Psychophysiological Responses of Humans during Seed-Sowing Activity Using Soil Inoculated with Streptomyces rimosus
Abstract
1. Introduction
2. Participants and Methods
2.1. Participants
2.2. Experimental Environment
2.3. Preparation of the Soil Sample
2.4. Seed Sowing Activity
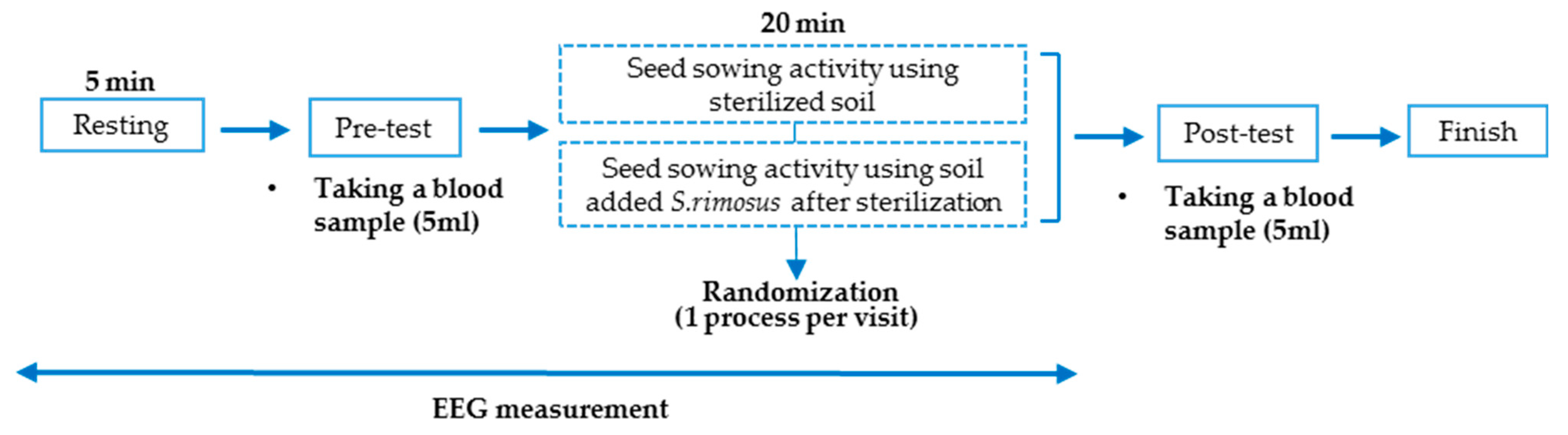
2.5. Experimental Protocol
2.6. Measurements
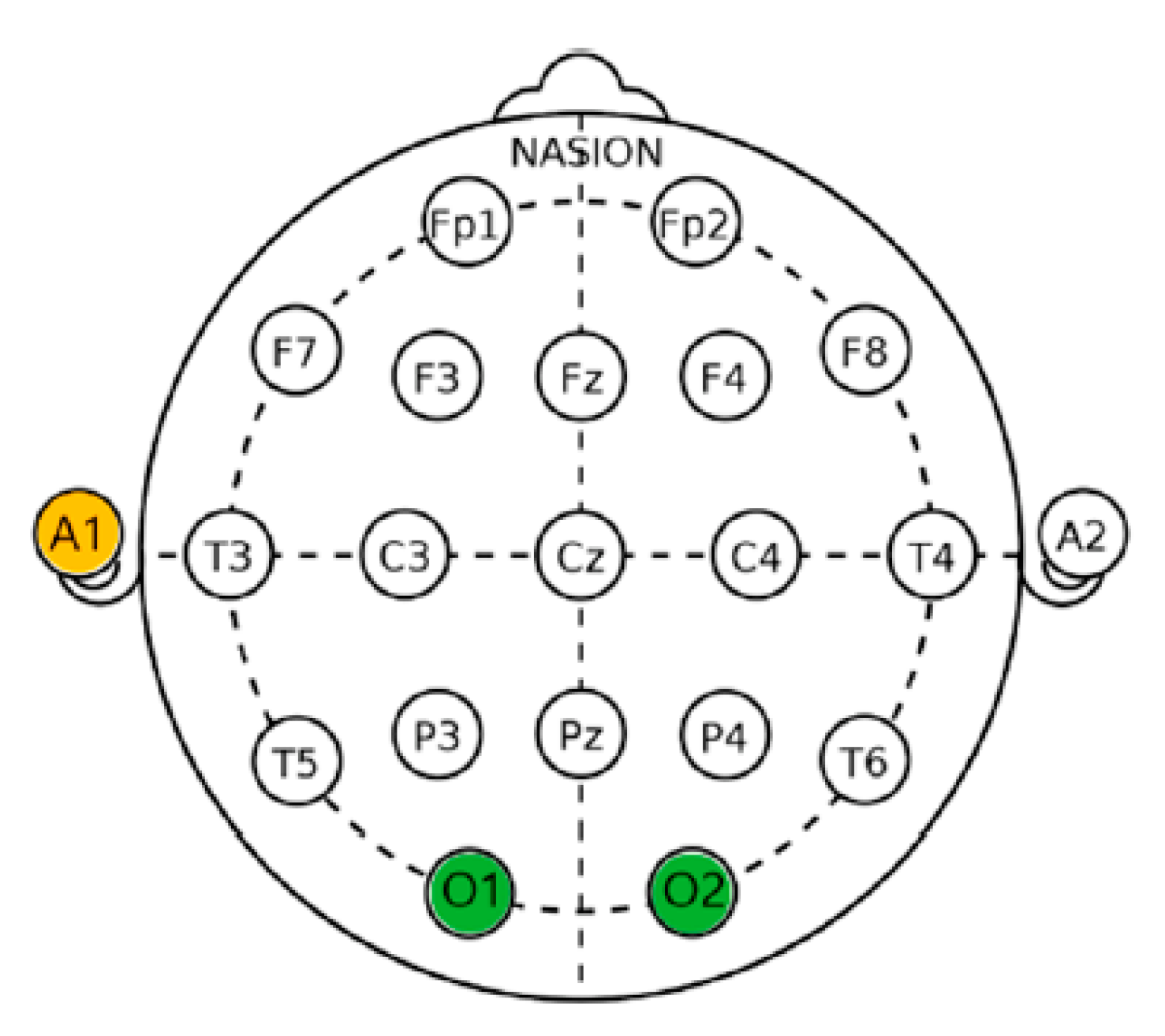
2.6.1. Electroencephalogram (EEG) Measurement
2.6.2. Measurement of Serum Metabolites
2.7. Data Analysis
2.7.1. EEG Data Analysis
2.7.2. Serum Metabolite Analysis
3. Results
3.1. Demographic Information
3.2. Results of EEG Responses
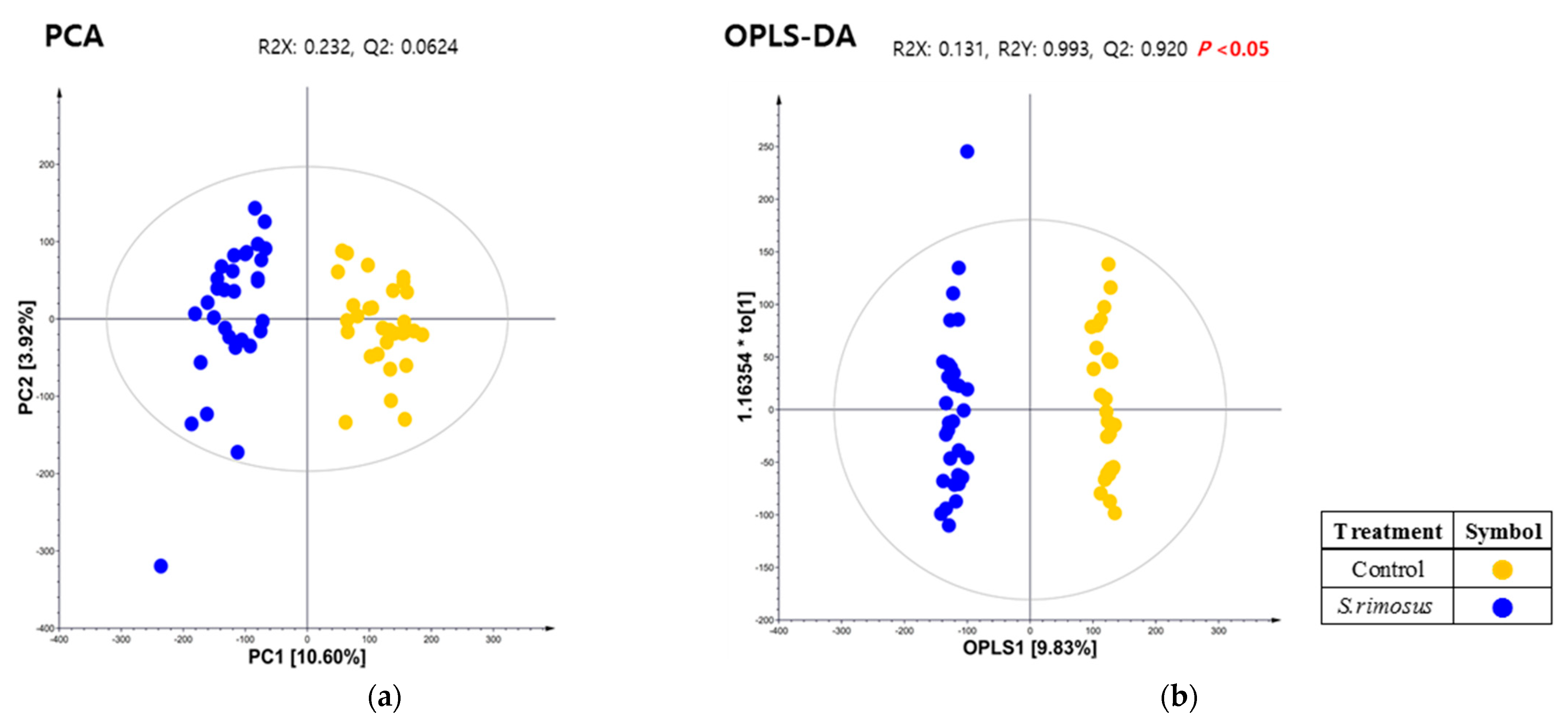
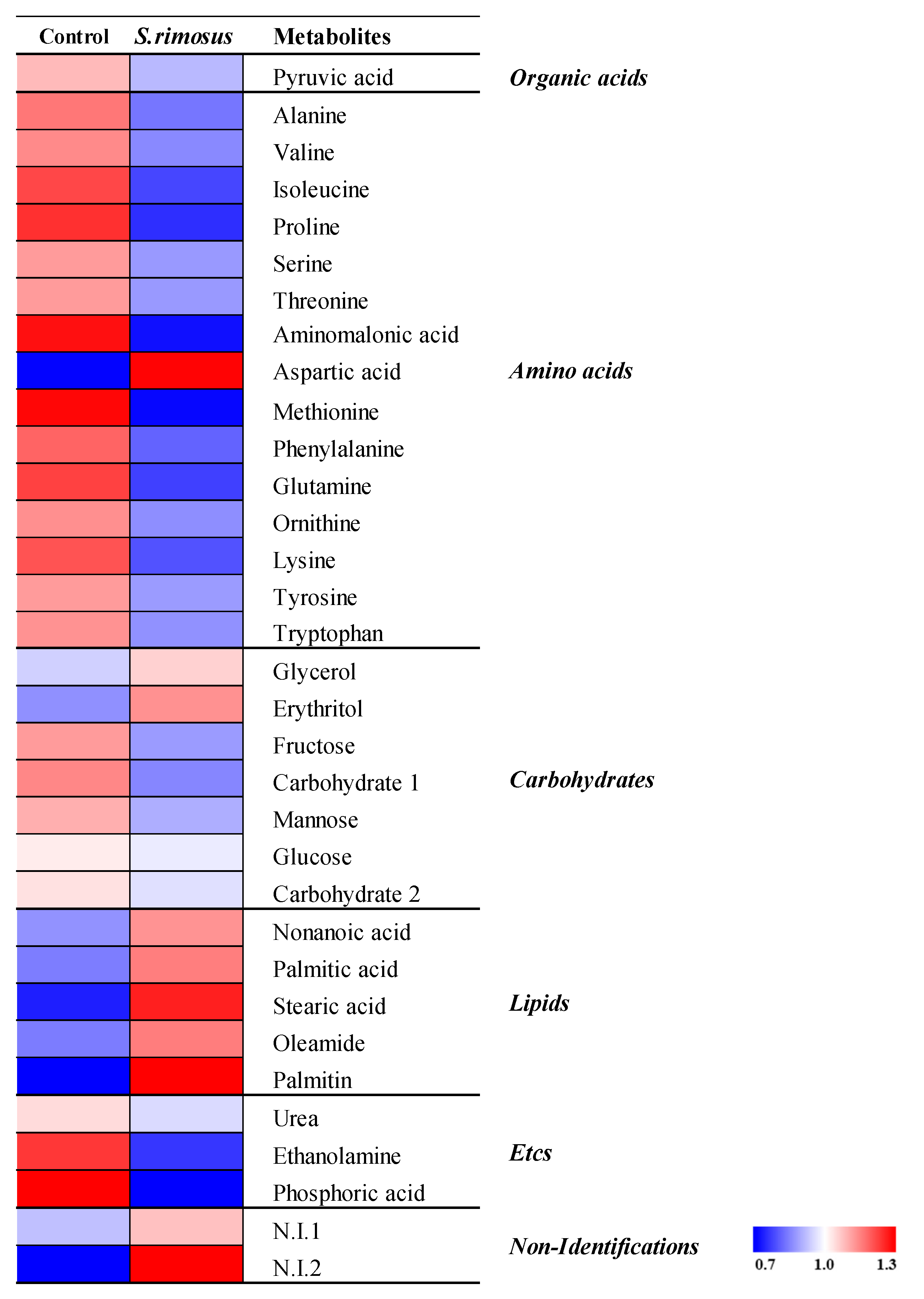
3.3. Results of Serum Metabolite
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ventriglio, A.; Torales, J.; Castaldelli-Maia, J.M.; De Berardis, D.; Bhugra, D. Urbanization and emerging mental health issues. CNS Spectr. 2021, 26, 43–50. [Google Scholar]
- Hanski, I.; von Hertzen, L.; Fyhrquist, N.; Koskinen, K.; Torppa, K.; Laatikainen, T.; Karisola, P.; Auvinen, P.; Paulin, L.; Mäkelä, M.J.; et al. Environmental biodiversity, human microbiota, and allergy are interrelated. Proc. Natl. Acad. Sci. USA 2012, 109, 8334–8339. [Google Scholar]
- Ruokolainen, L.; von Hertzen, L.; Fyhrquist, N.; Laatikainen, T.; Lehtomäki, J.; Auvinen, P.; Karvonen, A.M.; Hyvärinen, A.; Tillmann, V.; Niemelä, O.; et al. Green areas around homes reduce atopic sensitization in children. Allergy Eur. J. Allergy Clin. Immunol. 2015, 70, 195–202. [Google Scholar]
- Rook, G.A.W. Regulation of the immune system by biodiversity from the natural environment: An ecosystem service essential to health. Proc. Natl. Acad. Sci. USA 2013, 110, 18360–18367. [Google Scholar]
- Rook, G.A.W.; Raison, C.L.; Lowry, C.A. Microbiota, immunoregulatory old friends and psychiatric disorders. Adv. Exp. Med. Biol. 2014, 817, 319–356. [Google Scholar]
- Lowry, C.A.; Smith, D.G.; Siebler, P.H.; Schmidt, D.; Stamper, C.E.; Hassell, J.J.E.; Yamashita, P.S.; Fox, J.H.; Reber, S.O.; Brenner, L.A.; et al. The microbiota, immunoregulation, and mental health: Implications for public health. Curr. Environ. Health Rep. 2016, 3, 270–286. [Google Scholar]
- Turner, W.R.; Nakamura, T.; Dinetti, M. Global urbanization and the separation of humans from nature. BioScience 2004, 54, 585–590. [Google Scholar] [CrossRef]
- Daniel, R. The metagenomics of soil. Nat. Rev. Microbiol. 2005, 3, 470–478. [Google Scholar] [CrossRef]
- Blum, W.E.; Zechmeister-Boltenstern, S.; Keiblinger, K.M. Does soil contribute to the human gut microbiome? Microorganisms 2019, 7, 287. [Google Scholar]
- Roslund, M.I.; Puhakka, R.; Nurminen, N.; Oikarinen, S.; Siter, N.; Gronroos, M.; Cinek, O.; Kramna, L.; Jumpponen, A.; Laitinen, O.H.; et al. Long-term biodiversity intervention shapes health-associated commensal microbiota among urban day-care children. Environ. Int. 2021, 157, 106811. [Google Scholar]
- O’Brien, M.E.R.; Anderson, H.; Kaukel, E.; O’Byrne, K.; Pawlicki, M.; Von Pawel, J.; Reck, M. SRL172 (killed Mycobacterium vaccae) in addition to standard chemotherapy improves quality of life without affecting survival, in patients with advanced non-small-cell lung cancer: Phase III results. Ann. Oncol. 2004, 15, 906–914. [Google Scholar]
- Selway, C.A.; Mills, J.G.; Weinstein, P.; Skelly, C.; Yadav, S.; Lowe, A.; Breede, M.F.; Weyrich, L.S. Transfer of environmental microbes to the skin and respiratory tract of humans after urban green space exposure. Environ. Int. 2020, 145, 106084. [Google Scholar]
- Grenham, S.; Clarke, G.; Cryan, J.; Dinan, T. Brain-gut-microbe communication in health and disease. Front. Physiol. 2011, 2, 94. [Google Scholar]
- Lehrer, A.; Bressanelli, A.; Wachsmann, V.; Bottasso, O.; Bay, M.L.; Singh, M.; Stanford, C.; Stanford, J. Immunotherapy with Mycobacterium vaccae in the treatment of psoriasis. FEMS Immunol. Med. Microbiol. 1998, 21, 71–77. [Google Scholar]
- Kim, M.; Sowndhararajan, K.; Kim, T.; Kim, J.E.; Yang, J.E.; Kim, S. Gender differences in electroencephalographic activity in response to the earthy odorants geosmin and 2-methylisoborneol. Appl. Sci. 2017, 7, 876. [Google Scholar]
- Matthews, D.M.; Jenks, S.M. Ingestion of Myobacterium vaccae decreases anxiety-related behavior and improves learning in mice. Behav. Process. 2013, 96, 27–35. [Google Scholar]
- de Vasconcellos, R.L.F.; Cardoso, E.J.B.N. Rhizospheric streptomycetes as potential biocontrol agents of Fusarium and Armillaria pine rot and as PGPR for Pinus taeda. Biocontrol 2009, 54, 807–816. [Google Scholar]
- Nassar, A.H.; El-Tarabily, K.A.; Sivasithamparam, K. Growth promotion of bean (Phaseolus vulgaris L.) by a polyamine-producing isolate of Streptomyces griseoluteus. Plant Growth Regul. 2003, 40, 97–106. [Google Scholar]
- El-Tarabily, K.A. Promotion of tomato (Lycopersicon esculentum Mill.) plant growth by rhizosphere competent 1-aminocyclopropane-1-carboxylic acid deaminase-producing Streptomycete Actinomycetes. Plant Soil 2008, 308, 161–174. [Google Scholar]
- Dias, M.P.; Bastos, M.S.; Xavier, V.B.; Cassel, E.; Astarita, L.V.; Santarém, E.R. Plant growth and resistance promoted by Streptomyces spp. in tomato. Plant Physiol. Biochem. 2017, 118, 479–493. [Google Scholar]
- Qin, S.; Feng, W.W.; Wang, T.T.; Ding, P.; Xing, K.; Jiang, J.H. Plant growth-promoting effect and genomic analysis of the beneficial endophyte Streptomyces sp. KLBMP 5084 isolated from halophyte Limonium sinense. Plant Soil 2017, 416, 117–132. [Google Scholar]
- Berdy, J. Bioactive microbial metabolites. J. Antibiot. 2005, 58, 1–26. [Google Scholar]
- Kim, S.O.; Kim, M.J.; Choi, N.Y.; Kim, J.H.; Oh, M.S.; Lee, C.H.; Park, S.A. Psychophysiological and Metabolomics Responses of Adults during Horticultural Activities Using Soil Inoculated with Streptomyces rimosus: A Pilot Study. Int. J. Environ. Res. Public Health 2022, 19, 12901. [Google Scholar]
- Madigan, M.T. Brock Biology of Microorganisms, 11th ed.; Brock, T.D., Madigan, M.T., Martinko, J.M., Parker, J., Eds.; Prentice-Hall: Upper Saddle River, NJ, USA, 2005. [Google Scholar]
- Ham, K.J.; Park, K.Y.; Kim, M.S.; Song, J.M.; Lee, S.S.; Ok, Y.S. Research of monoterpenes content in the atmosphere of forest. Korean J. Soil Sci. Fertil. 2011, 44, 1126–1127. [Google Scholar]
- Shin, W.S.; Kim, S.K.; Yeon, P.S.; Lee, J.H. Effects phytoncides on psychophysical responses. J. Korean Inst. For. Recreat. 2010, 14, 85–89. [Google Scholar]
- Kim, S.O.; Son, S.Y.; Kim, M.J.; Lee, C.H.; Park, S.A. Physiological responses of adults during soil-mixing activities based on the presence of soil microorganisms: A metabolomics approach. J. Am. Soc. Hortic. Sci. 2022, 147, 135–144. [Google Scholar] [CrossRef]
- Tarkka, I.; Hallett, M. Cortical topography of premotor and motor potentials preceding self-paced, voluntary movement of dominant and non-dominant hands. Electroencephalogr. Clin. Neurophysiol. 1990, 75, 36–43. [Google Scholar] [CrossRef]
- Heckman, M.A.; Weil, J.; De Mejia, E.G. Caffeine (1, 3, 7-trimethylxanthine) in foods: A comprehensive review on consumption, functionality, safety, and regulatory matters. J. Food Sci. 2010, 75, R77–R87. [Google Scholar]
- Choi, N.-Y.; Wu, Y.-T.; Park, S.-A. Effects of Olfactory Stimulation with Aroma Oils on Psychophysiological Responses of Female Adults. Int. J. Environ. Res. Public Health 2022, 19, 5196. [Google Scholar] [CrossRef]
- Yang, Y.; Choi, H.R.; Cho, J.H.; Hong, S.C.; Kim, J.K. Clinical Feasibility of Scent Survey for Screening Test for Olfactory Function. J. Rhinol. 2018, 25, 14–20. [Google Scholar]
- Kim, S.O.; Oh, Y.A.; Park, S.A. Foliage plants improve concentration and emotional condition of elementary school students performing an intensive assignment. HortScience 2020, 55, 378–385. [Google Scholar] [CrossRef]
- Jasper, H.H. The ten-twenty electrode system of the International Federation. Electroencephalogr. Clin. Neurophysiol. 1958, 10, 370–495. [Google Scholar]
- Lorig, T.S. Human EEG and odor response. Prog. Neurobiol. 1989, 33, 387–398. [Google Scholar] [CrossRef]
- Park, S.A.; Son, S.Y.; Lee, A.Y.; Park, H.G.; Lee, W.L.; Lee, C.H. Metabolite profiling revealed that a gardening activity program improves cognitive ability correlated with BDNF levels and serotonin metabolism in the elderly. Int. J. Environ. Res. Public Health 2020, 17, 541. [Google Scholar]
- Yeon, B.R. Effect of Volatile Organic Chemicals in the Essential Oil of Cnidium Officinale Makino on Electroencephalogram. Master’s Thesis, Graduate School of Kangwon National University, Chuncheon, Republic of Korea, 2013. [Google Scholar]
- Cho, H.M. Effect of Essential Oil of Atractylodes Ovata (Thunb.) DC. Rhizome on Electroencephalographic Activity. Master’s Thesis, Graduate School of Kangwon National University, Chuncheon, Republic of Korea, 2014. [Google Scholar]
- Lee, C.Y. Comparison of changes in frontal EEG of women with anxiety disorder during clay activities of three kinds with different viscosity. J. Brain Educ. 2020, 25, 37–56. [Google Scholar]
- Lee, S.E. Effect of Volatile Fragrance Components of Citrus aurantiifolia and Eugenia caryophylla on electroencephalogram. Master’s Thesis, Graduate School of Kangwon University, Chuncheon, Republic of Korea, 2011. [Google Scholar]
- Antonelli, M.; Donelli, D.; Barbieri, G.; Valussi, M.; Maggini, V.; Firenzuoli, F. Forest volatile organic compounds and their effects on human health: A state-of-the-art review. Int. J. Environ. Res. Public Health 2020, 17, 6506. [Google Scholar] [CrossRef]
- Brevik, E.C.; Slaughter, L.; Singh, B.R.; Steffan, J.J.; Collier, D.; Barnhart, P.; Pereira, P. Soil and human health: Current status and future needs. Air Soil Water Res. 2020, 13, 1178622120934441. [Google Scholar] [CrossRef]
- Gerber, N.N.; Lechevalier, H.A. Geosmin, an earthly-smelling substance isolated from actinomycetes. Appl. Microbiol. 1965, 13, 935–938. [Google Scholar]
- Gerber, N.N. Geosmin, an earthy-smelling substance isolated from actinomycetes. Biotechnol. Bioeng. 1967, 9, 321–327. [Google Scholar]
- Gerber, N.N. A volatile metabolite of actinomycetes, 2-methylisoborneol. J. Antibiot. 1969, 22, 508–509. [Google Scholar]
- Gerber, N.N. Sesquiterpenoids from Actinomycetes: Cadin-4en-1-ol. Phytochemistry 1971, 10, 185–189. [Google Scholar]
- Gerber, N.N. Sesquiterpenoids from actinomycetes. Phytochemistry 1972, 11, 385–388. [Google Scholar]
- Gerber, N.N. Volatile lactones from Streptomyces. Tetrahedron Lett. 1973, 14, 771–774. [Google Scholar]
- Pollak, F.C.; Berger, R.G. Geosmin and related volatiles in bioreactor-cultured Streptomyces citreus CBS 109.60. Appl. Environ. Microbiol. 1996, 62, 1295–1299. [Google Scholar]
- Jáchymová, J.; Votruba, J.; Viden, I.; Řezanka, T. Identification of Streptomyces odor spectrum. Folia Microbiol. 2002, 47, 37–41. [Google Scholar]
- Wilkins, K.; Schöller, C. Volatile organic metabolites from selected Streptomyces strains. Actinomycetologica 2009, 23, 27–33. [Google Scholar]
- Schöller, C.E.; Gürtler, H.; Pedersen, R.; Molin, S.; Wilkins, K. Volatile metabolites from actinomycetes. J. Agric. Food Chem. 2002, 50, 2615–2621. [Google Scholar]
- Dickschat, J.S.; Wenzel, S.C.; Bode, H.B.; Müller, R.; Schulz, S. Biosynthesis of volatiles by the myxobacterium Myxococcus xanthus. ChemBioChem 2004, 5, 778–787. [Google Scholar]
- Dickschat, J.S.; Bode, H.B.; Wenzel, S.C.; Müller, R.; Schulz, S. Biosynthesis and identification of volatiles released by the myxobacterium Stigmatella aurantiaca. ChemBioChem 2005, 6, 2023–2033. [Google Scholar]
- Dickschat, J.S.; Martens, T.; Brinkhoff, T.; Simon, M.; Schulz, S. Volatiles released by a Streptomyces species isolated from the North Sea. Chem. Biodivers. 2005, 2, 837–865. [Google Scholar]
- Schulz, S.; Dickschat, J.S. Bacterial volatiles: The smell of small organisms. Nat. Prod. Rep. 2007, 24, 814–842. [Google Scholar]
- Rabe, P.; Citron, C.A.; Dickschat, J.S. Volatile terpenes from actinomycetes: A biosynthetic study correlating chemical analyses to genome data. ChemBioChem 2013, 14, 2345–2354. [Google Scholar]
- Gerber, N.N. Volatile substances from actinomycetes: Their role in the odor pollution of water. CRC Crit. Rev. Microbiol. 1979, 7, 191–214. [Google Scholar]
- Sowndhararajan, K.; Kim, S. Influence of fragrances on human psychophysiological activity: With special reference to human electroencephalographic response. Sci. Pharm. 2016, 84, 724–752. [Google Scholar] [CrossRef]
- Oliveira-Pinto, A.V.; Santos, R.M.; Coutinho, R.A.; Oliveira, L.M.; Santos, G.B.; Alho, A.T.; Leite, R.E.; Farfel, J.M.; Suemoto, C.K.; Grinberg, L.T.; et al. Sexual dimorphism in the human olfactory bulb: Females have more neurons and glial cells than males. PLoS ONE 2014, 9, e111733. [Google Scholar]
- Iijima, M.; Osawa, M.; Nishitani, N.; Iwata, M. Effects of incense on brain function: Evaluation using electroencephalograms and event–related potentials. Neuropsychobiology 2009, 59, 80–86. [Google Scholar] [CrossRef]
- Matsubara, E.; Fukagawa, M.; Okamoto, T.; Ohnuki, K.; Shimizu, K.; Kondo, R. The essential oil of Abies sibirica (Pinaceae) reduces arousal levels after visual display terminal work. Flavour Fragr. J. 2011, 26, 204–210. [Google Scholar]
- Jing, L.; Chengji, W. GC/MS-based metabolomics strategy to analyze the effect of exercise intervention in diabetic rats. Endocr. Connect. 2019, 8, 654–660. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, J.; Tang, G.; Cheng, K.; Yang, D.; Chen, G.; Liu, Z.; Zhang, R.; Zhou, J.; Fang, L.; Fang, Z.; et al. Peripheral blood mononuclear cell-based metabolomic profiling of a chronic unpredictable mild stress rat model of depression. Mol. BioSystems 2014, 10, 2994–3001. [Google Scholar]
- Fu, X.Y.; Lu, Y.R.; Wu, J.L.; Wu, X.Y.; Bao, A.M. Alterations of plasma aspartic acid, glycine and asparagine levels in patients with major depressive disorder. Zhejiang Xue Xue Bao Yi Xue Ban J. Zhejiang Univ. Med. Sci. 2012, 41, 132–138. [Google Scholar]


| Selection criteria |
|
| Exclusion criteria |
|
| Requirements of participation |
|
| Analysis Indicators | The Full Name of the EEG Power Spectrum Indicator | Wavelength Range (Hz) |
|---|---|---|
| RG | Relative power of gamma | (30–50)/(4–50) |
| RHB | Relative power of high beta | (20–30)/(4–50) |
| RST | Ratio of SMR to theta | (12–15)/(4–8) |
| RMT | Ratio of mid beta to theta | (15–20)/(4–8) |
| RSMT | Ratio of SMR—mid beta to theta | (12–20)/(4–8) |
| SEF50 | Spectral edge frequency 50% | 4–50 |
| SEF90 | Spectral edge frequency 90% | 4–50 |
| Variable | Male | Female | Total |
|---|---|---|---|
| Mean ± SD 1 | |||
| % (N) | 29.0 (9) | 71.0 (22) | 100 (31) |
| Age (years) | 27.13 ± 1.73 | 35.18 ± 11.39 | 32.66 ± 10.16 |
| Height (cm) | 172.93 ± 4.30 | 162.59 ± 5.42 | 165.94 ± 7.23 |
| Body weight (kg) | 75.31 ± 9.38 | 59.24 ± 8.39 | 64.27 ± 11.54 |
| Body mass index (kg·m−2) 2 | 25.12 ± 2.52 | 22.45 ± 3.24 | 23.25 ± 3.23 |
| SSS 3 | 76.0 ± 10.1 | 85.2 ± 10.1 | 82.2 ± 10.7 |
| VAS 4 | 7.5 ± 0.9 | 7.7 ± 1.2 | 7.6 ± 1.1 |
| EEG (O1) | RG 1 | RHB 2 | RST 3 | RMT 4 | RSMT 5 | SEF50 6 | SEF90 7 | |
| Mean ± SD 8 | ||||||||
| Total (N = 31) | Using sterilized soil (control) | 0.29 ± 0.07 | 0.18 ± 0.03 | 0.41 ± 0.12 | 0.57 ± 0.20 | 0.98 ± 0.32 | 18.61 ± 4.50 | 41.77 ± 3.21 |
| Using soil added S. rimosus after sterilization | 0.32 ± 0.05 | 0.19 ± 0.02 | 0.48 ± 0.12 | 0.68 ± 0.20 | 1.16 ± 0.31 | 20.53 ± 3.12 | 43.07 ± 1.19 | |
| Significance 9 | 0.062 NS | 0.058 NS | 0.046 * | 0.036 * | 0.038 * | 0.073 NS | 0.057 NS | |
| EEG (O2) | RG | RHB | RST | RMT | RSMT | SEF50 | SEF90 | |
| Mean ± SD | ||||||||
| Total (N = 31) | Using sterilized soil (control) | 0.28 ± 0.06 | 0.18 ± 0.03 | 0.41 ± 0.10 | 0.56 ± 0.17 | 0.97 ± 0.27 | 18.12 ± 3.81 | 41.76 ± 2.02 |
| Using soil added S. rimosus after sterilization | 0.31 ± 0.04 | 0.19 ± 0.02 | 0.49 ± 0.10 | 0.68 ± 0.17 | 1.17 ± 0.26 | 20.16 ± 2.82 | 42.84 ± 1.08 | |
| Significance | 0.024 * | 0.047 * | 0.003 ** | 0.003 ** | 0.003 ** | 0.026 * | 0.018 * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, N.-Y.; Park, S.-A.; Lee, Y.-R.; Lee, C.H. Psychophysiological Responses of Humans during Seed-Sowing Activity Using Soil Inoculated with Streptomyces rimosus. Int. J. Environ. Res. Public Health 2022, 19, 16275. https://doi.org/10.3390/ijerph192316275
Choi N-Y, Park S-A, Lee Y-R, Lee CH. Psychophysiological Responses of Humans during Seed-Sowing Activity Using Soil Inoculated with Streptomyces rimosus. International Journal of Environmental Research and Public Health. 2022; 19(23):16275. https://doi.org/10.3390/ijerph192316275
Chicago/Turabian StyleChoi, Na-Yoon, Sin-Ae Park, Ye-Rim Lee, and Choong Hwan Lee. 2022. "Psychophysiological Responses of Humans during Seed-Sowing Activity Using Soil Inoculated with Streptomyces rimosus" International Journal of Environmental Research and Public Health 19, no. 23: 16275. https://doi.org/10.3390/ijerph192316275
APA StyleChoi, N.-Y., Park, S.-A., Lee, Y.-R., & Lee, C. H. (2022). Psychophysiological Responses of Humans during Seed-Sowing Activity Using Soil Inoculated with Streptomyces rimosus. International Journal of Environmental Research and Public Health, 19(23), 16275. https://doi.org/10.3390/ijerph192316275









