Application of Percutaneous Needle Electrolysis Does Not Elicit Temperature Changes: An In Vitro Cadaveric Study
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Cadaveric Specimens
2.3. Temperature Assessment
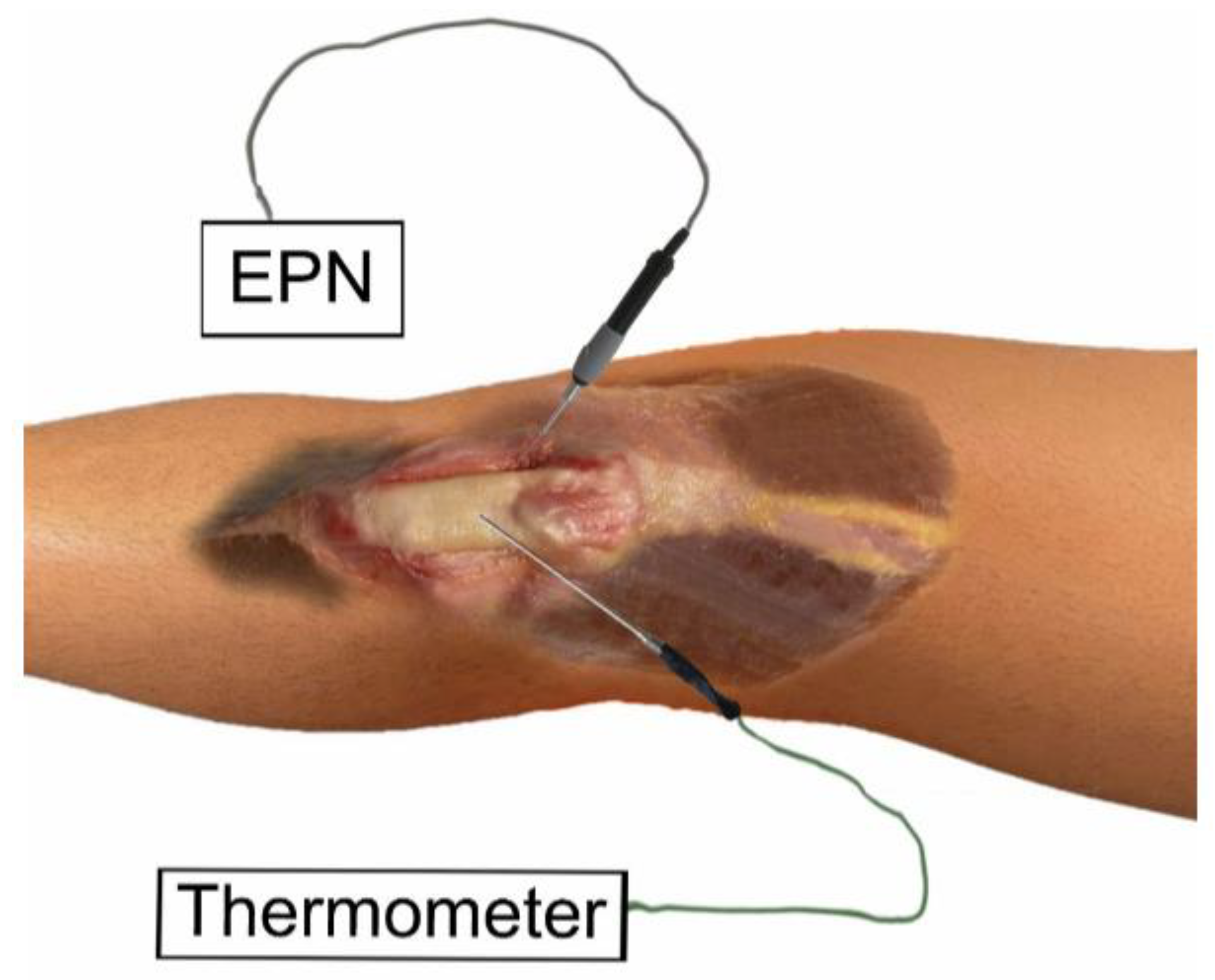
2.4. Needling Procedure
- (A)
- (B)
- (C)
2.5. Percutaneous Needle Electrolysis Procedure
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sánchez Ibañez, J.M. Clinical Course in the Treatment of Chronic Patellar Tendinopathy through Ultrasound Guided Percutaneous Electrolysis Intratissue (EPI®): Study of a Population Series of Cases in Sport. Ph.D. Thesis, Atlantic International University, Honolulu, HI, USA, 2009. [Google Scholar]
- Abat, F.; Valles, S.L.; Gelber, P.E.; Polidori, F.; Jorda, A.; García-Herreros, S.; Monllau, J.C.; Sanchez-Ibáñez, J.M. An Experimental Study of Muscular Injury Repair in a Mouse Model of Notexin-Induced Lesion with EPI® Technique. BMC Sports Sci. Med. Rehabil. 2015, 7, 7. [Google Scholar] [CrossRef]
- Mattiussi, G.; Moreno, C. Treatment of Proximal Hamstring Tendinopathy Related Sciatic Nerve Entrapment: Presentation of an Ultrasound-Guided “Intratissue Percutaneous Electrolysis” Application. Muscles Ligaments Tendons J. 2016, 6, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Margalef, R.; Valera-Garrido, F.; Minaya-Muñoz, F.; Bosque, M.; Ortiz, N.; Santafe, M.M. Percutaneous Needle Electrolysis Reverses Neurographic Signs of Nerve Entrapment by Induced Fibrosis in Mice. Evid.-Based Complement. Altern. Med. 2020, 2020, 6615563. [Google Scholar] [CrossRef] [PubMed]
- Colmena, C. New Technique in Tendon Sport Recovery. Percutaneous Electrolysis Intratissue (EPI®). Int. J. Phys. Med. R. habil. 2013, 1, 1000113. [Google Scholar] [CrossRef]
- Zhao, M. Electrical Fields in Wound Healing-An Overriding Signal That Directs Cell Migration. Semin. Cell Dev. Biol. 2009, 20, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Huguet, M.; Góngora-Rodríguez, J.; Rodríguez-Huguet, P.; Ibañez-Vera, A.J.; Rodríguez-Almagro, D.; Martín-Valero, R.; Díaz-Fernández, Á.; Lomas-Vega, R. Effectiveness of percutaneous electrolysis in supraspinatus tendinopathy: A single-blinded randomized controlled trial. J. Clin. Med. 2020, 6, 1837. [Google Scholar] [CrossRef] [PubMed]
- Abat, F.; Gelber, P.E.; Polidori, F.; Monllau, J.C.; Sanchez-Ibañez, J.M. Clinica Results after Ultrasound-Guided Intratissue Percutaneous Electrolysis (EPI®) and Eccentric Exercise in the Treatment of Patellar Tendinopathy. Knee Surg. Sport. Traumatol. Arthrosc. 2015, 23, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- García Naranjo, J.; Barroso Rosa, S.; Loro Ferrer, J.F.; Limiñana Cañal, J.M.; Suarez Hernández, E. A Novel Approach in the Treatment of Acute Whiplash Syndrome: Ultrasound-Guided Needle Percutaneous Electrolysis. A Randomized Controlled Trial. Orthop. Traumatol. Surg. Res. 2017, 103, 1229–1234. [Google Scholar] [CrossRef]
- Gómez-Chiguano, G.F.; Navarro-Santana, M.J.; Cleland, J.A.; Arias-Buría, J.L.; Fernández-de-Las-Peñas, C.; Ortega-Santiago, R.; Plaza-Manzano, G. Effectiveness of Ultrasound-Guided Percutaneous Electrolysis for Musculoskeletal Pain: A Systematic Review and Meta-Analysis. Pain Med. 2021, 22, 1055–1071. [Google Scholar] [CrossRef]
- Carvajal, O.; Alvarez, D.; Medina, F.; Minaya, F. Effects of Percutaneous Needle Electrolysis of the Patellar Tendon on Local and Contralateral Temperature, Measured with Infrared Thermography. Rev. Fisioter. Invasiva 2016, 1, 18–25. [Google Scholar]
- Margalef, R.; Bosque, M.; Minaya-Muñoz, F.; Valera-Garrido, F.; Santafe, M.M. Safety Analysis of Percutaneous Needle Electrolysis: A Study of Needle Composition, Morphology, and Electrical Resistance. Acupunct. Med. 2021, 39, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Sanz, J.; López-de-Celis, C.; Hidalgo-García, C.; González-Rueda, V.; Ragazzi, P.; Bueno-Gracia, E.; Llurda-Almuzara, L.; Pérez-Bellmunt, A. Is tecar therapy effective on biceps femoris and quadriceps rehabilitation? a cadaveric study. J. Sport Rehabil. 2022, 1, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Bellmunt, A.; Caballé-Serrano, J.; Rodríguez-Sanz, J.; Hidalgo-García, C.; González-Rueda, V.; Gassó-Villarejo, S.; Zegarra-Chávez, D.; López-de-Celis, C. Comparison of resistive capacitive energy transfer therapy on cadaveric molars and incisors with and without implants. Sci. Rep. 2022, 12, 11845. [Google Scholar] [CrossRef] [PubMed]
- McDaniels, A.; Pittman, D.; Cotter, A. Recommendations for Best Needling Practices with Respect to Skin Preparation. Med. Acupunct. 2012, 24, 67. [Google Scholar] [CrossRef]
- Standring, S. Gray’s Anatomy, 40th ed.; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Xian, Z.; Ali, S. Crossed Doubled Patellar Tendon: A Rare Anatomical Variant with Potential Clinical Significance. Radiol. Case Rep. 2021, 16, 3034–3038. [Google Scholar] [CrossRef]
- Macchi, V.; Stocco, E.; Stecco, C.; Belluzzi, E.; Favero, M.; Porzionato, A.; De Caro, R. The Infrapatellar Fat Pad and the Synovial Membrane: An Anatomo-Functional Unit. J. Anat. 2018, 233, 146–154. [Google Scholar] [CrossRef]
- Pierce, C.; Block, R.; Aguinis, H. Cautionary Note on Reporting Eta-Squared Values from Multifactor ANOVA Designs. Educ. Psychol. Meas. 2004, 64, 916–924. [Google Scholar] [CrossRef]
- Bryant Jeri, L.; Boughter John, D.; Gong, S.; LeDoux Mark, S.H.D.H. Current Body Composition Measurement Techniques. Physiol. Behav. 2010, 32, 41–52. [Google Scholar] [CrossRef]
- Rutkove, S.B.; Sanchez, B. Electrical Impedance Methods in Neuromuscular Assessment: An Overview. Cold Spring Harb. Perspect. Med. 2019, 9, a034405. [Google Scholar] [CrossRef]
- Lopez-Martos, R.; Gonzalez-Perez, L.M.; Ruiz-Canela-Mendez, P.; Urresti-Lopez, F.J.; Gutierrez-Perez, J.L.; Infante-Cossio, P. Randomized, Double-Blind Study Comparing Percutaneous Electrolysis and Dry Needling for the Management of Temporomandibular Myofascial Pain. Med. Oral Patol. Oral Cir. Bucal 2018, 23, e454–e462. [Google Scholar] [CrossRef]
- Varela-Rodríguez, S.; Sánchez-Sánchez, J.L.; Velasco, E.; Delicado-Miralles, M.; Sánchez-González, J.L. Endogenous Pain Modulation in Response to a Single Session of Percutaneous Electrolysis in Healthy Population: A Double-Blinded Randomized Clinical Trial. J. Clin. Med. 2022, 11, 2889. [Google Scholar] [CrossRef] [PubMed]
- Peñin-Franch, A.; García-Vidal, J.A.; Martínez, C.M.; Escolar-Reina, P.; Martínez-Ojeda, R.M.; Gómez, A.I.; Bueno, J.M.; Minaya-Muñoz, F.; Valera-Garrido, F.; Medina-Mirapeix, F.; et al. Galvanic Current Activates the NLRP3 Inflammasome to Promote Type I Collagen Production in Tendon. Elife 2022, 11, e73675. [Google Scholar] [CrossRef] [PubMed]
- Wemyss-Holden, S.A.; Berry, D.P.; Robertson, G.S.M.; Dennison, A.R.; Hall, P.D.L.M.; Maddern, G.J. Electrolytic Ablation as an Adjunct to Liver Resection: Safety and Efficacy in Patients. ANZ J. Surg. 2002, 72, 589–593. [Google Scholar] [CrossRef] [PubMed]


| (3:3:3) 1/(1.5:3:3) 2 | (1:24:1) | |
|---|---|---|
| Mean ± SD | Mean ± SD | |
| Tendon before (°C) | 31.2 ± 3.0 | 31.1 ± 1.2 |
| Tendon after (°C) | 31.2 ± 3.1 | 31.0 ± 1.3 |
| Fat before (°C) | 31.7 ± 2.6 | 31.1 ± 1.3 |
| Fat after (°C) | 31.7 ± 2.7 | 31.1 ± 1.3 |
| Muscle before (°C) | 31.5 ± 3.4 | 30.9 ± 1.1 |
| Muscle after (°C) | 31.9 ± 3.9 | 31.0 ± 1.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borrella-Andrés, S.; Malo-Urriés, M.; Pérez-Bellmunt, A.; Arias-Buría, J.L.; Rodríguez-Sanz, J.; Albarova-Corral, M.I.; González-Rueda, V.; Gallego-Sendarrubias, G.M.; Fernández-de-las-Peñas, C.; López-de-Celis, C. Application of Percutaneous Needle Electrolysis Does Not Elicit Temperature Changes: An In Vitro Cadaveric Study. Int. J. Environ. Res. Public Health 2022, 19, 15738. https://doi.org/10.3390/ijerph192315738
Borrella-Andrés S, Malo-Urriés M, Pérez-Bellmunt A, Arias-Buría JL, Rodríguez-Sanz J, Albarova-Corral MI, González-Rueda V, Gallego-Sendarrubias GM, Fernández-de-las-Peñas C, López-de-Celis C. Application of Percutaneous Needle Electrolysis Does Not Elicit Temperature Changes: An In Vitro Cadaveric Study. International Journal of Environmental Research and Public Health. 2022; 19(23):15738. https://doi.org/10.3390/ijerph192315738
Chicago/Turabian StyleBorrella-Andrés, Sergio, Miguel Malo-Urriés, Albert Pérez-Bellmunt, José L. Arias-Buría, Jacobo Rodríguez-Sanz, María Isabel Albarova-Corral, Vanessa González-Rueda, Gracia M. Gallego-Sendarrubias, César Fernández-de-las-Peñas, and Carlos López-de-Celis. 2022. "Application of Percutaneous Needle Electrolysis Does Not Elicit Temperature Changes: An In Vitro Cadaveric Study" International Journal of Environmental Research and Public Health 19, no. 23: 15738. https://doi.org/10.3390/ijerph192315738
APA StyleBorrella-Andrés, S., Malo-Urriés, M., Pérez-Bellmunt, A., Arias-Buría, J. L., Rodríguez-Sanz, J., Albarova-Corral, M. I., González-Rueda, V., Gallego-Sendarrubias, G. M., Fernández-de-las-Peñas, C., & López-de-Celis, C. (2022). Application of Percutaneous Needle Electrolysis Does Not Elicit Temperature Changes: An In Vitro Cadaveric Study. International Journal of Environmental Research and Public Health, 19(23), 15738. https://doi.org/10.3390/ijerph192315738










