Inhibitory Effects of Antipsychotic Chlorpromazine on the Survival, Reproduction and Population Growth Other Than Neurotransmitters of Zooplankton in Light of Global Warming
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Experimental Chemicals
2.3. Acute Toxicity Experiment
2.4. Determination of DA Concentration
2.5. Life Table Experiment
2.6. Population Growth Experiment
2.7. Statistical Analysis
3. Results
3.1. Acute Toxicity Test
3.2. Changes in DAC
3.3. Life Table Experiment
3.4. Population Growth Experiment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UNODC. World drug Report 2014. New York; United Nations Publication: Vienna, Austria, 2014. [Google Scholar]
- Zuccato, E.; Chiabrando, C.; Castiglioni, S.; Bagnati, R.; Fanelli, R. Estimating community drug abuse by wastewater analysis. Environ. Health Perspect. 2008, 116, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Postigo, C.; Lopez de Alda, M.J.; Barceló, D. Fully automated determination in the Low Nanogram per liter level of different classes of drugs of abuse in sewage water by On-Line Solid-Phase extraction-liquid chromatography-electrospray-tandem mass spectrometry. Anal. Chem. 2008, 80, 3123–3134. [Google Scholar] [CrossRef] [PubMed]
- Evgenidou, E.N.; Konstantinou, I.K.; Lambropoulou, D.A. Occurrence and removal of transformation products of PPCPs and illicit drugs in wastewaters: A review. Sci. Total Environ. 2015, 505, 905–926. [Google Scholar] [CrossRef] [PubMed]
- Boleda, M.R.; Galceran, M.T.; Ventura, F. Monitoring of opiates, cannabinoids and their metabolites in wastewater, surface water and finished water in Catalonia, Spain. Water Res. 2009, 43, 1126–1136. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.E.; Awad, G.A.; Rathbone, J.; Thornley, B.; Soares-Weiser, K. Chlorpromazine versus placebo for schizophrenia. Cochrane Database Syst. Rev. 2014, 1. [Google Scholar] [CrossRef]
- Bryan, J. How chlorpromazine improved the treatment of schizophrenic patients. Pharm. J. 2011, 286, 249–250. [Google Scholar]
- Dyall, J.; Coleman, C.M.; Hart, B.J.; Venkataraman, T.; Holbrook, M.R.; Kindrachuk, J.; Johnson, R.F.; Olinger, G.G.; Jahrling, P.B.; Laidlaw, M.; et al. Repurposing of clinically developed drugs for treatment of Middle East respiratory syndrome coronavirus infection. Antimicrob. Agents Chemother. 2014, 58, 4885–4893. [Google Scholar] [CrossRef]
- de Wilde, A.H.; Jochmans, D.; Posthuma, C.C.; Zevenhoven-Dobbe, J.C.; van Nieuwkoop, S.; Bestebroer, T.M.; van den Hoogen, B.G.; Neyts, J.; Snijder, E.J. Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrob. Agents Chemother. 2014, 58, 4875–4884. [Google Scholar] [CrossRef]
- Plaze, M.; Attali, D.; Petit, A.-C.; Blatzer, M.; Simon-Loriere, E.; Vinckier, F.; Cachia, A.; Chrétien, F.; Gaillard, R. Repurposing of chlorpromazine in COVID-19 treatment: The reCoVery study. Encephale 2020, 46, S35–S39. [Google Scholar] [CrossRef]
- de Alkimin, G.D.; Nunes, B.; Soares, A.M.V.M.; Bellot, M.; Gómez-Canela, C.; Barata, C. Daphnia magna responses to fish kairomone and chlorpromazine exposures. Chem. Biol. Interact. 2020, 325, 109123. [Google Scholar] [CrossRef]
- Oliveira, L.L.D.; Antunes, S.C.; Gonçalves, F.; Rocha, O.; Nunes, B. Evaluation of ecotoxicological effects of drugs on Daphnia magna using different enzymatic biomarkers. Ecotoxicol. Environ. Saf. 2015, 119, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Alkimin, G.D.; Daniel, D.; Frankenbach, S.; Serôdio, J.; Soares, A.M.V.M.; Barata, C.; Nunes, B. Evaluation of pharmaceutical toxic effects of non-standard endpoints on the macrophyte species Lemna minor and Lemna gibba. Sci. Total Environ. 2019, 657, 926–937. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhou, Q.; Zhang, N.; Luo, Y. Toxic effects of chlorpromazine on Carassius auratus and its oxidative stress. J. Environ. Sci. Health Part B 2008, 43, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Roberts, P.H.; Bersuder, P. Analysis of OSPAR priority pharmaceuticals using high-performance liquid chromatography-electrospray ionisation tandem mass spectrometry. J. Chromatogr. A 2006, 1134, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Jiang, X.; Xia, X.; Zhang, H.; Zheng, S. Detection, occurrence and fate of 22 psychiatric pharmaceuticals in psychiatric hospital and municipal wastewater treatment plants in Beijing, China. Chemosphere 2013, 90, 2520–2525. [Google Scholar] [CrossRef] [PubMed]
- Trautwein, C.; Kümmerer, K. Degradation of the tricyclic antipsychotic drug chlorpromazine under environmental conditions, identification of its main aquatic biotic and abiotic transformation products by LC-MSn and their effects on environmental bacteria. J. Chromatogr. B 2012, 889, 24–38. [Google Scholar] [CrossRef]
- Berry, H.L.; Bowen, K.; Kjellstrom, T. Climate change and mental health: A causal pathways framework. Int. J. Public Health 2010, 55, 123–132. [Google Scholar] [CrossRef]
- Bourque, F.; Cunsolo Willox, A. Climate change: The next challenge for public mental health? Int. Rev. Psychiatry 2014, 26, 415–422. [Google Scholar] [CrossRef]
- Noyes, P.D.; McElwee, M.K.; Miller, H.D.; Clark, B.W.; Van Tiem, L.A.; Walcott, K.C.; Erwin, K.N.; Levin, E.D. The toxicology of climate change: Environmental contaminants in a warming world. Environ. Int. 2009, 35, 971–986. [Google Scholar] [CrossRef]
- Obradovich, N.; Migliorini, R.; Paulus, M.P.; Rahwan, I. Empirical evidence of mental health risks posed by climate change. Proc. Natl. Acad. Sci. USA 2018, 115, 10953–10958. [Google Scholar] [CrossRef]
- Berry, H.L.; Waite, T.D.; Dear, K.B.G.; Capon, A.G.; Murray, V. The case for systems thinking about climate change and mental health. Nat. Clim. 2018, 8, 282–290. [Google Scholar] [CrossRef]
- Manciocco, A.; Calamandrei, G.; Alleva, E. Global warming and environmental contaminants in aquatic organisms: The need of the etho-toxicology approach. Chemosphere 2014, 100, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mehta, K. Impact of temperature on contaminants toxicity in fish fauna: A review. Indian, J. Sci. Technol. 2017, 10, 1–6. [Google Scholar] [CrossRef]
- Capkin, E.; Altinok, I.; Karahan, S. Water quality and fish size affect toxicity of endosulfan, an organochlorine pesticide, to rainbow trout. Chemosphere 2006, 64, 1793–1800. [Google Scholar] [CrossRef]
- Lydy, M.J.; Belden, J.B.; Ternes, M.A. Effects of temperature on the toxicity of M-parathion, chlorpyrifos, and pentachlorobenzene to Chironomus tentans. Arch. Environ. Contam. Toxicol. 1999, 37, 542–547. [Google Scholar] [CrossRef]
- DeLorenzo, M.E.; Wallace, S.C.; Danese, L.E.; Baird, T.D. Temperature and salinity effects on the toxicity of common pesticides to the grass shrimp, Palaemonetes pugio. J. Environ. Sci. Health Part B 2009, 44, 455–460. [Google Scholar] [CrossRef]
- Wallace, R.; Snell, T. Rotifera. In Ecology and Classification of North American Freshwater Invertebrates; Academic Press: Cambridge, MA, USA, 1991; pp. 173–235. [Google Scholar]
- Isibor, P.O.; Imoobe, T.O.T.; Dedeke, G.A.; Adagunodo, T.A.; Taiwo, O.S. Health risk indices and zooplankton-based assessment of a tropical rainforest river contaminated with iron, lead, cadmium, and chromium. Sci. Rep. 2020, 10, 16896. [Google Scholar] [CrossRef]
- de Oliveira, L.L.D.; Antunes, S.C.; Gonçalves, F.; Rocha, O.; Nunes, B. Acute and chronic ecotoxicological effects of four pharmaceuticals drugs on cladoceran Daphnia magna. Drug Chem. Toxicol. 2016, 39, 13–21. [Google Scholar] [CrossRef]
- Oropesa, A.L.; Floro, A.M.; Palma, P. Assessment of the effects of the carbamazepine on the endogenous endocrine system of Daphnia magna. Environ. Sci. Pollut. Res. 2016, 23, 17311–17321. [Google Scholar] [CrossRef]
- Janssen, C.; Ferrando, M.; Persoone, G. Ecotoxicological studies with the freshwater rotifer Brachionus calyciflorus: IV. Rotifer behavior as a sensitive and rapid sublethal test criterion. Ecotoxicol. Environ. Saf. 1994, 28, 244–255. [Google Scholar] [CrossRef]
- Snell, T.W.; Janssen, C.R. Rotifers in ecotoxicology: A review. Hydrobiologia 1995, 313, 231–247. [Google Scholar] [CrossRef]
- Dahms, H.-U.; Hagiwara, A.; Lee, J.S. Ecotoxicology, ecophysiology, and mechanistic studies with rotifers. Aquat. Toxicol. 2011, 101, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Niu, J.; Wang, Y. Full life-cycle toxicity assessment on triclosan using rotifer Brachionus calyciflorus. Ecotoxicol. Environ. Saf. 2016, 127, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.L.; Xi, Y.L.; Qian, F.P.; Zhang, G.; Xiang, X.L. Comparative analysis of rotifer community structure in five subtropical shallow lakes in East China: Role of physical and chemical conditions. Hydrobiologia 2011, 661, 303–316. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, A.; Xi, Y.L.; Sun, Q.; Ning, L.F.; Xie, P.; Wen, X.L.; Xiang, X.L. Temporal patterns and processes of genetic differentiation of the Brachionus calyciflorus (Rotifera) complex in a subtropical shallow lake. Hydrobiologia 2018, 807, 313–331. [Google Scholar] [CrossRef]
- Peltier, W.H.; Weber, C.I. Methods for Measuring the Acute Toxicity of Effluents to Freshwater and Marine Organisms; U.S. Environmental Protection Agency: Washington, WA, USA, 1985.
- Li, S.H.; Zhu, H.; Xia, Y.Z.; Yu, M.J.; Lin, K.E.; Liu, K.S.; Le, Z.Y.; Chen, Y.X. The mass culture of unicellular green algae. Acta Hydrobiol. Sin. 1959, 4, 462–472. [Google Scholar]
- Jiménez, J.J.; Muñoz, B.E.; Sánchez, M.I.; Pardo, R.; Vega, M.S. Fate of the drug chlorpromazine in river water according to laboratory assays. Identification and evolution over time of degradation products. Sorption to sediment. Chemosphere 2016, 162, 285–292. [Google Scholar] [CrossRef]
- IJO; Finney, D.J.; Tattersfield, F. Probit Analysis: A Statistical Treatment of the Sigmoid Response Curve. J. R. Stat. Soc. 1947, 110, 263–266. [Google Scholar] [CrossRef]
- Poole, R.W. Introduction to Quantitative Ecology; McGraw-Hill: New York, NY, USA, 1974. [Google Scholar]
- Krebs, C.J. Ecology: The Experimental Analysis of Distribution and Abundance, 3rd ed.; Harper and Row Publisher: New York, NY, USA, 1985. [Google Scholar]
- Ricci, C.; Snell, T.W. Rotifer Symposium V: Proceedings of the Fifth Rotifer Symposium. Held in Gargnano, Italy, September 11–18; King, C.E., Ed.; Springer: Dordrecht, The Netherlands, 1989. [Google Scholar]
- Medrano, J. Historical Perspective of Psychotropic Drug Use Geriatrics; Lertxundi, U., Medrano, J., Hernández, R., Eds.; Bentham Science Publishers: Sharjah, United Arab Emirates, 2015; pp. 3–15. [Google Scholar]
- Reichert, J.F.; Souza, D.M.; Martins, A.F. Antipsychotic drugs in hospital wastewater and a preliminary risk assessment. Ecotoxicol. Environ. Saf. 2019, 170, 559–567. [Google Scholar] [CrossRef]
- Nakada, N.; Komori, K.; Suzuki, Y.; Konishi, C.; Houwa, I.; Tanaka, H. Occurrence of 70 pharmaceutical and personal care products in Tone River basin in Japan. Water Sci. Technol. 2007, 56, 133–140. [Google Scholar] [CrossRef]
- Nałęcz-Jawecki, G.; Kaza, M.; Sawicki, J. Evaluation of the toxicity of psychoactive compounds with the battery of bioassays. Fresenius Environ. Bull. 2008, 17, 1257–1263. [Google Scholar]
- Arkhipova, S.S.; Gainutdinova, T.K.; Ismailova, A.I.; Gainutdinov, K.L. Comparative studies of the effects of chlorpromazine and 5, 6-dihydroxytryptamine on locomotion, defensive reactions in the snail Helix Lucorum, and command neuron excitability in long-term sensitization. Neurosci. Behav. Physiol. 2006, 36, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Rihel, J.; Prober, D.A.; Arvanites, A.; Lam, K.; Zimmerman, S.; Jang, S.; Haggarty, S.J.; Kokel, D.; Rubin, L.L.; Peterson, R.T.; et al. Zebrafish behavioral profiling links drugs to biological targets and rest/wake regulation. Science 2010, 327, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Atama, C.I.; Nnaji, E.C.; Christian Ezeoyili, I.; Udeani, F.O.; Onovo, C.J.; Ike Ossai, N.; Oscar Aguzie, I.; Nwani, C.D. Neuromodulatory and oxidative stress evaluations in African catfish Clarias gariepinus exposed to antipsychotic drug chlorpromazine. Drug Chem. Toxicol. 2022, 45, 1318–1324. [Google Scholar] [CrossRef] [PubMed]
- Keplinger, M.L.; Lanier, G.E.; Deichmann, W.B. Effects of environmental temperature on the acute toxicity of a number of compounds in rats. Toxicol. Appl. Pharmacol. 1959, 1, 156–161. [Google Scholar] [CrossRef]
- Shimojo, G.; Joseph, B.; Shah, R.; Consolim-Colombo, F.M.; De Angelis, K.; Ulloa, L. Exercise activates vagal induction of dopamine and attenuates systemic inflammation. Brain. Behav. Immun. 2019, 75, 181–191. [Google Scholar] [CrossRef]
- Flack, K.; Pankey, C.; Ufholz, K.; Johnson, L.; Roemmich, J.N. Genetic variations in the dopamine reward system influence exercise reinforcement and tolerance for exercise intensity. Behav. Brain Res. 2019, 375, 112148. [Google Scholar] [CrossRef]
- Hove, M.J.; Martinez, S.A.; Shorrock, S.R. Physical exercise increases perceived musical pleasure: Modulatory roles of arousal, affect, or dopamine? Psychol. Music 2022, 50, 849–861. [Google Scholar] [CrossRef]
- Nieoullon, A. Dopamine and the regulation of cognition and attention. Prog. Neurobiol. 2002, 67, 53–83. [Google Scholar] [CrossRef]
- Bäckman, L.; Nyberg, L.; Lindenberger, U.; Li, S.-C.; Farde, L. The correlative triad among aging, dopamine, and cognition: Current status and future prospects. Neurosci. Biobehav. Rev. 2006, 30, 791–807. [Google Scholar] [CrossRef]
- Lee, M.; Fatouros-Bergman, H.; Plavén-Sigray, P.; Ikonen Victorsson, P.; Sellgren, C.M.; Erhardt, S.; Flyckt, L.; Farde, L.; Cervenka, S. No association between cortical dopamine D2 receptor availability and cognition in antipsychotic-naive first-episode psychosis. NPJ Schizophr. 2021, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Meaney, M.J.; Brake, W.; Gratton, A. Environmental regulation of the development of mesolimbic dopamine systems: A neurobiological mechanism for vulnerability to drug abuse? Psychoneuroendocrinology 2002, 27, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Hoops, D.; Reynolds, L.M.; Restrepo-Lozano, J.M.; Flores, C. Dopamine development in the mouse orbital prefrontal cortex is protracted and sensitive to amphetamine in adolescence. Eneuro 2018, 5. [Google Scholar] [CrossRef] [PubMed]
- Malekzadeh Viayeh, R.; Mohammadi, H.; Shafiei, A.B. Population growth of six Iranian Brachionus rotifer strains in response to salinity and food type. Int. Rev. Hydrobiol. 2010, 95, 461–470. [Google Scholar] [CrossRef]
- Xu, X.P.; Chen, T.; Wei, X.Y.; Yang, X.F.; Xi, Y.L.; Wang, X.M. Effects of bromate on life history parameters, swimming speed and antioxidant biomarkers in Brachionus calyciflorus. Ecotoxicol. Environ. Saf. 2021, 208, 111705. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.O.; Kim, B.M.; Jeong, C.B.; Lee, J.S.; Rhee, J.S. Effects of chlorpyrifos on life cycle parameters, cytochrome P450S expression, and antioxidant systems in the monogonont rotifer Brachionus koreanus. Environ. Toxicol. Chem. 2016, 35, 1449–1457. [Google Scholar] [CrossRef]
- Xu, X.P.; Xi, Y.L.; Wang, X.M. Effects of DDT and dicofol on life table demography of the freshwater rotifer Brachionus calyciflorus Pallas. Pol. J. Environ. Stud. 2020, 29, 1945–1951. [Google Scholar] [CrossRef]
- Heinle, D.R. Temperature and zooplankton. Chesap. Sci. 1969, 10, 186–209. [Google Scholar] [CrossRef]
- Dong, L.L.; Wang, H.X.; Ding, T.; Li, W.; Zhang, G. Effects of TiO2 nanoparticles on the life-table parameters, antioxidant indices, and swimming speed of the freshwater rotifer Brachionus calyciflorus. J. Exp. Zool. Part Ecol. Integr. Physiol. 2020, 333, 230–239. [Google Scholar] [CrossRef]
- Wen, Y.; Cao, M.M.; Huang, Z.Y.; Xi, Y.L. Combined Effects of Warming and Imidacloprid on Survival, Reproduction and Population Growth of Brachionus calyciflorus (Rotifera). Bull. Environ. Contam. Toxicol. 2022, 109, 990–995. [Google Scholar] [CrossRef]
- Brecken-Folse, J.A.; Mayer, F.L.; Pedigo, L.E.; Marking, L.L. Acute toxicity of 4-nitrophenol, 2, 4-dinitrophenol, terbufos and trichlorfon to grass shrimp (Palaemonetes spp.) and sheepshead minnows (Cyprinodon variegatus) as affected by salinity and temperature. Environ. Toxicol. Chem. 1994, 13, 67–77. [Google Scholar] [CrossRef]
- Camp, A.A.; Buchwalter, D.B. Can’t take the heat: Temperature-enhanced toxicity in the mayfly Isonychia bicolor exposed to the neonicotinoid insecticide imidacloprid. Aquat. Toxicol. 2016, 178, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Laetz, C.A.; Baldwin, D.H.; Hebert, V.R.; Stark, J.D.; Scholz, N.L. Elevated temperatures increase the toxicity of pesticide mixtures to juvenile coho salmon. Aquat. Toxicol. 2014, 146, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Park, J.C.; Hwang, U.K.; Lee, J.S.; Han, J. Adverse effects of the insecticides chlordecone and fipronil on population growth and expression of the entire cytochrome P450 (CYP) genes in the freshwater rotifer Brachionus calyciflorus and the marine rotifer Brachionus plicatilis. Aquat. Toxicol. 2018, 202, 181–187. [Google Scholar] [CrossRef]
- Iqbal, M.S.; Zhu, H.; Xi, Y.L. Effect of chloramphenicol on the life table demography of Brachionus calyciflorus (Rotifera): A multigenerational study. Ecotoxicol. Environ. Saf. 2022, 237, 113525. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Huang, Z.Y.; Jiang, S.; Pan, L.; Xi, Y.L. Rapid adaptation of Brachionus dorcas (Rotifera) to tetracycline antibiotic stress. Aquat. Toxicol. 2022, 245, 106126. [Google Scholar] [CrossRef] [PubMed]
- de Coen, W.M.D.; Janssen, C.R. The use of biomarkers in Daphnia magna toxicity testing. IV. Cellular energy allocation: A new methodology to assess the energy budget of toxicant-stressed Daphnia populations. J. Aquat. Ecosyst. Stress Recovery 1997, 6, 43–55. [Google Scholar] [CrossRef]
- Zhu, L.Y.; Huang, R.; Zhou, L.B.; Xi, Y.L.; Xiang, X.L. Responses of the ecological characteristics and antioxidant enzyme activities in Rotaria rotatoria to UV-B radiation. Hydrobiologia 2021, 848, 4749–4761. [Google Scholar] [CrossRef]
- Xiang, X.L.; Chen, Y.Y.; Xu, Q.L.; Zhu, L.Y.; Wen, X.L.; Xi, Y.L. Combined effects of temperature and the microcystin MC-LR on the feeding behavior of the rotifer Brachionus calyciflorus. Bull. Environ. Contam. Toxicol. 2017, 99, 493–499. [Google Scholar] [CrossRef]
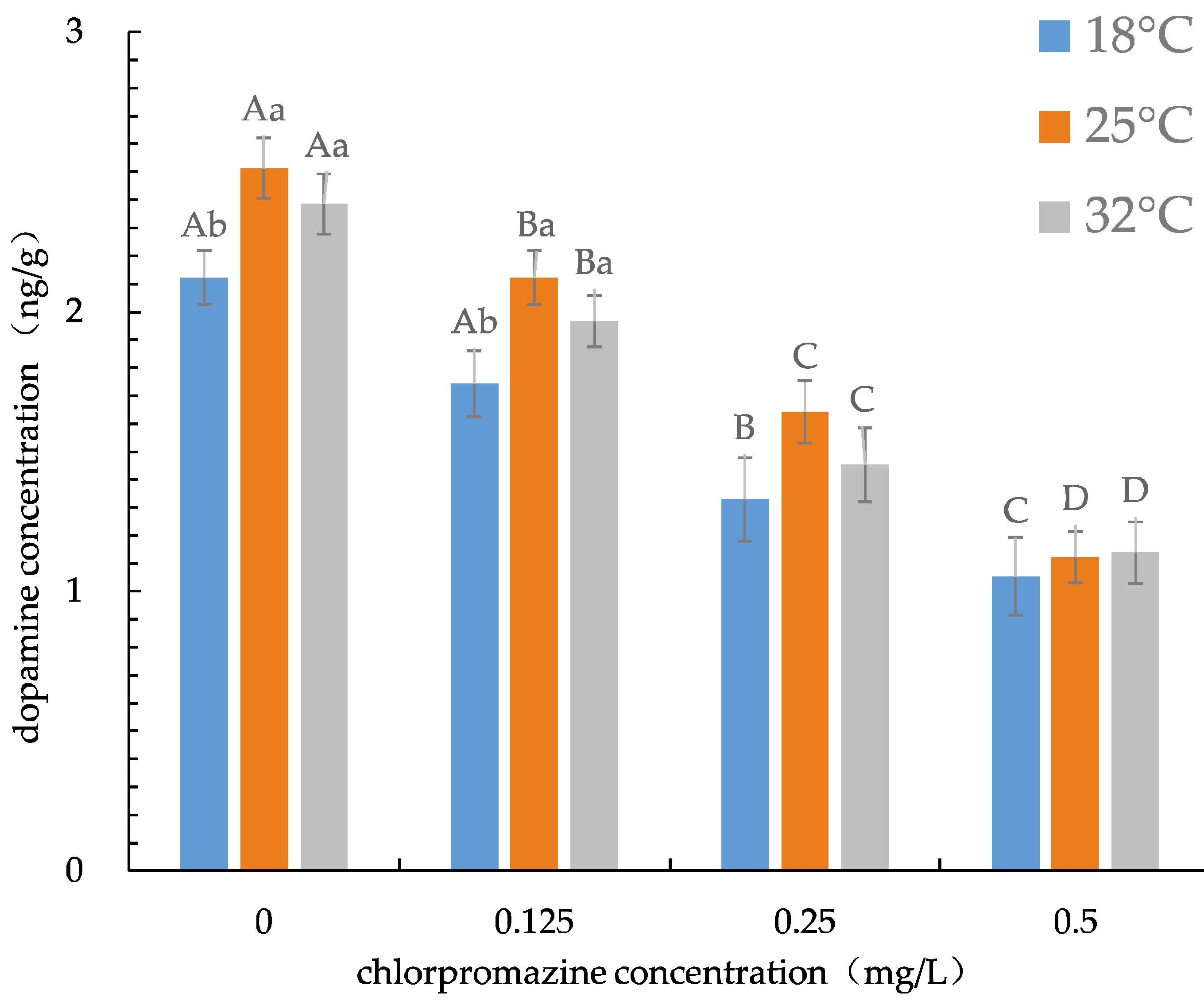
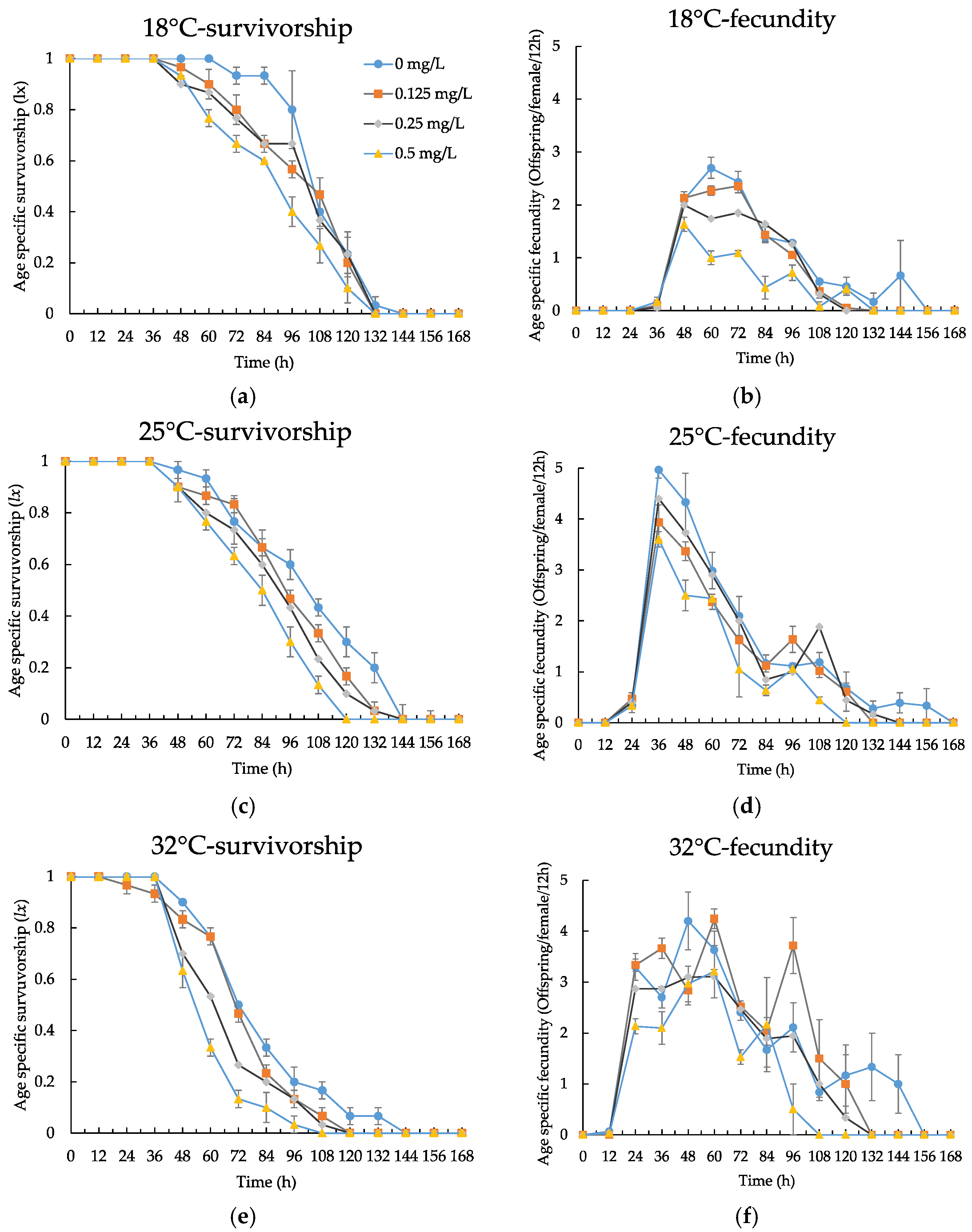
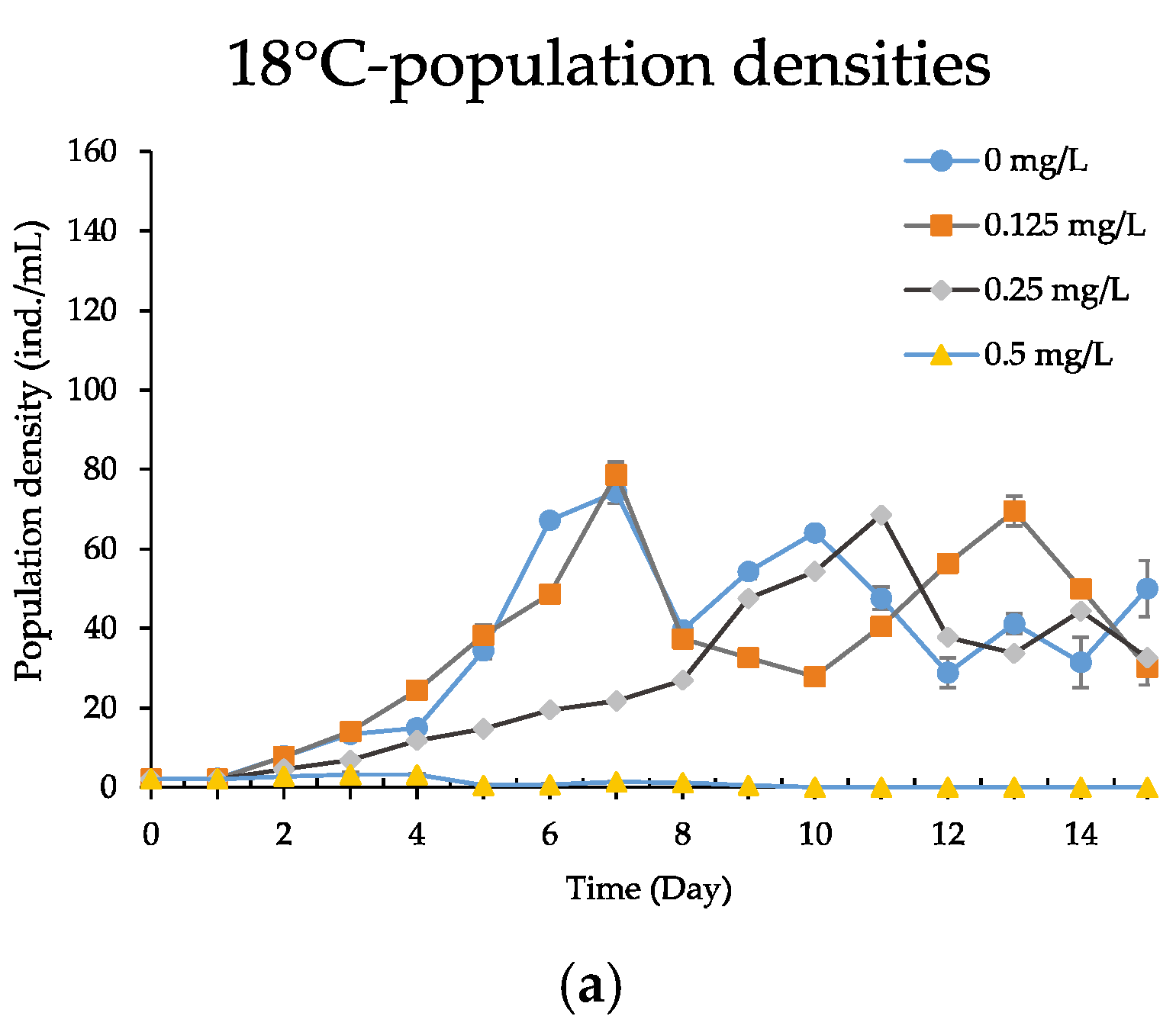
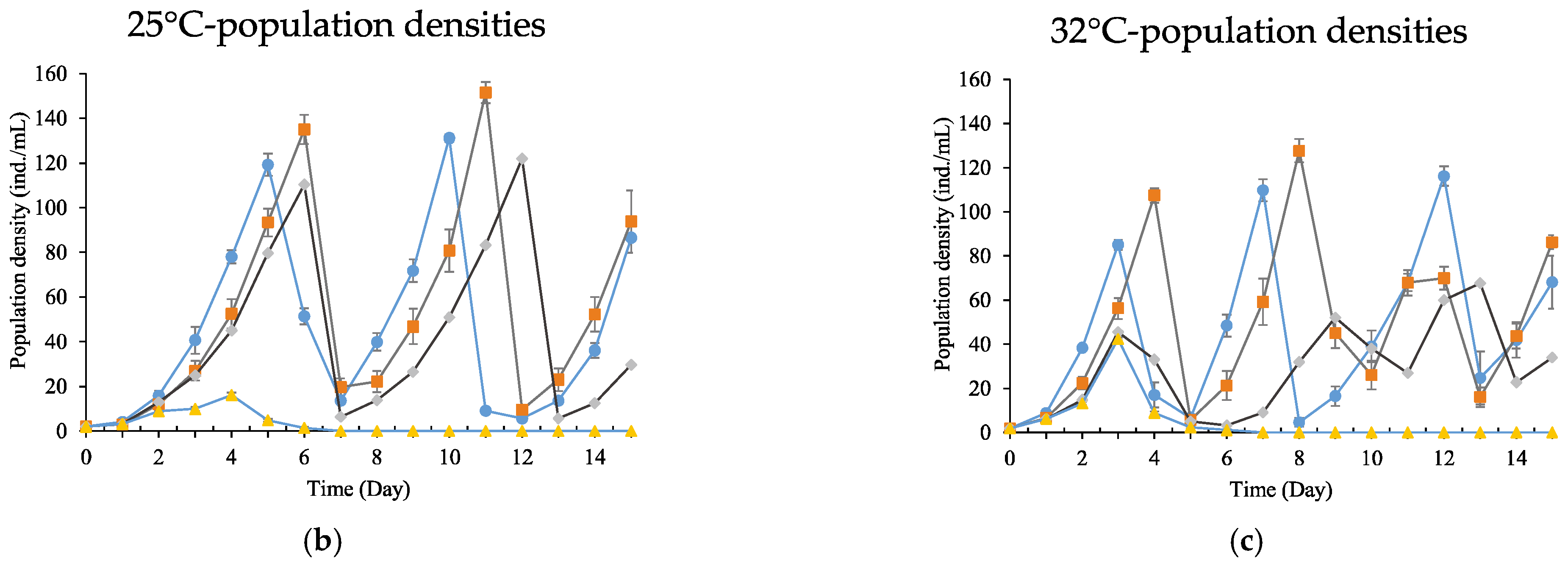
| Temperature (°C) | 24 h LC50 (mg/L) | 95% Confidence Limits | Regressive Equations | Significant Tests |
|---|---|---|---|---|
| 18 | 1.795 | 1.669~1.918 | y = 0.375x − 0.178 | R2 = 0.907, p < 0.001 |
| 25 | 1.242 | 0.795~1.644 | y = 0.390x − 0.054 | R2 = 0.847, p < 0.001 |
| 32 | 0.833 | 0.728~0.933 | y = 0.3412x + 0.142 | R2 = 0.795, p < 0.001 |
| Temperature (°C) | Parameters | Regressive Equations | Significant Tests |
|---|---|---|---|
| 18 | e0 | y = 67.491x2 − 71.156x + 105.629 | R2 = 0.744, p < 0.01 |
| R0 | y = 8.000x2 − 16.120x + 9.837 | R2 = 0.953, p < 0.001 | |
| T | y = −34.764x2 + 5.332x + 68.094 | R2 = 0.696, p < 0.01 | |
| rm | y = 0.00034x2 − 0.027x + 0.036 | R2 = 0.903, p < 0.001 | |
| DAC | y = 14.749x2 − 16.110x + 8.564 | R2 = 0.831, p < 0.001 | |
| 25 | e0 | y = 59.927x2 − 71.811x + 101.793 | R2 = 0.786, p < 0.001 |
| R0 | y = 5.479x2 − 15.752x + 16.005 | R2 = 0.686, p < 0.01 | |
| T | y = −17.745x2 − 2.422x + 55.252 | R2 = 0.621, p < 0.05 | |
| rm | y = −0.005x2 − 0.014x + 0.059 | R2 = 0.657, p < 0.01 | |
| DAC | y = 9.202x2 − 15.940x + 10.134 | R2 = 0.926, p < 0.001 | |
| 32 | e0 | y = 45.964x2 − 65.585x + 78.076 | R2 = 0.925, p < 0.001 |
| R0 | y = −1.552x2 − 15.059x + 15.538 | R2 = 0.909, p < 0.001 | |
| T | y = 6.012x2 − 18.352x + 49.255 | R2 = 0.618, p < 0.05 | |
| rm | y = −0.039x2 − 0.011x + 0.072 | R2 = 0.905, p < 0.001 | |
| DAC | y = 16.931x2 − 18.730x + 9.652 | R2 = 0.893, p < 0.001 |
| Parameters | CPZ Concen. (mg/L) | Temperature (°C) | ||
|---|---|---|---|---|
| 18 | 25 | 32 | ||
| Life expectancyat hatching (h) | 0 | 106.0 ± 4.2 Aa | 102.0 ± 4.8 Aa | 78.0 ± 2.1 Ab |
| 0.125 | 96.8 ± 2.8 ABa | 93.2 ± 1.7 ABa | 70.8 ± 0.7 Bb | |
| 0.25 | 92.8 ± 2.6 BCa | 88.0 ± 0.8 BCa | 64.4 ± 2.2 Cb | |
| 0.5 | 86.8 ± 0.4 Ca | 80.8 ± 2.2 Cb | 56.8 ± 0.4 Dc | |
| Generationtime (h) | 0 | 68.8 ± 1.0 Aa | 55.1 ± 1.6 Ab | 49.0 ± 2.3 Ac |
| 0.125 | 66.3 ± 0.4 Aa | 55.2 ± 0.4 Ab | 47.7 ±0.9 Ac | |
| 0.25 | 68.7 ± 0.9 Aa | 53.2 ± 1.2 ABb | 44.6 ± 1.8 ABc | |
| 0.5 | 61.9 ± 0.8 Ba | 49.7 ± 1.2 Bb | 41.7 ± 0.3 Bc | |
| Net reproductiverate | 0 | 9.9 ± 0.2 Ab | 16.5 ± 1.6 Aa | 15.2 ± 0.7 Aa |
| 0.125 | 7.9 ± 0.3 Bc | 12.8 ± 0.5 Bb | 14.5 ± 0.3 Aa | |
| 0.25 | 6.4 ± 0.4 Cc | 13.4 ± 0.9 ABa | 11.0 ± 0.2 Bb | |
| 0.5 | 3.8 ± 0.3 Db | 9.4 ± 0.6 Ca | 7.7 ± 0.6 Ca | |
| Intrinsic rate of population increase (h) | 0 | 0.0357 ± 0.0005 Ac | 0.0598 ± 0.0014 Ab | 0.0714 ± 0.0016 Aa |
| 0.125 | 0.0330 ± 0.0010 Ac | 0.0549 ± 0.0014 Bb | 0.0705 ± 0.0003 Aa | |
| 0.25 | 0.0288 ± 0.0011 Bc | 0.0568 ± 0.0007 ABb | 0.0660 ± 0.0010 Ba | |
| 0.5 | 0.0225 ± 0.0017 Cc | 0.0504 ± 0.0011 Cb | 0.0566 ± 0.0017 Ca | |
| Temperature (℃) | CPZ Concen. (mg/L) | Parameters | ||
|---|---|---|---|---|
| Day of Maximum Density | Maximum Rotifer Density (ind./mL) | Population Growth RATE | ||
| 18 | 0 | 7 | 74.30 ± 2.66 | 0.40 ± 0.02 |
| 0.125 | 7 | 78.63 ± 3.39 | 0.29 ± 0.02 ** | |
| 0.25 | 12 | 68.67 ± 1.76 *** | 0.23 ± 0.01 *** | |
| 0.5 | 4 | 3.30 ± 0.49 | 0.12 ± 0.04 | |
| 25 | 0 | 10 | 131.13 ± 1.68 | 0.57 ± 0.03 |
| 0.125 | 11 | 151.57 ± 4.79 ** | 0.42 ± 0.04 ** | |
| 0.25 | 12 | 121.93 ± 4.30 | 0.38 ± 0.01 ** | |
| 0.5 | 4 | 16.17 ± 1.13 | 0.20 ± 0.01 | |
| 32 | 0 | 12 | 116.20 ± 4.39 | 0.67 ± 0.08 |
| 0.125 | 12 | 103.33 ± 5.29 | 0.48 ± 0.07 | |
| 0.25 | 13 | 91.17 ± 2.60 ** | 0.41 ± 0.01 * | |
| 0.5 | 3 | 17.77 ± 0.62 | 0.35 ± 0.02 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, S.; Zhang, Y.; Gao, F.; Li, M.; Zhu, L.; Wen, H.; Xi, Y.; Xiang, X. Inhibitory Effects of Antipsychotic Chlorpromazine on the Survival, Reproduction and Population Growth Other Than Neurotransmitters of Zooplankton in Light of Global Warming. Int. J. Environ. Res. Public Health 2022, 19, 16167. https://doi.org/10.3390/ijerph192316167
Feng S, Zhang Y, Gao F, Li M, Zhu L, Wen H, Xi Y, Xiang X. Inhibitory Effects of Antipsychotic Chlorpromazine on the Survival, Reproduction and Population Growth Other Than Neurotransmitters of Zooplankton in Light of Global Warming. International Journal of Environmental Research and Public Health. 2022; 19(23):16167. https://doi.org/10.3390/ijerph192316167
Chicago/Turabian StyleFeng, Sen, Yongzhi Zhang, Fan Gao, Meng Li, Lingyun Zhu, Hao Wen, Yilong Xi, and Xianling Xiang. 2022. "Inhibitory Effects of Antipsychotic Chlorpromazine on the Survival, Reproduction and Population Growth Other Than Neurotransmitters of Zooplankton in Light of Global Warming" International Journal of Environmental Research and Public Health 19, no. 23: 16167. https://doi.org/10.3390/ijerph192316167
APA StyleFeng, S., Zhang, Y., Gao, F., Li, M., Zhu, L., Wen, H., Xi, Y., & Xiang, X. (2022). Inhibitory Effects of Antipsychotic Chlorpromazine on the Survival, Reproduction and Population Growth Other Than Neurotransmitters of Zooplankton in Light of Global Warming. International Journal of Environmental Research and Public Health, 19(23), 16167. https://doi.org/10.3390/ijerph192316167








