine 2018, 36, 6556–6558. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. EMA Recommends Approval of Second Adapted Spikevax Vaccine. Available online: https://www.ema.europa.eu/en/news/ema-recommends-approval-second-adapted-spikevax-vaccine#:~:text=The%20adapted%20vaccine%2C%20Spikevax%20bivalent,EMA%20has%20recommended%20for%20approval. (accessed on 20 October 2022).
- Dube, E.; Gagnon, D.; MacDonald, N.E.; SAGE Working Group on Vaccine Hesitancy. Strategies intended to address vaccine hesitancy: Review of published reviews. Vaccine 2015, 33, 4191–4203. [Google Scholar] [CrossRef] [PubMed]
- Paterson, P.; Meurice, F.; Stanberry, L.R.; Glismann, S.; Rosenthal, S.L.; Larson, H.J. Vaccine hesitancy and healthcare providers. Vaccine 2016, 34, 6700–6706. [Google Scholar] [CrossRef] [PubMed]
- Kassianos, G.; Kuchar, E.; Nitsch-Osuch, A.; Kyncl, J.; Galev, A.; Humolli, I.; Falup-Pecurariu, O.; Thomson, A.; Klein, C.; Vallée-Tourangeau, G. Motors of influenza vaccination uptake and vaccination advocacy in healthcare workers: A comparative study in six European countries. Vaccine 2018, 36, 6546–6552. [Google Scholar] [CrossRef]
- Vigezzi, G.P.; Lume, A.; Minerva, M.; Nizzero, P.; Biancardi, A.; Gianfredi, V.; Odone, A.; Signorelli, C.; Moro, M. Safety surveillance after BNT162b2 mRNA COVID-19 vaccination: Results from a cross-sectional survey among staff of a large Italian teaching hospital. Acta Bio-Med. Atenei Parm. 2021, 92, e2021450. [Google Scholar] [CrossRef]
- Hrehova, L.; Seifert, B.; de Sire, A.; Mezian, K. Physicians perceptions of working in mass vaccination sites during COVID-19 pandemic. Bratisl. Lek. Listy 2022, 123, 470–474. [Google Scholar] [CrossRef]
- Nadler, J.T.; Weston, R.; Voyles, E.C. Stuck in the middle: The use and interpretation of mid-points in items on questionnaires. J. Gen. Psychol. 2015, 142, 71–89. [Google Scholar] [CrossRef]
- Goda, K.; Kenzaka, T.; Yahata, S.; Okayama, M.; Nishisaki, H. Association between Adverse Reactions to the First and Second Doses of COVID-19 Vaccine. Vaccines 2022, 10, 1232. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Son, N.H.; Park, Y.S.; Lee, J.H.; Kim, D.A.; Kim, Y.C. Effect of a hospital-wide campaign on COVID-19 vaccination uptake among healthcare workers in the context of raised concerns for life-threatening side effects. PLoS ONE 2021, 16, e0258236. [Google Scholar] [CrossRef]
- Mardiko, A.A.; Heinemann, S.; Bludau, A.; Kaba, H.E.J.; Leha, A.; von Maltzahn, N.; Mutters, N.T.; Leistner, R.; Mattner, F.; Scheithauer, S. COVID-19 vaccination strategy for hospital staff in Germany: A cross-sectional study in March-April 2021. J. Hosp. Infect. 2022, 126, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Robert Koch-Institut. KROCO-die Krankenhausbasierte Online-Befragung zur COVID-19-Impfung. Ergebnisbericht Erste Welle. Available online: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Projekte_RKI/KROCO.html (accessed on 16 July 2021).



| LMU University Hospital Staff Vaccinated at the In-Hospital Vaccination Centre | Vaccination Centre Staff ° | ||||||
|---|---|---|---|---|---|---|---|
| n | % | Satisfaction with Vaccination Process | AEFIs Following 1st Vaccine | AEFIs Following 2nd Vaccine | n | % | |
| Age * | p < 0.001 | p < 0.001 | p < 0.001 | ||||
| <29 years | 188 | 14.2 | 4 | 7.4 | |||
| 30–39 years | 297 | 22.5 | 9 | 16.7 | |||
| 40–59 years | 269 | 20.3 | 13 | 24.1 | |||
| 50–69 years | 367 | 27.8 | 21 | 38.9 | |||
| >60 years | 189 | 14.3 | 7 | 13.0 | |||
| No answer | 12 | 0.9 | 0 | - | |||
| Sex ** | p = 0.027 | p < 0.001 | p < 0.001 | ||||
| Male | 318 | 24.1 | 20 | 37.0 | |||
| Female | 1001 | 75.7 | 34 | 63.0 | |||
| Other | 3 | 0.2 | 0 | - | |||
| Education | p = 0.314 | p = 0.219 | p = 0.583 | ||||
| Secondary/elementary school | 31 | 2.3 | 1 | 1.9 | |||
| Middle school | 198 | 15.0 | 7 | 13.0 | |||
| High school/technical diploma | 222 | 16.8 | 9 | 16.7 | |||
| Vocational training | 278 | 21.0 | 2 | 3.7 | |||
| Academic degree (bachelor) | 94 | 7.1 | 1 | 1.9 | |||
| Academic degree (master’s/diploma) | 203 | 15.4 | 4 | 7.4 | |||
| Academic degree (doctorate or higher) | 274 | 20.7 | 30 | 55.6 | |||
| Other training | 21 | 1.6 | 0 | - | |||
| No diploma | 1 | 0.1 | 0 | - | |||
| Occupation (dichotomous) *** | p = 0.006 | p = 0.012 | p = 0.124 | ||||
| Medical staff | 784 | 59.3 | 31 | 57.4 | |||
| Non-medical staff | 538 | 40.7 | 23 | 42.6 | |||
| Work with COVID-19 patients **** | p = 0.916 | p = 0.123 | p = 0.699 | ||||
| Yes | 213 | 16.1 | 53 | 98.1 | |||
| No | 1109 | 83.9 | 1 | 1.9 | |||
| All | 1322 | 54° | |||||
| To What Extent Do You Agree with the following Statements? (In Absolute Numbers) | |||||
|---|---|---|---|---|---|
| General satisfaction α = 0.801 | Disagree | Rather disagree | Partly agree | Rather agree | Agree |
| The vaccination process at LMU Hospital was generally well organised. | 12 | 10 | 64 | 222 | 1014 |
| The registration and vaccination process were well organised. | 15 | 27 | 83 | 239 | 958 |
| The different stations in the vaccination centre were logically arranged. | 7 | 8 | 17 | 175 | 1115 |
| The vaccination appointment was easy to organise. | 26 | 38 | 107 | 246 | 905 |
| Satisfaction with the individual aspects of the vaccination process α = 0.808 | Disagree | Rather disagree | Partly agree | Rather agree | Agree |
| Prioritisation of departments to be vaccinated | 16 | 82 | 203 | 517 | 504 |
| Availability of the vaccine | 28 | 160 | 409 | 400 | 325 |
| Organisation of appointment booking | 13 | 53 | 116 | 438 | 702 |
| Scheduling of the administration of the second vaccination dose (availability of appointment options) | 9 | 29 | 97 | 328 | 859 |
| Process of registration at the vaccination centre | 5 | 32 | 107 | 417 | 761 |
| Possibility of a medical consultation at the vaccination centre | 7 | 21 | 209 | 352 | 733 |
| Preparation of the vaccine doses | 4 | 6 | 222 | 278 | 812 |
| Inoculation | 5 | 7 | 37 | 266 | 1007 |
| Follow-up after the inoculation | 6 | 43 | 306 | 434 | 533 |
| The vaccination process at LMU Hospital was generally well organised. (item used for testing of general satisfaction) | |||||
| To what extent do you agree with the following statements? | Disagree/rather disagree | Partly agree | Rather agree/agree (ref.) | ||
| Location α = 0.164 AIC = 77.531 BIC = 129.400 | n (RR; p-value) | n (RR; p-value) | n | ||
| The vaccination centre at the LMU hospital was easily accessible in terms of location | |||||
| Disagree/Rather disagree | 8 (9.542; 0.000) | 11 (5.519; 0.000) | 51 | ||
| Partly agree | 2 (1.478; 0.616) | 7 (1.492; 0.339) | 111 | ||
| Rather agree/agree (ref.) | 12 | 46 | 1074 | ||
| Even if it had taken me over 1 h to get there to receive the vaccine, I would still have taken the time to get there. | |||||
| Disagree/rather disagree | 7 (9.502; 0.000) | 0 | 38 | ||
| Partly agree | 1 (1.568; 0.669) | 5 (1.775; 0.241) | 58 | ||
| Rather agree/agree (ref.) | 14 | 59 | 1140 | ||
| Waiting time * α = 0.706AIC = 94.242 BIC = 166.699 | Disagree/rather disagree | Partly agree | Rather agree/agree (ref.) | ||
| How long was the waiting time from registration at the vaccination centre until you received the inoculation? | n (RR; p-value) | n (RR; p-value) | n | ||
| Less than 10 min | 5 (0.027; 0.000) | 34 (0.565; 0.473) | 696 | ||
| Between 10 and 20 min | 8 (0.100; 0.006) | 20 (0.453; 0.315) | 437 | ||
| Between 20 and 30 min | 2 (0.234; 0.112) | 6 (0.991; 0.991) | 65 | ||
| Over 30 min (ref.) | 6 | 3 | 29 | ||
| I cannot remember | 1 | 1 | 9 | ||
| How much time did you spend at the LMU Hospital vaccination centre in total? | |||||
| Less than 30 min | 8 (0.846; 0.856) | 23 (0.507; 0.411) | 570 | ||
| Between 30 and 45 min | 7 (0.427; 0.331) | 32 (0.806; 0.785) | 520 | ||
| Between 45 and 60 min | 1 (0.107; 0.054) | 6 (0.437; 0.316) | 119 | ||
| Over 1 h (ref.) | 5 | 3 | 23 | ||
| I cannot remember | 1 | 0 | 4 | ||
| Effect of the Observation of AEFIs on the General Satisfaction + | RR, p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Did you observe any adverse reactions after the first vaccination dose?—Yes (n = 676) | −0.479, 0.001 | ||||||||
| Did you observe any adverse reactions after the second vaccination dose?—Yes (n = 924) | −0.052, 0.745 | ||||||||
| AEFIs following 1st Vaccine * n = 687 | Intensity of adverse reaction | Age ° | Sex ** °° | Occupation (med vs. non-med) °° | |||||
| n | Not at all | Very mild | Mild | Strong | Very strong | Kendall Tau p-value | Cramér’s V p-value | Cramér’s V p-value | |
| Pain at the injection site | 591 | 21 | 96 | 185 | 186 | 103 | −0.090 p = 0.004 | 0.153 p = 0.008 | 0.099 p = 0.212 |
| Redness | 571 | 415 | 91 | 44 | 14 | 7 | −0.008 p = 0.413 | p = 0.178 ° | p = 0.163 ° |
| Haematoma | 566 | 509 | 25 | 16 | 12 | 4 | −0.010 p = 0.393 | p = 0.377 ° | p = 0.689 ° |
| Fatigue | 581 | 193 | 103 | 115 | 91 | 79 | 0.013 p = 0.347 | 0.130 p = 0.044 | 0.113 p = 0.115 |
| Flu-like symptoms (e.g., aching limbs, chills) | 568 | 392 | 74 | 42 | 25 | 35 | 0.021 p = 0.283 | 0.088 p = 0.357 | 0.039 p = 0.933 |
| Headache | 578 | 331 | 79 | 73 | 48 | 47 | −0.046 p = 0.094 | 0.105 p = 0.179 | p = 0.540 |
| Known migraine (triggering of an attack within 24 h) | 561 | 533 | 7 | 7 | 4 | 10 | −0.069 p = 0.035 | p = 1.000 ° | p = 0.878 ° |
| Known tension headache (triggering an attack within 24 h) | 556 | 521 | 13 | 8 | 5 | 9 | −0.015 p = 0.350 | p = 0.297 ° | p = 0.367 ° |
| Dizziness/balance problems | 568 | 468 | 45 | 29 | 15 | 11 | 0.026 p = 0.244 | p = 0.168 ° | 0.123 p = 0.071 |
| Circulatory weakness | 567 | 496 | 35 | 18 | 10 | 8 | −0.033 p = 0.192 | p = 0.246 ° | p = 0.949 ° |
| Fever ≥ 38 °C | 568 | 518 | 21 | 11 | 8 | 10 | −0.038 p = 0.153 | p = 0.060 | p = 0.065 ° |
| Nausea, vomiting | 569 | 523 | 22 | 14 | 5 | 5 | 0.058 p = 0.063 | p = 0.511 ° | p = 0.877 ° |
| Diarrhoea | 563 | 525 | 23 | 6 | 6 | 3 | 0.056 p = 0.068 | p = 0.789 ° | p = 0.501 ° |
| AEFIs following 2nd Vaccine * n = 935 | n | Not at all | Very mild | Mild | Strong | Very strong | Age | Sex ** ° | - |
| Pain at the injection site | 827 | 64 | 217 | 255 | 170 | 121 | −0.155 p < 0.001 | 0.142 p = 0.002 | - |
| Redness | 795 | 595 | 115 | 52 | 15 | 18 | 0.011 p = 0.361 | p = 0.137 ° | - |
| Haematoma | 786 | 714 | 37 | 16 | 11 | 9 | −0.014 p = 0.326 | p = 0.018 ° | - |
| Fatigue | 820 | 97 | 87 | 175 | 216 | 246 | −0.054 p = 0.029 | 0.129 p = 0.009 | - |
| Flu-like symptoms (e.g., aching limbs, chills) | 796 | 269 | 103 | 103 | 133 | 189 | −0.090 p = 0.001 | 0.131 p = 0.009 | - |
| Headache | 807 | 287 | 117 | 141 | 127 | 135 | −0.091 p = 0.001 | 0.192 p < 0.001 | - |
| Known migraine (triggering of an attack within 24 h) | 764 | 710 | 11 | 7 | 14 | 22 | −0.065 p = 0.022 | p = 0.629 ° | - |
| Known tension headache (triggering an attack within 24 h) | 769 | 670 | 20 | 14 | 35 | 30 | 0.002 p = 0.477 | p = 0.018 ° | - |
| Dizziness/balance problems | 792 | 582 | 71 | 55 | 51 | 33 | −0.047 p = 0.064 | 0.147 p = 0.002 | - |
| Circulatory weakness | 781 | 600 | 76 | 50 | 38 | 18 | −0.050 p = 0.055 | 0.133 p = 0.008 | - |
| Fever ≥ 38 °C | 778 | 538 | 54 | 57 | 58 | 71 | −0.064 p = 0.018 | 0.078 p = 0.312 | - |
| Nausea, vomiting | 794 | 671 | 47 | 35 | 25 | 16 | 0.032 p = 0.150 | p = 0.004 ° | - |
| Diarrhoea | 781 | 705 | 28 | 24 | 14 | 10 | 0.035 p = 0.134 | p = 0.448 ° | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhelyazkova, A.; Adorjan, K.; Kim, S.; Klein, M.; Prueckner, S.; Kressirer, P.; Choukér, A.; Coenen, M.; Horster, S. Are We Prepared for the Next Pandemic? Management, Systematic Evaluation and Lessons Learned from an In-Hospital COVID-19 Vaccination Centre for Healthcare Workers. Int. J. Environ. Res. Public Health 2022, 19, 16326. https://doi.org/10.3390/ijerph192316326
Zhelyazkova A, Adorjan K, Kim S, Klein M, Prueckner S, Kressirer P, Choukér A, Coenen M, Horster S. Are We Prepared for the Next Pandemic? Management, Systematic Evaluation and Lessons Learned from an In-Hospital COVID-19 Vaccination Centre for Healthcare Workers. International Journal of Environmental Research and Public Health. 2022; 19(23):16326. https://doi.org/10.3390/ijerph192316326
Chicago/Turabian StyleZhelyazkova, Ana, Kristina Adorjan, Selina Kim, Matthias Klein, Stephan Prueckner, Philipp Kressirer, Alexander Choukér, Michaela Coenen, and Sophia Horster. 2022. "Are We Prepared for the Next Pandemic? Management, Systematic Evaluation and Lessons Learned from an In-Hospital COVID-19 Vaccination Centre for Healthcare Workers" International Journal of Environmental Research and Public Health 19, no. 23: 16326. https://doi.org/10.3390/ijerph192316326
APA StyleZhelyazkova, A., Adorjan, K., Kim, S., Klein, M., Prueckner, S., Kressirer, P., Choukér, A., Coenen, M., & Horster, S. (2022). Are We Prepared for the Next Pandemic? Management, Systematic Evaluation and Lessons Learned from an In-Hospital COVID-19 Vaccination Centre for Healthcare Workers. International Journal of Environmental Research and Public Health, 19(23), 16326. https://doi.org/10.3390/ijerph192316326











