Comparative Perceptual, Affective, and Cardiovascular Responses between Resistance Exercise with and without Blood Flow Restriction in Older Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Methods
2.2.1. Predicted One Repetition Maximum
2.2.2. Determination of Arterial Occlusion Pressure
2.2.3. Resistance Exercise Protocols
2.2.4. Perceptual Responses
2.2.5. Affective Responses
2.2.6. Cardiovascular Responses
2.2.7. Statistical Analysis
3. Results
3.1. Adverse Events
3.2. Total Repetitons Completed
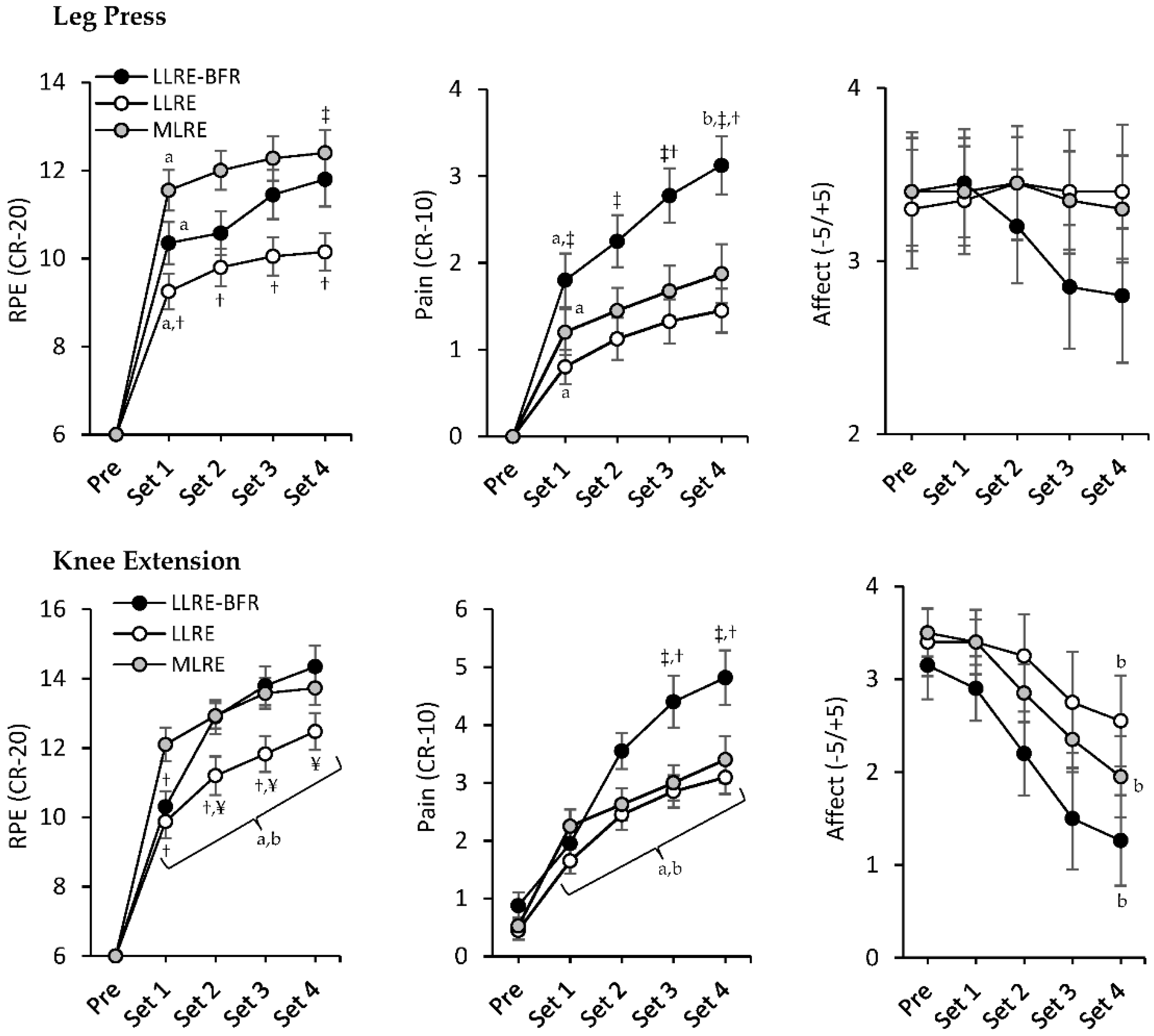
3.3. RPE, Pain, and Affect
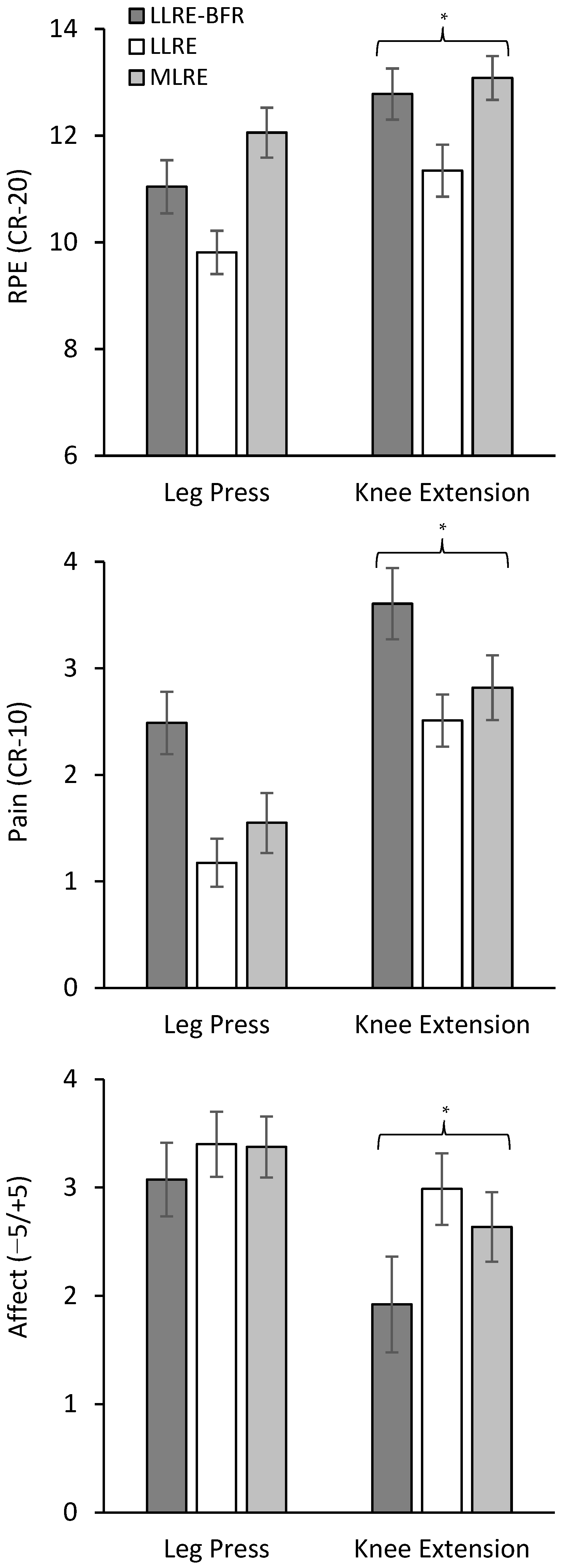
3.4. Session-RPE
3.5. Cardiovascular Responses
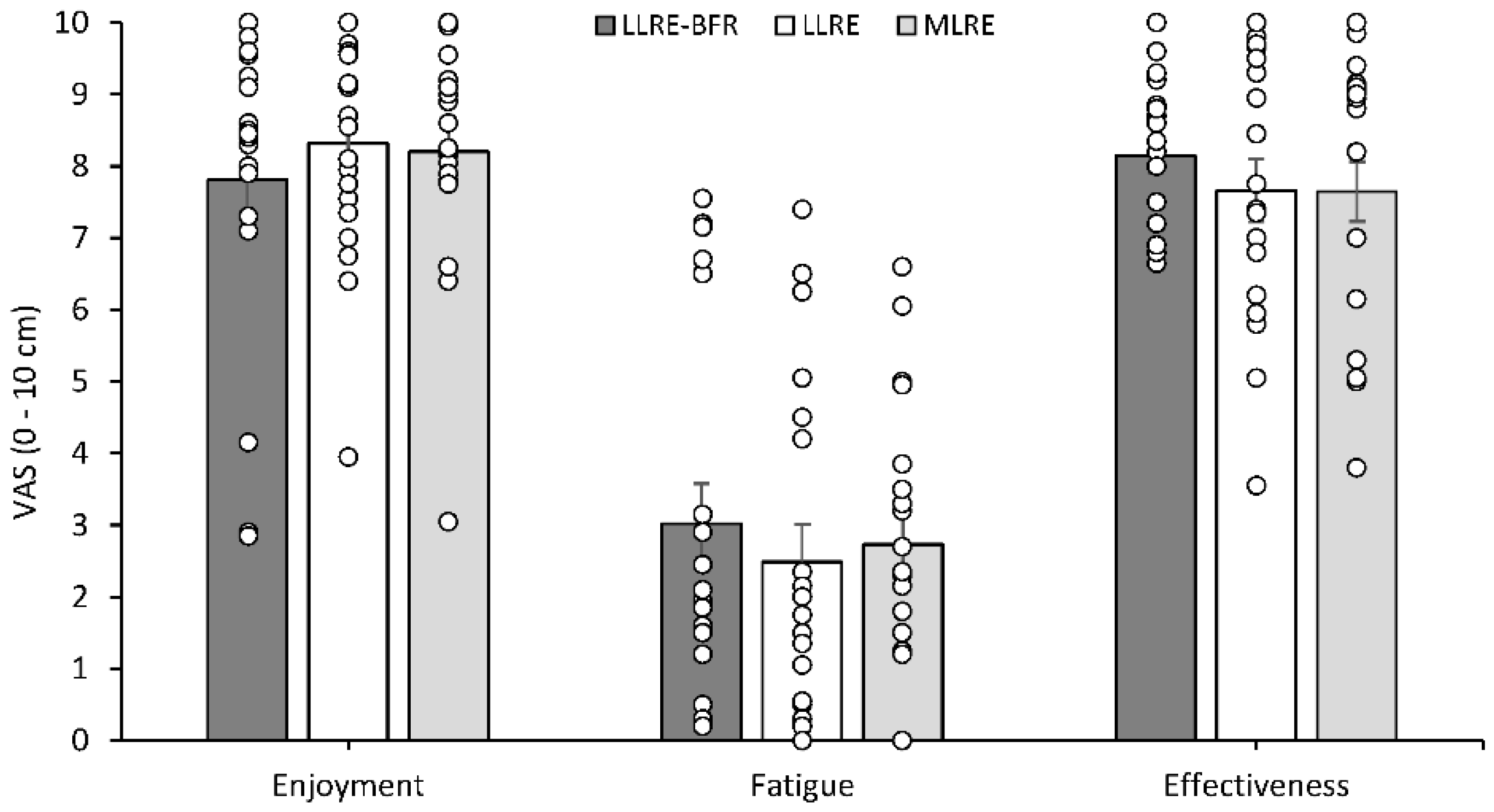
3.6. Physical Activity Enjoyment Scale
3.7. Physical Activity Affect Scale
3.8. Visual Analogue Scales
3.9. Muscle Soreness
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hairi, N.N.; Cumming, R.G.; Naganathan, V.; Handelsman, D.J.; le Couteur, D.G.; Creasey, H.; Waite, L.M.; Seibel, M.J.; Sambrook, P.N. Loss of Muscle Strength, Mass (Sarcopenia), and Quality (Specific Force) and Its Relationship with Functional Limitation and Physical Disability: The Concord Health and Ageing in Men Project. J. Am. Geriatr. Soc. 2010, 58, 2055–2062. [Google Scholar] [CrossRef]
- Volaklis, K.A.; Halle, M.; Meisinger, C. Muscular Strength as a Strong Predictor of Mortality: A Narrative Review. Eur. J. Intern. Med. 2015, 26, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Fragala, M.S.; Cadore, E.L.; Dorgo, S.; Izquierdo, M.; Kraemer, W.J.; Peterson, M.D.; Ryan, E.D. Resistance Training for Older Adults: Position Statement From the National Strength and Conditioning Association. J. Strength Cond. Res. 2019, 33, 2019–2052. [Google Scholar] [CrossRef] [PubMed]
- Peterson, M.D.; Rhea, M.R.; Sen, A.; Gordon, P.M. Resistance Exercise for Muscular Strength in Older Adults: A Meta-Analysis. Ageing Res. Rev. 2010, 9, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Kujala, U.M. Evidence on the Effects of Exercise Therapy in the Treatment of Chronic Disease. Br. J. Sports Med. 2009, 43, 550–555. [Google Scholar] [CrossRef]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.M.; Nieman, D.C.; Swain, D.P. Quantity and Quality of Exercise for Developing and Maintaining Cardiorespiratory, Musculoskeletal, and Neuromotor Fitness in Apparently Healthy Adults: Guidance for Prescribing Exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef]
- Centner, C.; Wiegel, P.; Gollhofer, A.; König, D. Effects of Blood Flow Restriction Training on Muscular Strength and Hypertrophy in Older Individuals: A Systematic Review and Meta Analysis. Sport. Med. 2019, 49, 95–108. [Google Scholar] [CrossRef]
- Hughes, L.; Paton, B.; Rosenblatt, B.; Gissane, C.; Patterson, S.D. Blood Flow Restriction Training in Clinical Musculoskeletal Rehabilitation: A Systematic Review and Meta-Analysis. Br. J. Sports Med. 2017, 51, 1003–1011. [Google Scholar] [CrossRef]
- Patterson, S.D.; Hughes, L.; Warmington, S.; Burr, J.; Scott, B.R.; Owens, J.; Abe, T.; Nielsen, J.L.; Libardi, C.A.; Laurentino, G.; et al. Blood Flow Restriction Exercise Position Stand: Considerations of Methodology, Application, and Safety. Front. Physiol. 2019, 10, 1332. [Google Scholar] [CrossRef]
- Pearson, S.J.; Hussain, S.R. A Review on the Mechanisms of Blood-Flow Restriction Resistance Training-Induced Muscle Hypertrophy. Sports Med. 2015, 45, 187–200. [Google Scholar] [CrossRef]
- Manini, T.M.; Yarrow, J.F.; Buford, T.W.; Clark, B.C.; Conover, C.F.; Borst, S.E. Growth Hormone Responses to Acute Resistance Exercise with Vascular Restriction in Young and Old Men. Growth Horm. IGF Res. 2012, 22, 167–172. [Google Scholar] [CrossRef]
- Sieljacks, P.; Wang, J.; Groennebaek, T.; Rindom, E.; Jakobsgaard, J.E.; Herskind, J.; Gravholt, A.; Møller, A.B.; Musci, R.V.; de Paoli, F.V.; et al. Six Weeks of Low-Load Blood Flow Restricted and High-Load Resistance Exercise Training Produce Similar Increases in Cumulative Myofibrillar Protein Synthesis and Ribosomal Biogenesis in Healthy Males. Front. Physiol. 2019, 10, 649. [Google Scholar] [CrossRef]
- Groennebaek, T.; Jespersen, N.R.; Jakobsgaard, J.E.; Sieljacks, P.; Wang, J.; Rindom, E.; Musci, R.V.; Bøtker, H.E.; Hamilton, K.L.; Miller, B.F.; et al. Skeletal Muscle Mitochondrial Protein Synthesis and Respiration Increase with Low-Load Blood Flow Restricted as Well as High-Load Resistance Training. Front. Physiol. 2018, 9, 1796. [Google Scholar] [CrossRef]
- Larkin, K.; Macneil, R.; Dirain, M.; Sandesara, B.; Manini, T.; Buford, T. Blood Flow Restriction Enhances Post–Resistance Exercise Angiogenic Gene Expression. Med. Sci. Sports Exerc. 2012, 44, 2077–2083. [Google Scholar] [CrossRef]
- Loenneke, J.P.; Wilson, J.M.; Marín, P.J.; Zourdos, M.C.; Bemben, M.G. Low Intensity Blood Flow Restriction Training: A Meta-Analysis. Eur. J. Appl. Physiol. 2012, 112, 1849–1859. [Google Scholar] [CrossRef]
- Lixandrão, M.E.; Ugrinowitsch, C.; Berton, R.; Vechin, F.C.; Conceição, M.S.; Damas, F.; Libardi, C.A.; Roschel, H. Magnitude of Muscle Strength and Mass Adaptations Between High-Load Resistance Training Versus Low-Load Resistance Training Associated with Blood-Flow Restriction: A Systematic Review and Meta-Analysis. Sport. Med. 2018, 48, 361–378. [Google Scholar] [CrossRef]
- Rodrigues, R.; Ferraz, R.B.; Kurimori, C.O.; Guedes, L.K.; Lima, F.R.; de Sá-Pinto, A.L.; Gualano, B.; Roschel, H. Low-Load Resistance Training With Blood-Flow Restriction in Relation to Muscle Function, Mass, and Functionality in Women With Rheumatoid Arthritis. Arthritis Care Res. 2020, 72, 787–797. [Google Scholar] [CrossRef]
- Corrêa, H.L.; Neves, R.V.P.; Deus, L.A.; Souza, M.K.; Haro, A.S.; Costa, F.; Silva, V.L.; Santos, C.A.R.; Moraes, M.R.; Simões, H.G.; et al. Blood Flow Restriction Training Blunts Chronic Kidney Disease Progression in Humans. Med. Sci. Sports Exerc. 2021, 53, 249–257. [Google Scholar] [CrossRef]
- Centner, C.; Zdzieblik, D.; Roberts, L.; Gollhofer, A.; König, D. Effects of Blood Flow Restriction Training with Protein Supplementation on Muscle Mass And Strength in Older Men. J. Sports Sci. Med. 2019, 18, 471–478. [Google Scholar]
- Suga, T.; Dora, K.; Mok, E.; Sugimoto, T.; Tomoo, K.; Takada, S.; Hashimoto, T.; Isaka, T. Exercise Adherence-Related Perceptual Responses to Low-Load Blood Flow Restriction Resistance Exercise in Young Adults: A Pilot Study. Physiol. Rep. 2021, 9, e15122. [Google Scholar] [CrossRef]
- Loenneke, J.P.; Kim, D.; Fahs, C.A.; Thiebaud, R.S.; Abe, T.; Larson, R.D.; Bemben, D.A.; Bemben, M.G. The Effects of Resistance Exercise with and without Different Degrees of Blood-Flow Restriction on Perceptual Responses. J. Sports Sci. 2015, 33, 1472–1479. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lang, J.A.; Pilania, N.; Franke, W.D. Effects of Blood Flow Restricted Exercise Training on Muscular Strength and Blood Flow in Older Adults. Exp. Gerontol. 2017, 99, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, R.E.; Kates, A. Can the Affective Response to Exercise Predict Future Motives and Physical Activity Behavior? A Systematic Review of Published Evidence. Ann. Behav. Med. 2015, 49, 715–731. [Google Scholar] [CrossRef] [PubMed]
- Scott, B.R.; Peiffer, J.J.; Thomas, H.J.; Marston, K.J.; Hill, K.D. Hemodynamic Responses to Low-Load Blood Flow Restriction and Unrestricted High-Load Resistance Exercise in Older Women. Front. Physiol. 2018, 9, 1324. [Google Scholar] [CrossRef]
- Pinto, R.R.; Karabulut, M.; Poton, R.; Polito, M.D. Acute Resistance Exercise with Blood Flow Restriction in Elderly Hypertensive Women: Haemodynamic, Rating of Perceived Exertion and Blood Lactate. Clin. Physiol. Funct. Imaging 2018, 38, 17–24. [Google Scholar] [CrossRef]
- Spranger, M.D.; Krishnan, A.C.; Levy, P.D.; O’Leary, D.S.; Smith, S.A. Blood Flow Restriction Training and the Exercise Pressor Reflex: A Call for Concern. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1440–H1452. [Google Scholar] [CrossRef]
- Staunton, C.A.; May, A.K.; Brandner, C.R.; Warmington, S.A. Haemodynamics of Aerobic and Resistance Blood Flow Restriction Exercise in Young and Older Adults. Eur. J. Appl. Physiol. 2015, 115, 2293–2302. [Google Scholar] [CrossRef]
- Poton, R.; Polito, M.D. Hemodynamic Responses during Lower-Limb Resistance Exercise with Blood Low Restriction in Healthy Subjects Hemodynamic Response to Resistance Exercise with and without Blood Flow Restriction in Healthy Subjects. J. Sports Med. Phys. Fit. 2014, 55, 1571–1577. [Google Scholar]
- Dankel, S.J.; Jessee, M.B.; Mattocks, K.T.; Buckner, S.L.; Mouser, J.G.; Bell, Z.W.; Abe, T.; Loenneke, J.P. Perceptual and Arterial Occlusion Responses to Very Low Load Blood Flow Restricted Exercise Performed to Volitional Failure. Clin. Physiol. Funct. Imaging 2019, 39, 29–34. [Google Scholar] [CrossRef]
- Lixandrão, M.E.; Roschel, H.; Ugrinowitsch, C.; Miquelini, M.; Alvarez, I.F.; Libardi, C.A. Blood-Flow Restriction Resistance Exercise Promotes Lower Pain and Ratings of Perceived Exertion Compared with Either High- or Low-Intensity Resistance Exercise Performed to Muscular Failure. J. Sport Rehabil. 2019, 28, 706–710. [Google Scholar] [CrossRef]
- Mattocks, K.T.; Mouser, J.G.; Jessee, M.B.; Buckner, S.L.; Dankel, S.J.; Bell, Z.W.; Loenneke, J.P. Perceptual Changes to Progressive Resistance Training with and without Blood Flow Restriction. J. Sport Sci. 2019, 37, 1857–1864. [Google Scholar] [CrossRef]
- Freitas, E.D.S.; Miller, R.M.; Heishman, A.D.; Aniceto, R.R.; Silva, J.G.C.; Bemben, M.G. Perceptual Responses to Continuous versus Intermittent Blood Flow Restriction Exercise: A Randomized Controlled Trial. Physiol. Behav. 2019, 212, 112717. [Google Scholar] [CrossRef]
- Silva, J.C.G.; Aniceto, R.R.; Oliota-Ribeiro, L.S.; Neto, G.R.; Leandro, L.S.; Cirilo-Sousa, M.S. Mood Effects of Blood Flow Restriction Resistance Exercise among Basketball Players. Percept. Mot. Ski. 2018, 125, 788–801. [Google Scholar] [CrossRef]
- Motykie, G.D.; Zebala, L.P.; Caprini, J.A.; Lee, C.E.; Arcelus, J.I.; Reyna, J.J.; Cohen, E.B.; Courtney, T.; Sullivan, L.J. A Guide to Venous Thromboembolism Risk Factor Assessment. J. Thromb. Thrombolysis 2000, 9, 253–262. [Google Scholar] [CrossRef]
- Cook, S.B.; LaRoche, D.P.; Villa, M.R.; Barile, H.; Manini, T.M. Blood Flow Restricted Resistance Training in Older Adults at Risk of Mobility Limitations. Exp. Gerontol. 2017, 99, 138–145. [Google Scholar] [CrossRef]
- Kilgas, M.A.; Lytle, L.L.M.; Drum, S.N.; Elmer, S.J. Exercise with Blood Flow Restriction to Improve Quadriceps Function Long after ACL Reconstruction. Int. J. Sports Med. 2019, 40, 650–656. [Google Scholar] [CrossRef]
- Brzycki, M. Strength Testing—Predicting a One-Rep Max from Reps-to-Fatigue. J. Phys. Act. Recreat. Danc. 1993, 64, 88–90. [Google Scholar] [CrossRef]
- McNair, P.J.; Colvin, M.; Reid, D. Predicting Maximal Strength of Quadriceps from Submaximal Performance in Individuals with Knee Joint Osteoarthritis. Arthritis Care Res. 2011, 63, 216–222. [Google Scholar] [CrossRef]
- Laurentino, G.C.; Loenneke, J.P.; Mouser, J.G.; Buckner, S.L.; Counts, B.R.; Dankel, S.J.; Jessee, M.B.; Mattocks, K.T.; Iared, W.; Tavares, L.D.; et al. Validity of the Handheld Doppler to Determine Lower-Limb Blood Flow Restriction Pressure for Exercise Protocols. J. Strength Cond. Res. 2020, 34, 2693–2696. [Google Scholar] [CrossRef]
- Borg, G. Borg’s Perceived Exertion and Pain Scales; Human Kinetics: Champaign, IL, USA, 1998. [Google Scholar]
- Borg, G. Psychophysical Bases of Perceived Exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef]
- Hardy, C.J.; Rejeski, W.J. Not What, but How One Feels: The Measurement of Affect during Exercise. J. Sport Exerc. Psychol. 1989, 11, 304–317. [Google Scholar] [CrossRef]
- Richardson, D.L.; Duncan, M.J.; Jimenez, A.; Jones, V.M.; Juris, P.M.; Clarke, N.D. The Perceptual Responses to High-Velocity, Low-Load and Low-Velocity, High-Load Resistance Exercise in Older Adults. J. Sports Sci. 2018, 36, 1594–1601. [Google Scholar] [CrossRef] [PubMed]
- Lox, C.L.; Jackson, S.; Tuholski, S.W.; Wasley, D. Revisiting the Measurement of Exercise-Induced Feeling States: The Physical Activity Affect Scale (PAAS). Meas. Phys. Educ. Exerc. Sci. 2000, 4, 79–95. [Google Scholar] [CrossRef]
- Kwan, B.M.; Bryan, A. In-Task and Post-Task Affective Response to Exercise: Translating Exercise Intentions into Behaviour. Br. J. Health Psychol. 2010, 15, 115–131. [Google Scholar] [CrossRef]
- Carpenter, L.C.; Tompkins, S.A.; Schmiege, S.J.; Nilsson, R.; Bryan, A. Affective Response to Physical Activity: Testing for Measurement Invariance of the Physical Activity Affect Scale across Active and Non-Active Individuals. Meas. Phys. Educ. Exerc. Sci. 2010, 14, 1–14. [Google Scholar] [CrossRef]
- Spitz, R.W.; Wong, V.; Bell, Z.W.; Viana, R.B.; Chatakondi, R.N.; Abe, T.; Loenneke, J.P. Blood Flow Restricted Exercise and Discomfort: A Review. J. Strength Cond. Res. 2022, 36, 871–879. [Google Scholar] [CrossRef]
- Soligon, S.D.; Lixandrão, M.E.; Biazon, T.M.P.C.; Angleri, V.; Roschel, H.; Libardi, C.A. Lower Occlusion Pressure during Resistance Exercise with Blood-Flow Restriction Promotes Lower Pain and Perception of Exercise Compared to Higher Occlusion Pressure When the Total Training Volume Is Equalized. Physiol. Int. 2018, 105, 276–284. [Google Scholar] [CrossRef]
- Husmann, F.; Mittlmeier, T.; Bruhn, S.; Zschorlich, V.; Behrens, M. Impact of Blood Flow Restriction Exercise on Muscle Fatigue Development and Recovery. Med. Sci. Sports Exerc. 2018, 50, 436–446. [Google Scholar] [CrossRef]
- Suga, T.; Okita, K.; Morita, N.; Yokota, T.; Hirabayashi, K.; Horiuchi, M.; Takada, S.; Takahashi, T.; Omokawa, M.; Kinugawa, S.; et al. Intramuscular Metabolism during Low-Intensity Resistance Exercise with Blood Flow Restriction. J. Appl. Physiol. 2009, 106, 1119–1124. [Google Scholar] [CrossRef]
- Vechin, F.C.; Libardi, C.A.; Conceição, M.S.; Damas, F.R.; Conceição, M.E.; Berton, R.P.B.; Tricoli, V.A.A.; Roschel, H.A.; Cavaglieri, C.R.; Patricia, M.; et al. Comparisons between Low-Intensity Resistance Training with Blood Flow Restriction and High-Intensity Resistance Training on Quadriceps Muscle Mass and Strength in Elderly. J. Strength Cond. Res. 2015, 29, 1071–1076. [Google Scholar] [CrossRef]
- Ekkekakis, P.; Parfitt, G.; Petruzzello, S.J. The Pleasure and Displeasure People Feel When They Exercise at Different Intensities. Sports Med. 2011, 41, 641–671. [Google Scholar] [CrossRef]
- Martín-Hernández, J.; Ruiz-Aguado, J.; Herrero, A.J.; Loenneke, J.P.; Aagaard, P.; Cristi-Montero, C.; Menéndez, H.; Marín, P.J. Adaptation of Perceptual Responses to Low-Load Blood Flow Restriction Training. J. Strength Cond. Res. 2017, 31, 765–772. [Google Scholar] [CrossRef]
- Devereux-fitzgerald, A.; Powell, R.; Dewhurst, A.; French, D.P. The Acceptability of Physical Activity Interventions to Older Adults: A Systematic Review and Meta-Synthesis. Soc. Sci. Med. 2016, 158, 14–23. [Google Scholar] [CrossRef]
- Mitropoulos, A.; Gumber, A.; Crank, H.; Akil, M.; Klonizakis, M. Exploring the Feasibility of an Exercise Programme Including Aerobic and Resistance Training in People with Limited Cutaneous Systemic Sclerosis. Clin. Rheumatol. 2020, 39, 1889–1898. [Google Scholar] [CrossRef]
- Mok, E.; Suga, T.; Sugimoto, T.; Tomoo, K.; Dora, K.; Takada, S.; Hashimoto, T.; Isaka, T. Negative Effects of Blood Flow Restriction on Perceptual Responses to Walking in Healthy Young Adults: A Pilot Study. Heliyon 2020, 6, e04745. [Google Scholar] [CrossRef]
- Jessee, M.B.; Mouser, J.G.; Buckner, S.L.; Dankel, S.J.; Mattocks, K.T.; Abe, T.; Loenneke, J.P. Effects of Load on the Acute Response of Muscles Proximal and Distal to Blood Flow Restriction. J. Physiol. Sci. 2018, 68, 769–779. [Google Scholar] [CrossRef]
- Neto, G.R.; Novaes, J.S.; Dias, I.; Brown, A.; Vianna, J.; Cirilo-Sousa, M.S. Effects of Resistance Training with Blood Flow Restriction on Haemodynamics: A Systematic Review. Clin. Physiol. Funct. Imaging 2017, 37, 567–574. [Google Scholar] [CrossRef]
- Manini, T.M.; Clark, B.C. Blood Flow Restricted Exercise and Skeletal Muscle Health. Exerc. Sport Sci. Rev. 2009, 37, 78–85. [Google Scholar] [CrossRef]
- Domingos, E.; Polito, M.D. Blood Pressure Response between Resistance Exercise with and without Blood Flow Restriction: A Systematic Review and Meta-Analysis. Life Sci. 2018, 209, 122–131. [Google Scholar] [CrossRef]
- Fletcher, G.F.; Balady, G.J.; Amsterdam, E.A.; Chaitman, B.; Eckel, R.; Fleg, J.; Froelicher, V.F.; Leon, A.S.; Piña, I.L.; Rodney, R.; et al. Exercise standards for testing and training: A statement for healthcare professionals from the American Heart Association. Circulation 2001, 104, 1694–1740. [Google Scholar] [CrossRef]
- Patterson, S.D.; Brandner, C.R. The Role of Blood Flow Restriction Training for Applied Practitioners: A Questionnaire-Based Survey. J. Sports Sci. 2018, 36, 123–130. [Google Scholar] [CrossRef]
- Nakajima, T.; Kurano, M.; Iida, H.; Takano, H.; Oonuma, H.; Morita, T.; Meguro, K.; Sato, Y.; Nagata, T.; KAATSU Training Group. Use and Safety of KAATSU Training:Results of a National Survey. Int. J. KAATSU Train. Res. 2006, 2, 5–13. [Google Scholar] [CrossRef]
- Singer, T.J.; Stavres, J.; Elmer, S.J.; Kilgas, M.A.; Pollock, B.S.; Kearney, S.G.; McDaniel, J. Knee Extension with Blood Flow Restriction: Impact of Cuff Pressure on Hemodynamics. Eur. J. Appl. Physiol. 2020, 120, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Rossow, L.M.; Fahs, C.A.; Loenneke, J.P.; Thiebaud, R.S.; Sherk, V.D.; Abe, T.; Bemben, M.G. Cardiovascular and Perceptual Responses to Blood-Flow-Restricted Resistance Exercise with Differing Restrictive Cuffs. Clin. Physiol. Funct. Imaging 2012, 32, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Spitz, R.W.; Bell, Z.W.; Wong, V.; Viana, R.B.; Dankel, S.J.; Abe, T.; Yamada, Y. Blood Flow Restriction Exercise: Effects of Sex, Cuff Width, and Cuff Pressure on Perceived Lower Body Discomfort. Percept. Mot. Ski. 2021, 128, 353–375. [Google Scholar] [CrossRef]
- Miller, R.M.; Galletti, B.A.R.; Koziol, K.J.; Freitas, E.D.S.; Heishman, A.D.; Black, C.D.; Larson, D.J.; Bemben, D.A.; Bemben, M.G. Perceptual Responses: Clinical versus Practical Blood Flow Restriction Resistance Exercise. Physiol. Behav. 2020, 227, 113137. [Google Scholar] [CrossRef]
- Shimano, T.; Kraemer, W.J.; Spiering, B.A.; Volek, J.S.; Hatfield, D.L.; Silvestre, R.; Vingren, J.L.; Fragala, M.S.; Maresh, C.M.; Fleck, S.J.; et al. Relationship between the Number of Repetitions and Selected Percentages of One Repetition Maximum in Free Weight Exercises in Trained and Untrained Men. J. Strength Cond. Res. 2006, 20, 819–823. [Google Scholar]
- Tibana, R.A.; Prestes, J.; da Cunha Nascimento, D.; Balsamo, S. Comparison of the Number of Repetitions and Perceived Exertion Between Multi-Joint and Single-Joint Exercise At Different Intensities in Untrained Women. Braz. J. Biomotricity 2011, 5, 96–105. [Google Scholar]
- Chen, T.C.; Chen, H.L.; Lin, M.J.; Wu, C.J.; Nosaka, K. Potent Protective Effect Conferred by Four Bouts of Low-Intensity Eccentric Exercise. Med. Sci. Sports Exerc. 2010, 42, 1004–1012. [Google Scholar] [CrossRef]


| Variable | Total (n = 20) | Males (n = 10) | Females (n = 10) |
|---|---|---|---|
| Age (years) | 64.3 ± 4.2 | 63.6 ± 3.2 | 64.9 ± 5.2 |
| Stature (cm) | 171.3 ± 9.8 | 179.0 ± 4.9 | 163.7 ± 6.8 |
| Body mass (kg) | 75.1 ± 11.5 | 81.2 ± 11.5 | 68.9 ± 7.9 |
| BMI (kg/m2) | 25.6 ± 3.7 | 25.3 ± 3.4 | 25.9 ± 4.1 |
| Fat mass (%) | 24.8 ± 10.6 | 21.6 ± 8.0 | 28.0 ± 12.3 |
| Muscle mass (%) | 39.1 ± 10.6 | 44.0 ± 4.6 | 34.2 ± 12.8 |
| Waist circumference (cm) | 83.2 ± 8.7 | 86.2 ± 9.6 | 79.9 ± 6.6 |
| Lipid profile | |||
| Total | 5.4 ± 0.8 | 5.1 ± 0.5 | 5.7 ± 1.0 |
| LDL | 3.1 ± 0.8 | 2.8 ± 0.6 | 3.6 ± 0.9 |
| HDL | 1.5 ± 0.4 | 1.5 ± 0.5 | 1.6 ± 0.4 |
| TRI | 1.5 ± 0.9 | 1.8 ± 1.0 | 1.2 ± 0.5 |
| Blood glucose (mmol/L) | 5.4 ± 0.8 | 5.5 ± 0.7 | 5.3 ± 1.0 |
| SBP (mmHg) | 131.8 ± 8.0 | 129.4 ± 7.7 | 133.6 ± 7.8 |
| DBP (mmHg) | 84.0 ± 8.3 | 83.5 ± 9.5 | 84.5 ± 8.0 |
| MAP (mmHg) | 99.9 ± 7.7 | 98.8 ± 8.7 | 101.2 ± 6.7 |
| PP (mmHg) | 47.8 ± 6.2 | 45.9 ± 4.5 | 50.0 ± 7.4 |
| ABPI | 1.2 ± 0.1 | 1.2 ± 0.1 | 1.2 ± 0.1 |
| IPAQ-SF | |||
| Physical activity category | moderate | moderate | moderate |
| Leg Press | LLRE-BFR | LLRE | MLRE |
|---|---|---|---|
| HRmean | 86 ± 3 | 84 ± 3 | 84 ± 3 |
| HRpeak | 94 ± 3 | 93 ± 3 | 96 ± 3 |
| SBP | 145 ± 3 | 131 ± 4 | 140 ± 4 |
| DBP | 87 ± 2 | 83 ± 2 | 85 ± 3 |
| MAP | 106 ± 2 | 99 ± 3 | 103 ± 3 |
| RPP | 132 ± 6 | 117 ± 4 | 134 ± 7 |
| PP | 58 ± 3 | 48 ± 2 | 54 ± 3 |
| Knee Extension | |||
| HRmean | 88 ± 3 | 83 ± 2 | 83 ± 2 |
| HRpeak | 101 ± 3 ‡†* | 93 ± 3 | 94 ± 3 |
| SBP | 144 ± 5 | 143 ± 4 | 146 ± 3 |
| DBP | 86 ± 2 * | 84 ± 2 * | 84 ± 2 * |
| MAP | 106 ± 3 | 104 ± 2 | 105 ± 2 |
| RPP | 146 ± 6 * | 131 ± 5 * | 135 ± 5 * |
| PP | 58 ± 4 * | 53 ± 3 * | 61 ± 3 * |
| LLRE-BFR | LLRE | MLRE | ||||
|---|---|---|---|---|---|---|
| PAAS (0–4) | Pre | Post | Pre | Post | Pre | Post |
| Positive affect | 2.5 ± 0.3 | 2.9 ± 0.2 a | 2.8 ± 0.2 | 2.9 ± 0.2 a | 2.8 ± 0.2 | 3.0 ± 0.2 a |
| Negative affect | 0.2 ± 0.1 | 0.1 ± 0.1 a | 0.2 ± 0.1 | 0.1 ± 0.1 a | 0.3 ± 0.1 | 0.1 ± 0.1 a |
| Fatigue | 0.6 ± 0.1 | 0.8 ± 0.2 | 0.6 ± 0.1 | 0.5 ± 0.1 | 0.6 ± 0.1 | 0.7 ± 0.1 |
| Tranquillity | 2.6 ± 0.2 | 2.9 ± 0.2a | 2.7 ± 0.2 | 2.8 ± 0.2 a | 2.6 ± 0.2 | 3.1 ± 0.2 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parkington, T.; Maden-Wilkinson, T.; Klonizakis, M.; Broom, D. Comparative Perceptual, Affective, and Cardiovascular Responses between Resistance Exercise with and without Blood Flow Restriction in Older Adults. Int. J. Environ. Res. Public Health 2022, 19, 16000. https://doi.org/10.3390/ijerph192316000
Parkington T, Maden-Wilkinson T, Klonizakis M, Broom D. Comparative Perceptual, Affective, and Cardiovascular Responses between Resistance Exercise with and without Blood Flow Restriction in Older Adults. International Journal of Environmental Research and Public Health. 2022; 19(23):16000. https://doi.org/10.3390/ijerph192316000
Chicago/Turabian StyleParkington, Thomas, Thomas Maden-Wilkinson, Markos Klonizakis, and David Broom. 2022. "Comparative Perceptual, Affective, and Cardiovascular Responses between Resistance Exercise with and without Blood Flow Restriction in Older Adults" International Journal of Environmental Research and Public Health 19, no. 23: 16000. https://doi.org/10.3390/ijerph192316000
APA StyleParkington, T., Maden-Wilkinson, T., Klonizakis, M., & Broom, D. (2022). Comparative Perceptual, Affective, and Cardiovascular Responses between Resistance Exercise with and without Blood Flow Restriction in Older Adults. International Journal of Environmental Research and Public Health, 19(23), 16000. https://doi.org/10.3390/ijerph192316000






