Effects of Curcumin and Lactoferrin to Inhibit the Growth and Migration of Prostatic Cancer Cells
Abstract
1. Introduction
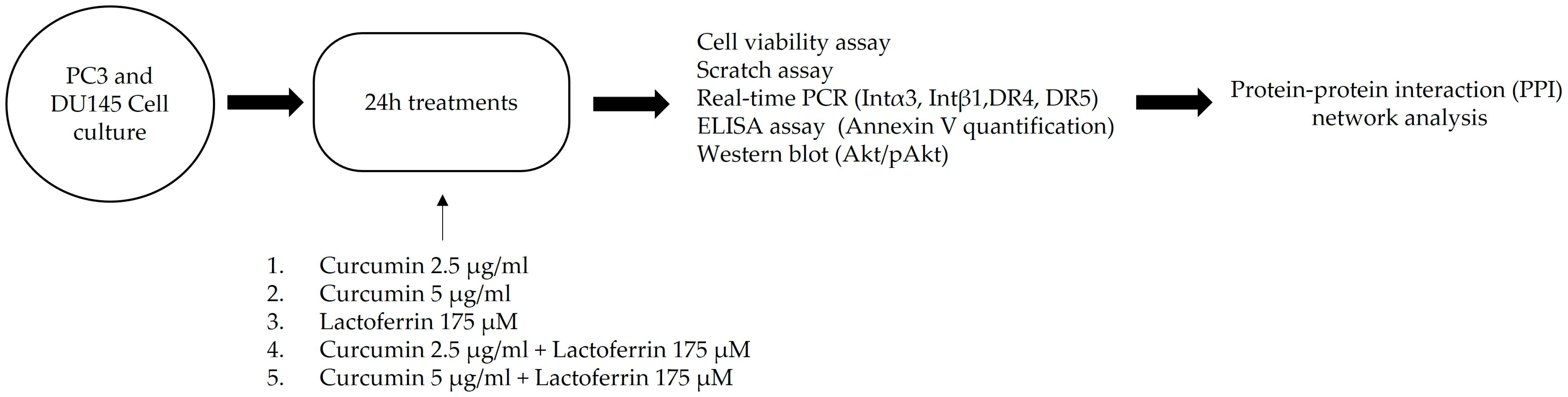
2. Materials and Methods
2.1. Reagents
2.2. Cells
2.3. Cell Viability Assay
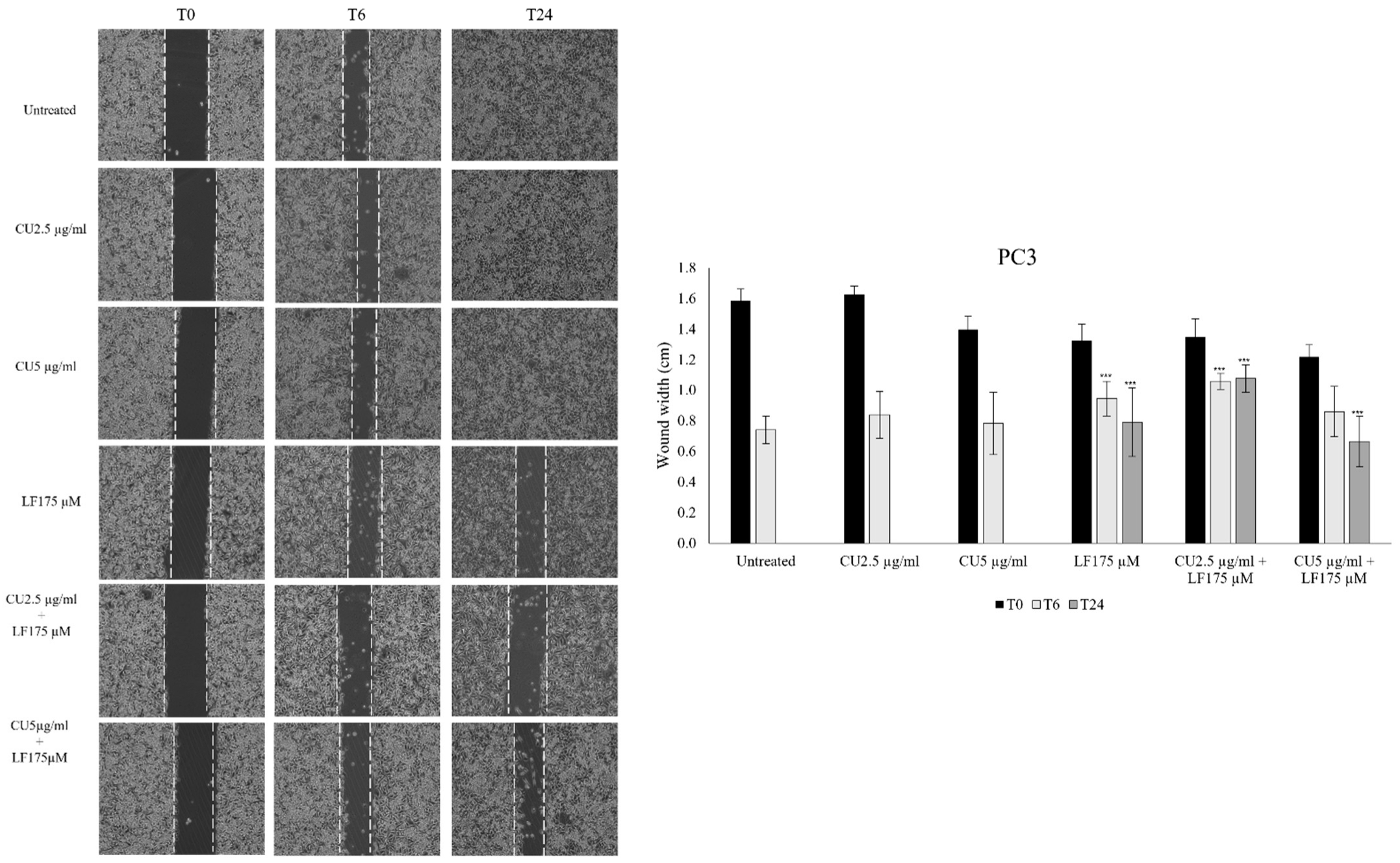
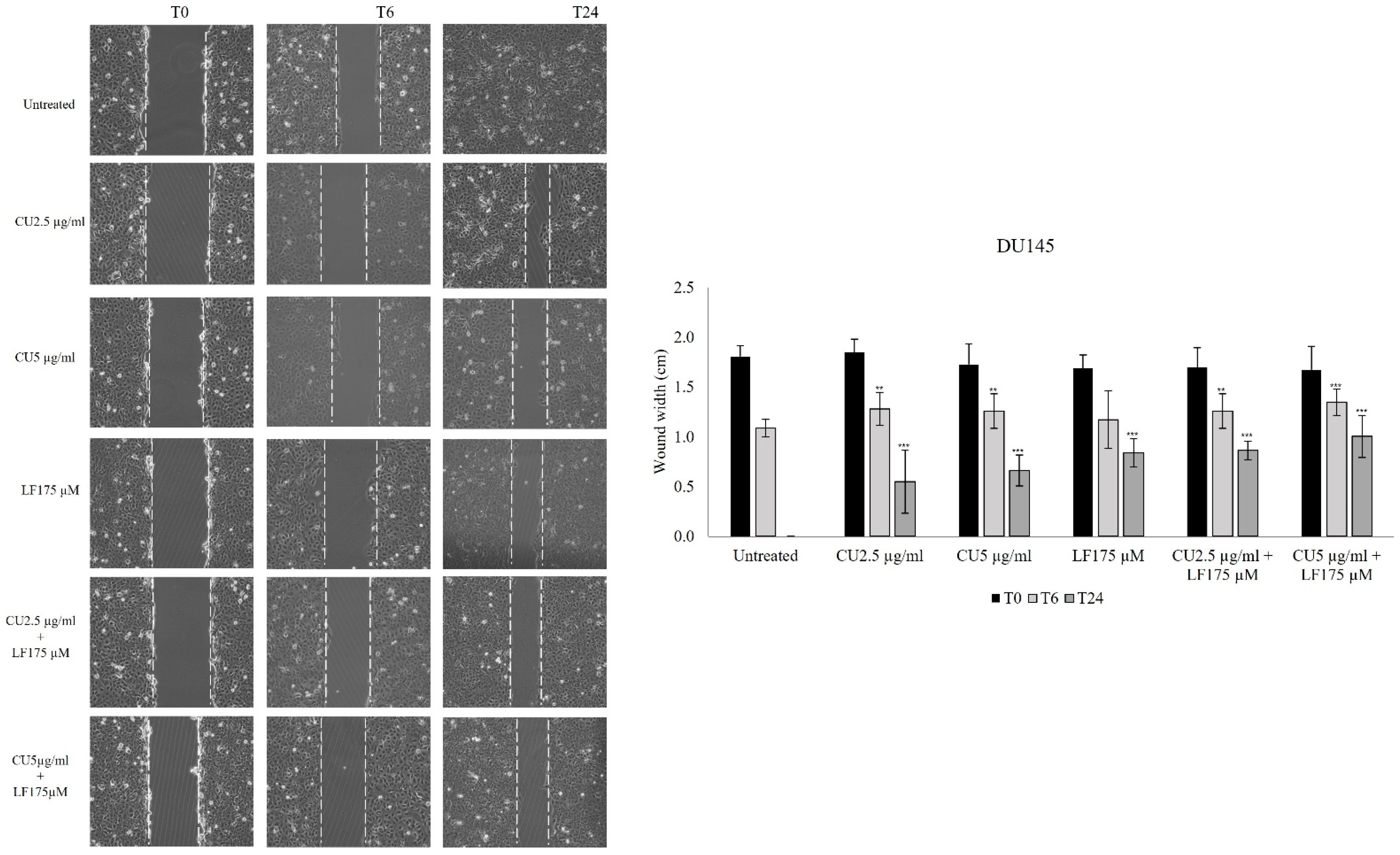
2.4. Scratch Assay
2.5. RNA Extraction, Purification, Retro-Transcription, and Real-Time PCR Analysis
2.6. Annexin V Detection
2.7. Western Blot
2.8. Statistical Analysis
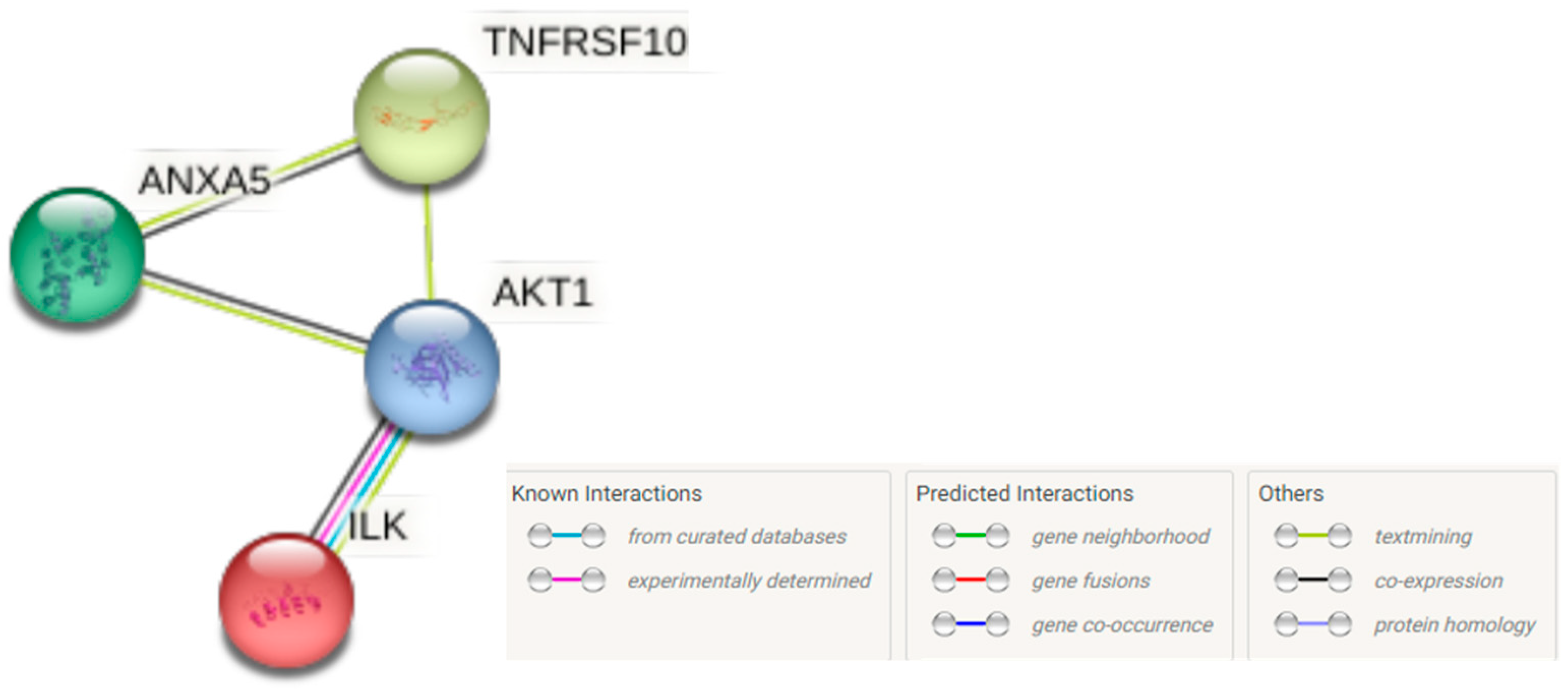
2.9. Protein-Protein Interaction (PPI) Network Analysis
3. Results
3.1. Effects of Curcumin and Lactoferrin on Cell Growth
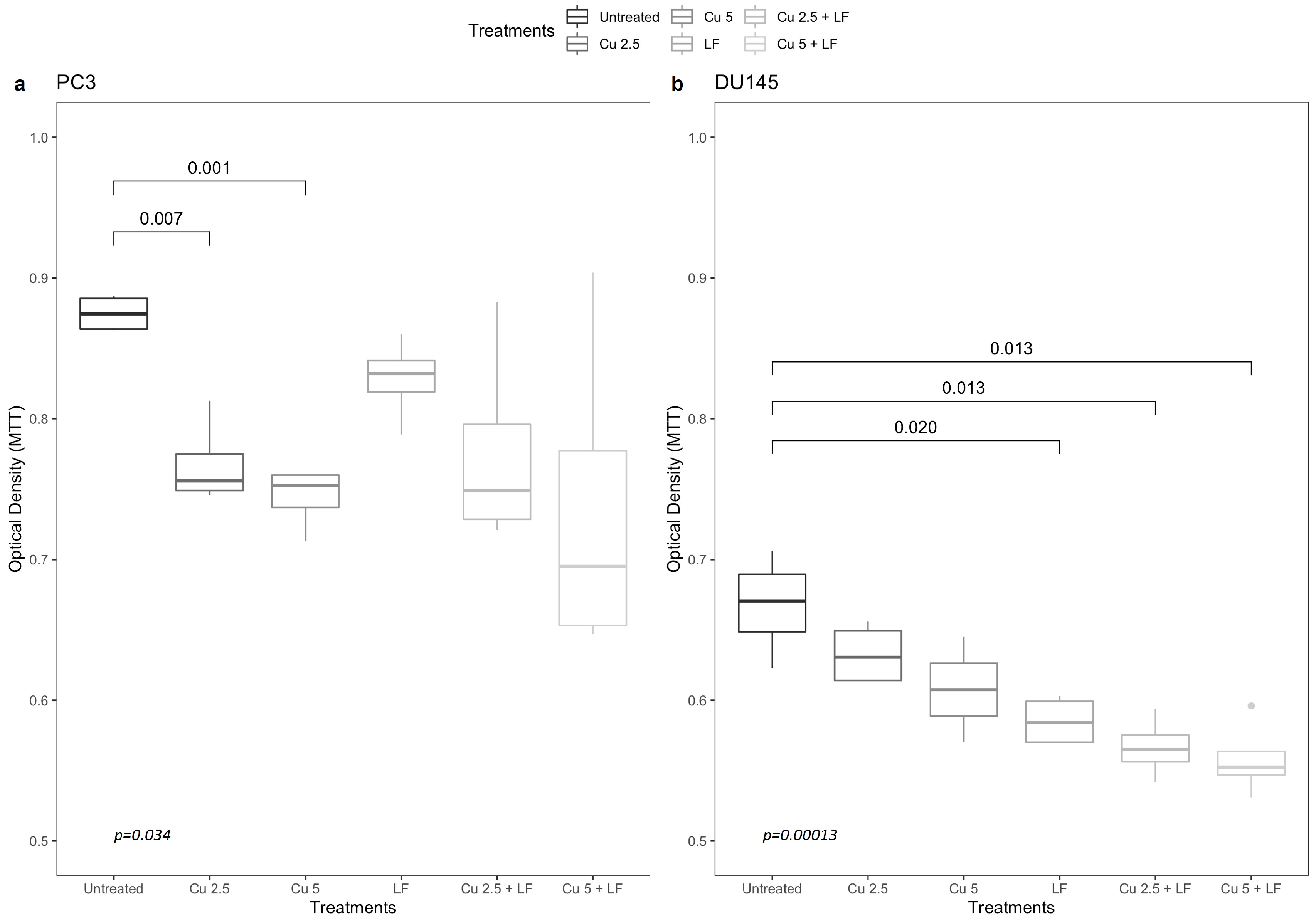
3.2. Migration Assay
3.3. Effect of CU 2.5 μg/mL, CU 5 μg/mL, and LF 175 μM on Integrin Gene Expression
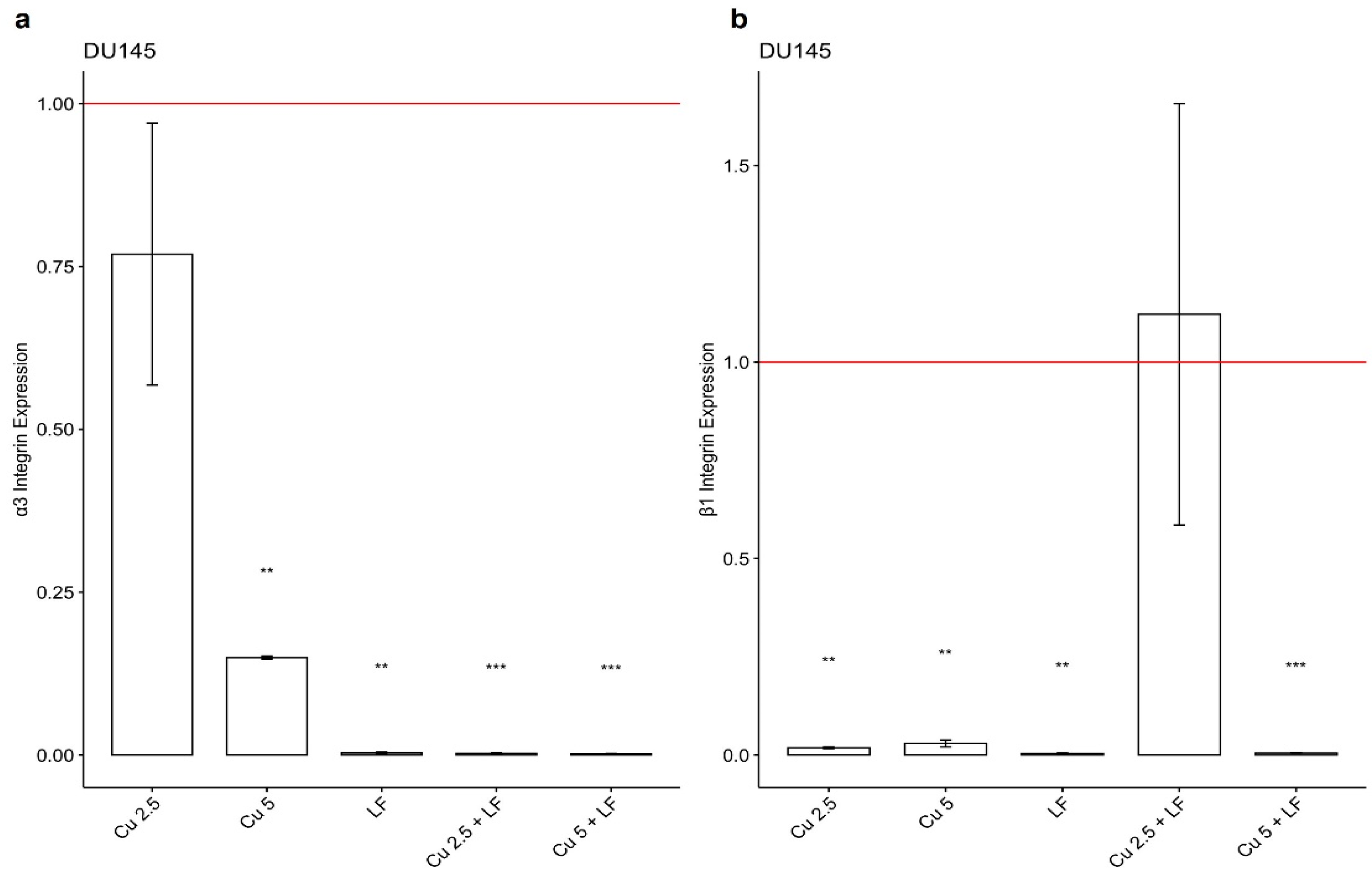
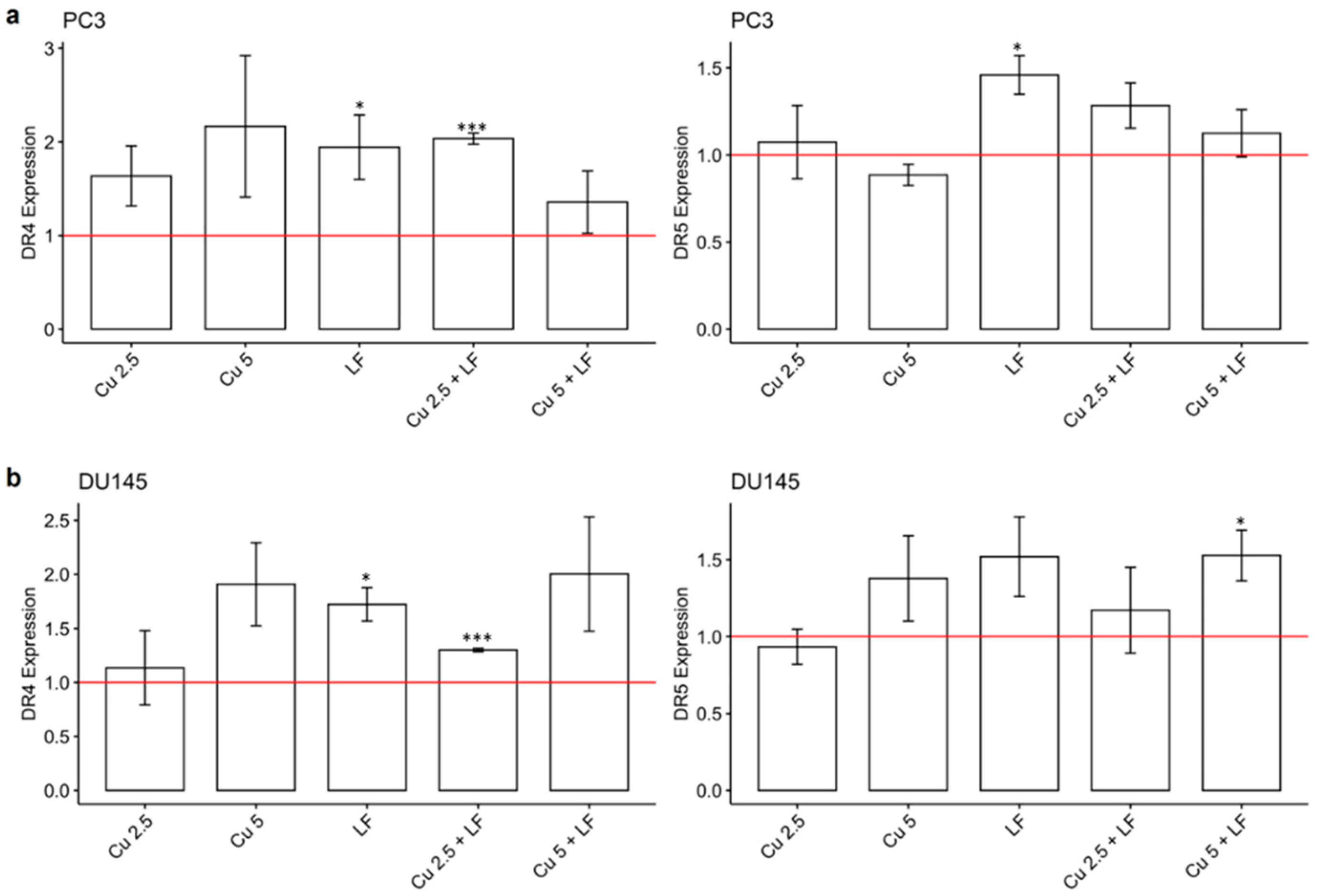
3.4. Effect of CU 2.5 μg/mL, CU 5 μg/mL, and LF 175 μM on DR4 and DR5 Gene Expression
3.5. Effect of Treatments on annexin V
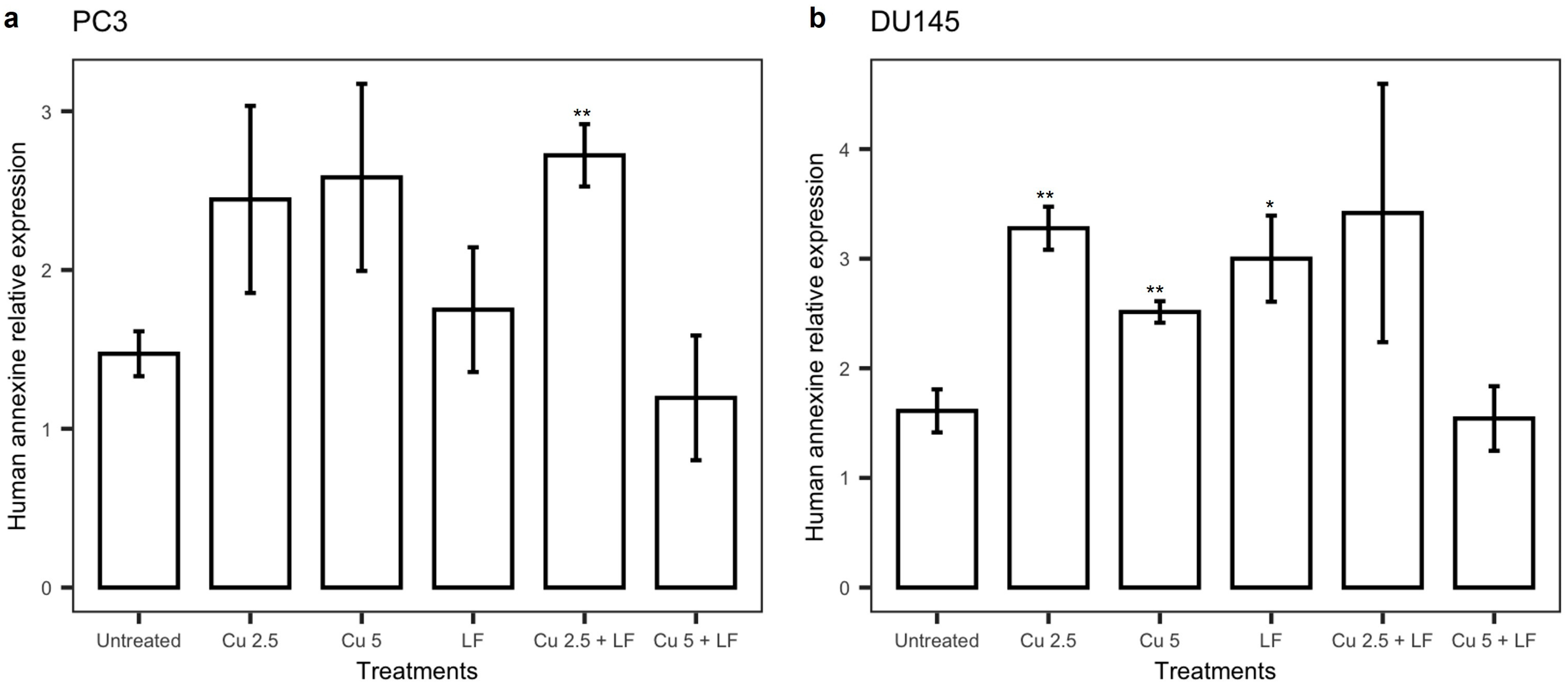
3.6. Effects of Treatments on Akt Pathway
3.7. The PPI Network
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics. Cancer J. Clin. 2013, 63, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Marchioni, M.; Di Nicola, M.; Primiceri, G.; Novara, G.; Castellan, P.; Paul, A.K.; Veccia, A.; Autorino, R.; Cindolo, L.; Schips, L. New Antiandrogen Compounds Compared to Docetaxel for Metastatic Hormone Sensitive Prostate Cancer: Results from a Network Meta-Analysis. J. Urol. 2020, 203, 751–759. [Google Scholar] [CrossRef]
- Abd Wahab, N.A.; Lajis, N.H.; Abas, F.; Othman, I.; Naidu, R. Mechanism of Anti-Cancer Activity of Curcumin on Androgen-Dependent and Androgen-Independent Prostate Cancer. Nutrients 2020, 12, 679. [Google Scholar] [CrossRef] [PubMed]
- Thomford, N.E.; Senthebane, D.A.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Natural Products for Drug Discovery in the 21st Century: Innovations for Novel Drug Discovery. Int. J. Mol. Sci. 2018, 19, 1578. [Google Scholar] [CrossRef]
- Costantini, E.; Jarlapoodi, S.; Serra, F.; Aielli, L.; Khan, H.; Belwal, T.; Falasca, K.; Reale, M. Neuroprotective Potential of Bacopa monnieri: Modulation of Inflammatory Signals. CNS Neurol. Disord.-Drug Targets 2022, 21. [Google Scholar] [CrossRef]
- Cianciosi, D.; Forbes-Hernandez, T.Y.; Ansary, J.; Gil, E.; Amici, A.; Bompadre, S.; Simal-Gandara, J.; Giampieri, F.; Battino, M. Phenolic compounds from Mediterranean foods as nutraceutical tools for the prevention of cancer: The effect of honey polyphenols on colorectal cancer stem-like cells from spheroids. Food Chem. 2020, 325, 126881. [Google Scholar] [CrossRef]
- Kratz, F.; Müller, I.A.; Ryppa, C.; Warnecke, A. Prodrug strategies in anticancer chemotherapy. ChemMedChem 2008, 3, 20–53. [Google Scholar] [CrossRef]
- Collins, I.; Workman, P. New approaches to molecular cancer therapeutics. Nat. Chem. Biol. 2006, 2, 689–700. [Google Scholar] [CrossRef]
- Urošević, M.; Nikolić, L.; Gajić, I.; Nikolić, V.; Dinić, A.; Miljković, V. Curcumin: Biological Activities and Modern Pharmaceutical Forms. Antibiotics 2022, 11, 135. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Alves, H.; Marques, V.; Durazzo, A.; Lucarini, M.; Alves, T.F.; Morsink, M.; Willemen, N.; Eder, P.; Chaud, M.V.; et al. Properties, Extraction Methods, and Delivery Systems for Curcumin as a Natural Source of Beneficial Health Effects. Medicina 2020, 56, 336. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Ying, Q.; Liu, H.; Yu, S.Q.; Bu, L.P.; Shao, L.; Li, X.Y. Curcumin enhances anti-cancer efficacy of either gemcitabine or docetaxel on pancreatic cancer cells. Oncol. Rep. 2020, 44, 1393–1402. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.S.; Acharya, A.; Ray, R.S.; Agrawal, R.; Raghuwanshi, R.; Jain, P. Cellular and molecular mechanisms of curcumin in prevention and treatment of disease. Crit. Rev. Food Sci. Nutr. 2020, 60, 887–939. [Google Scholar] [CrossRef] [PubMed]
- Belluti, S.; Orteca, G.; Semeghini, V.; Rigillo, G.; Parenti, F.; Ferrari, E.; Imbriano, C. Potent Anti-Cancer Properties of Phthalimide-Based Curcumin Derivatives on Prostate Tumor Cells. Int. J. Mol. Sci. 2018, 20, 28. [Google Scholar] [CrossRef]
- Kunwar, A.; Barik, A.; Mishra, B.; Rathinasamy, K.; Pandey, R.; Priyadarsini, K.I. Quantitative cellular uptake, localization and cytotoxicity of curcumin in normal and tumor cells. Biochim. Biophys. Acta 2008, 1780, 673–679. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Wang, D. Liposome-encapsulated curcumin suppresses growth of head and neck squamous cell carcinoma in vitro and in xenografts through the inhibition of nuclear factor kappa B by an AKT-independent pathway. Clin. Cancer Res. 2008, 14, 6228–6236. [Google Scholar] [CrossRef]
- Das, R.K.; Kasoju, N.; Bora, U. Encapsulation of curcumin in alginate-chitosan-pluronic composite nanoparticles for delivery to cancer cells. Nanomedicine 2010, 6, 153–160. [Google Scholar] [CrossRef]
- Gupta, V.; Aseh, A.; Ríos, C.N.; Aggarwal, B.B.; Mathur, A.B. Fabrication and characterization of silk fibroin derived curcumin nanoparticles for cancer therapy. Int. J. Nanomed. 2009, 4, 115–122. [Google Scholar] [CrossRef]
- Vogel, H.J. Lactoferrin, a bird’s eye view. Biochem. Cell Biol. 2012, 90, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Actor, J.K.; Hwang, S.A.; Kruzel, M.L. Lactoferrin as a natural immune modulator. Curr. Pharm. Des. 2009, 15, 1956–1973. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Matsuda, E.; Sekine, K.; Iigo, M.; Tsuda, H. Lactoferrin modifies apoptosis-related gene expression in the colon of the azoxymethane-treated rat. Cancer Lett. 2004, 213, 21–29. [Google Scholar] [CrossRef]
- Cutone, A.; Rosa, L.; Ianiro, G.; Lepanto, M.S.; Bonaccorsi di Patti, M.C.; Valenti, P.; Musci, G. Lactoferrin’s Anti-Cancer Properties: Safety, Selectivity, and Wide Range of Action. Biomolecules 2020, 10, 456. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.R.; Araújo, A.M.; Pinto, J.; Jerónimo, C.; Henrique, R.; Bastos, M.L.; Carvalho, M.; Guedes de Pinho, P. Discrimination between the human prostate normal and cancer cell exometabolome by GC-MS. Sci. Rep. 2018, 8, 5539. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, S.; Kunwar, A.; Sandur, S.K.; Pandey, B.N.; Chaubey, R.C. Differential response of DU145 and PC3 prostate cancer cells to ionizing radiation: Role of reactive oxygen species, GSH and Nrf2 in radiosensitivity. Biochim. Biophys. Acta 2014, 1840, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Grada, A.; Otero-Vinas, M.; Prieto-Castrillo, F.; Obagi, Z.; Falanga, V. Research techniques made simple: Analysis of collective cell migration using the wound healing assay. J. Investig. Dermatol. 2017, 137, e11–e16. [Google Scholar] [CrossRef]
- Rao, X.; Huang, X.; Zhou, Z.; Lin, X. An improvement of the 2ˆ(-delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat. Bioinform. Biomath. 2013, 3, 71–85. [Google Scholar]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef]
- Nevedomskaya, E.; Baumgart, S.J.; Haendler, B. Recent Advances in Prostate Cancer Treatment and Drug Discovery. Int. J. Mol. Sci. 2018, 19, 1359. [Google Scholar] [CrossRef]
- Wade, C.A.; Kyprianou, N. Profiling Prostate Cancer Therapeutic Resistance. Int. J. Mol. Sci. 2018, 19, 904. [Google Scholar] [CrossRef] [PubMed]
- Rakauskas, A.; Marra, G.; Heidegger, I.; Kasivisvanathan, V.; Kretschmer, A.; Zattoni, F.; Preisser, F.; Tilki, D.; Tsaur, I.; van den Bergh, R.; et al. Focal Therapy for Prostate Cancer: Complications and Their Treatment. Front. Surg. 2021, 8, 696242. [Google Scholar] [CrossRef] [PubMed]
- Pucci, C.; Martinelli, C.; Ciofani, G. Innovative approaches for cancer treatment: Current perspectives and new challenges. Ecancermedicalscience 2019, 13, 961. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, C.; Zhang, Z.; Chen, X.; Jia, Y.; Wang, B.; Kong, T. Curcumin inhibits the survival and metastasis of prostate cancer cells via the Notch-1 signaling pathway. APMIS 2017, 125, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Kumar, A.; Bharti, A.C. Anticancer potential of curcumin: Preclinical and clinical studies. Anticancer Res. 2003, 23, 363–398. [Google Scholar] [PubMed]
- Bashang, H.; Tamma, S. The use of curcumin as an effective adjuvant to cancer therapy: A short review. Biotechnol. Appl. Biochem. 2020, 67, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Rutz, J.; Benchellal, A.; Kassabra, W.; Maxeiner, S.; Bernd, A.; Kippenberger, S.; Zöller, N.; Chun, F.K.; Juengel, E.; Blaheta, R.A. Growth, Proliferation and Metastasis of Prostate Cancer Cells Is Blocked by Low-Dose Curcumin in Combination with Light Irradiation. Int. J. Mol. Sci. 2021, 22, 9966. [Google Scholar] [CrossRef] [PubMed]
- Iyer, A.K.; Khaled, G.; Fang, J.; Maeda, H. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov. Today 2006, 11, 812–818. [Google Scholar] [CrossRef]
- Chaharband, F.; Kamalinia, G.; Atyabi, F.; Mortazavi, S.A.; Mirzaie, Z.H.; Dinarvand, R. Formulation and in vitro evaluation of curcumin-lactoferrin conjugated nanostructures for cancerous cells. Artif. Cells Nanomed. Biotechnol. 2018, 46, 626–636. [Google Scholar] [CrossRef]
- Altwaijry, N.; Somani, S.; Parkinson, J.A.; Tate, R.J.; Keating, P.; Warzecha, M.; Mackenzie, G.R.; Leung, H.Y.; Dufès, C. Regression of prostate tumors after intravenous administration of lactoferrin-bearing polypropylenimine dendriplexes encoding TNF-α, TRAIL, and interleukin-12. Drug Deliv. 2018, 25, 679–689. [Google Scholar] [CrossRef]
- Olszewska, P.; Pazdrak, B.; Kruzel, M.L. A Novel Human Recombinant Lactoferrin Inhibits Lung Adenocarcinoma Cell Growth and Migration with No Cytotoxic Effect on Normal Human Epithelial Cells. Arch. Immunol. Ther. Exp. 2021, 69, 33. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Yoon, S.Y.; Park, S.H.; Hwang, J.H. Anti-Migration and Anti-Invasion Effects of Curcumin via Suppression of Fascin Expression in Glioblastoma Cells. Brain Tumor Res. Treat. 2019, 7, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Sha, J.; Lin, W.; Wang, Y.; Bian, T.; Guo, J. Curcumin inhibits prostate cancer progression by regulating the miR-30a-5p/PCLAF axis. Exp. Ther. Med. 2021, 22, 969. [Google Scholar] [CrossRef] [PubMed]
- Desgrosellier, J.S.; Cheresh, D.A. Integrins in cancer: Biological implications and therapeutic opportunities. Nat. Rev. Cancer 2010, 10, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Atsumi, T.; Murakami, Y.; Shibuya, K.; Tonosaki, K.; Fujisawa, S. Induction of cytotoxicity and apoptosis and inhibition of cyclooxygenase-2 gene expression, by curcumin and its analog, alpha-diisoeugenol. Anticancer. Res. 2005, 25, 4029–4036. [Google Scholar] [PubMed]
- Chan, W.H.; Wu, H.Y.; Chang, W.H. Dosage effects of curcumin on cell death types in a human osteoblast cell line. Food Chem. Toxicol. 2006, 44, 1362–1371. [Google Scholar] [CrossRef]
- Sidhu, G.S.; Mani, H.; Gaddipati, J.P.; Singh, A.K.; Seth, P.; Banaudha, K.K.; Patnaik, G.K.; Maheshwari, R.K. Curcumin enhances wound healing in streptozotocin induced diabetic rats and genetically diabetic mice. Wound Repair Regen. 1999, 7, 362–374. [Google Scholar] [CrossRef]
- Iglesias-Figueroa, B.F.; Siqueiros-Cendón, T.S.; Gutierrez, D.A.; Aguilera, R.J.; Espinoza-Sánchez, E.A.; Arévalo-Gallegos, S.; Varela-Ramirez, A.; Rascón-Cruz, Q. Recombinant human lactoferrin induces apoptosis, disruption of F-actin structure and cell cycle arrest with selective cytotoxicity on human triple negative breast cancer cells. Apoptosis 2019, 24, 562–577. [Google Scholar] [CrossRef]
- Zhong, H.H.; Wang, H.Y.; Li, J.; Huang, Y.Z. TRAIL-based gene delivery and therapeutic strategies. Acta Pharmacol. Sin. 2021, 42, 843. [Google Scholar] [CrossRef]
- Yoshida, T.; Yamasaki, K.; Tadagaki, K.; Kuwahara, Y.; Matsumoto, A.; Sofovic, A.E.; Kondo, N.; Sakai, T.; Okuda, T. Tumor necrosis factor-related apoptosis-inducing ligand is a novel transcriptional target of runt-related transcription factor 1. Int. J. Oncol. 2022, 60, 6. [Google Scholar] [CrossRef]
- Dai, X.; Zhang, J.; Arfuso, F.; Chinnathambi, A.; Zayed, M.E.; Alharbi, S.A.; Kumar, A.P.; Ahn, K.S.; Sethi, G. Targeting TNF-related apoptosis-inducing ligand (TRAIL) receptor by natural products as a potential therapeutic approach for cancer therapy. Exp. Biol. Med. 2015, 240, 760–773. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Ma, X.; Wang, Z.; Zeng, X.; Hu, Z.; Ye, Z.; Shen, G. Curcumin induces apoptosis and protective autophagy in castration-resistant prostate cancer cells through iron chelation. Drug Des. Devel. Ther. 2017, 11, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Guedes, J.P.; Pereira, C.S.; Rodrigues, L.R.; Côrte-Real, M. Bovine Milk Lactoferrin Selectively Kills Highly Metastatic Prostate Cancer PC-3 and Osteosarcoma MG-63 Cells In Vitro. Front. Oncol. 2018, 8, 200. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costantini, E.; Di Nicola, M.; Marchioni, M.; Aielli, L.; Reale, M.; Schips, L. Effects of Curcumin and Lactoferrin to Inhibit the Growth and Migration of Prostatic Cancer Cells. Int. J. Environ. Res. Public Health 2022, 19, 16193. https://doi.org/10.3390/ijerph192316193
Costantini E, Di Nicola M, Marchioni M, Aielli L, Reale M, Schips L. Effects of Curcumin and Lactoferrin to Inhibit the Growth and Migration of Prostatic Cancer Cells. International Journal of Environmental Research and Public Health. 2022; 19(23):16193. https://doi.org/10.3390/ijerph192316193
Chicago/Turabian StyleCostantini, Erica, Marta Di Nicola, Michele Marchioni, Lisa Aielli, Marcella Reale, and Luigi Schips. 2022. "Effects of Curcumin and Lactoferrin to Inhibit the Growth and Migration of Prostatic Cancer Cells" International Journal of Environmental Research and Public Health 19, no. 23: 16193. https://doi.org/10.3390/ijerph192316193
APA StyleCostantini, E., Di Nicola, M., Marchioni, M., Aielli, L., Reale, M., & Schips, L. (2022). Effects of Curcumin and Lactoferrin to Inhibit the Growth and Migration of Prostatic Cancer Cells. International Journal of Environmental Research and Public Health, 19(23), 16193. https://doi.org/10.3390/ijerph192316193







