In-Vitro Assessment of the Corrosion Potential of an Oral Strain of Sulfate-Reducing Bacteria on Metallic Orthodontic Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Selection
2.2. Assembly of Samples
2.3. Assessment of Corrosion Potential
3. Results
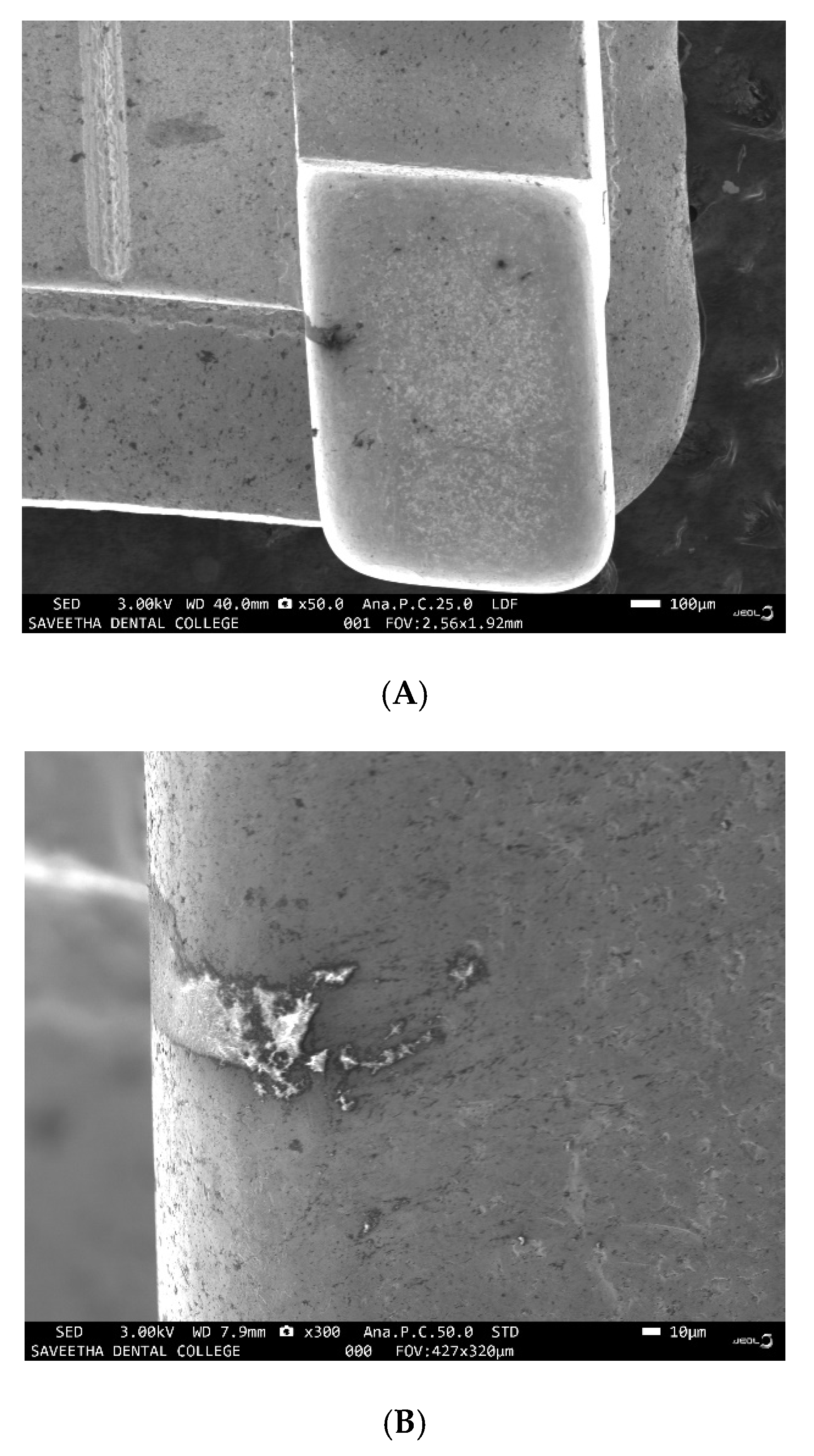
3.1. Evaluation of Corrosion Potential of Artificial Saliva and Culture Medium
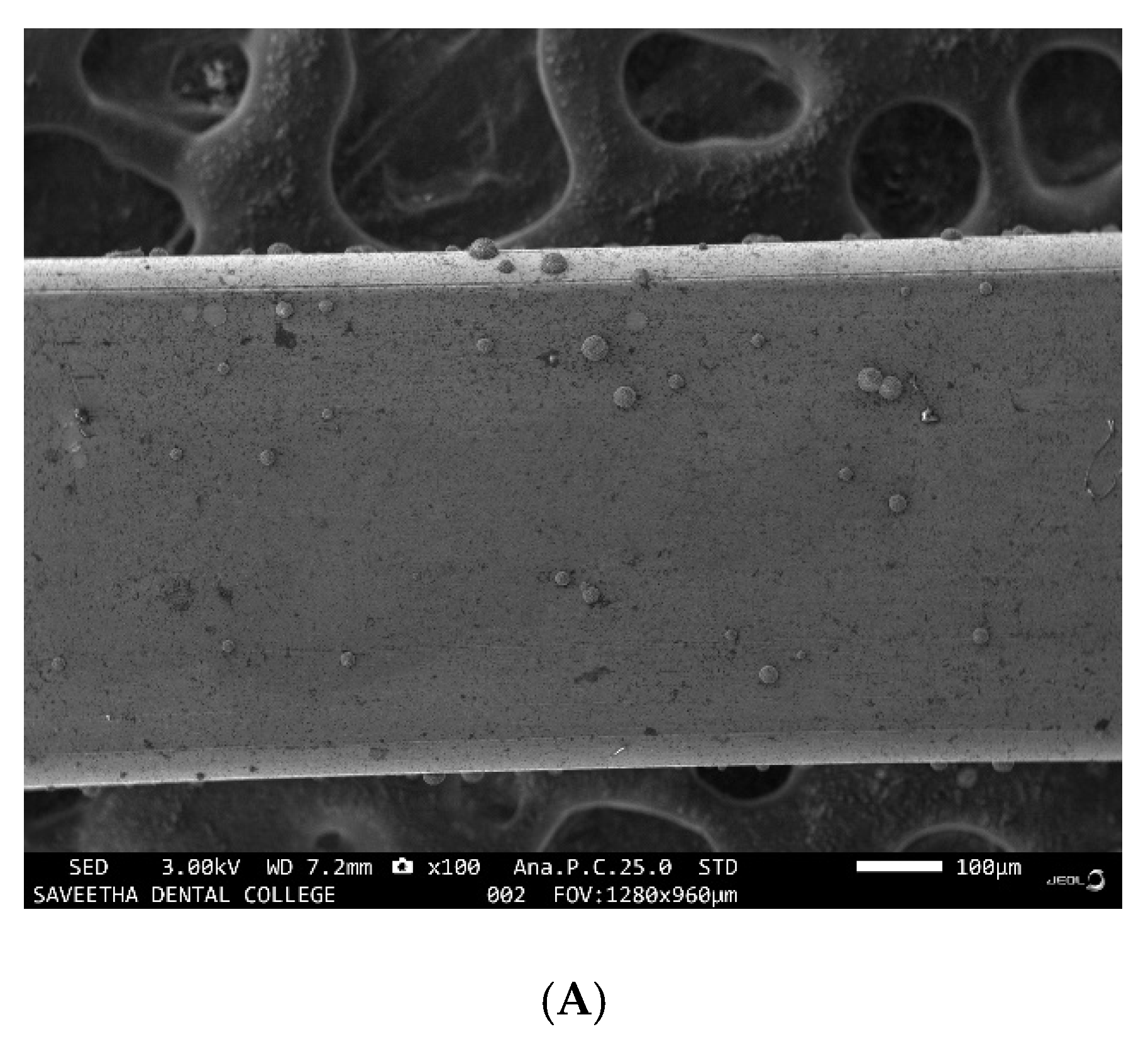
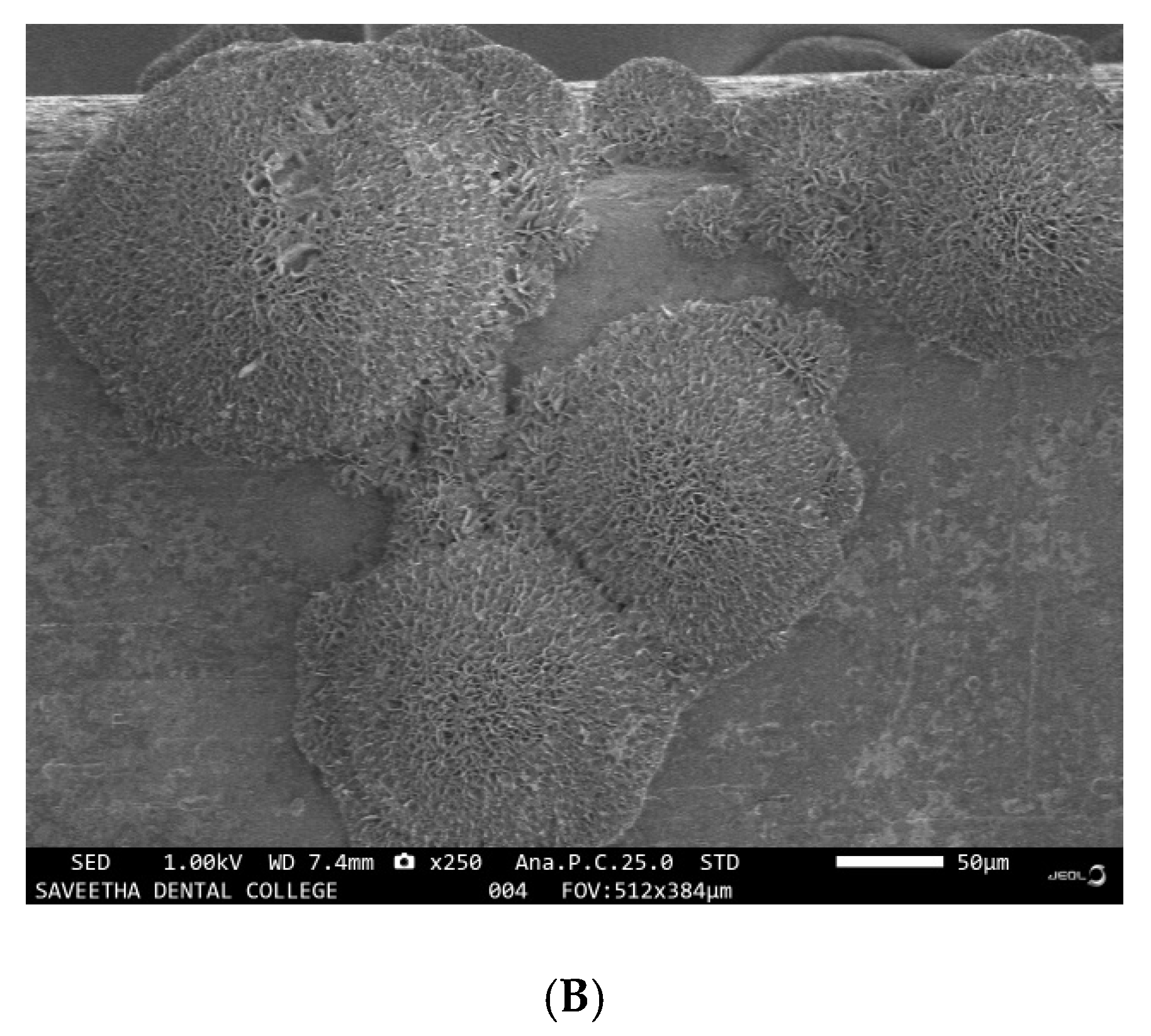
3.2. Evaluation of the Corrosive Potential of the Genus Desulfovibrio
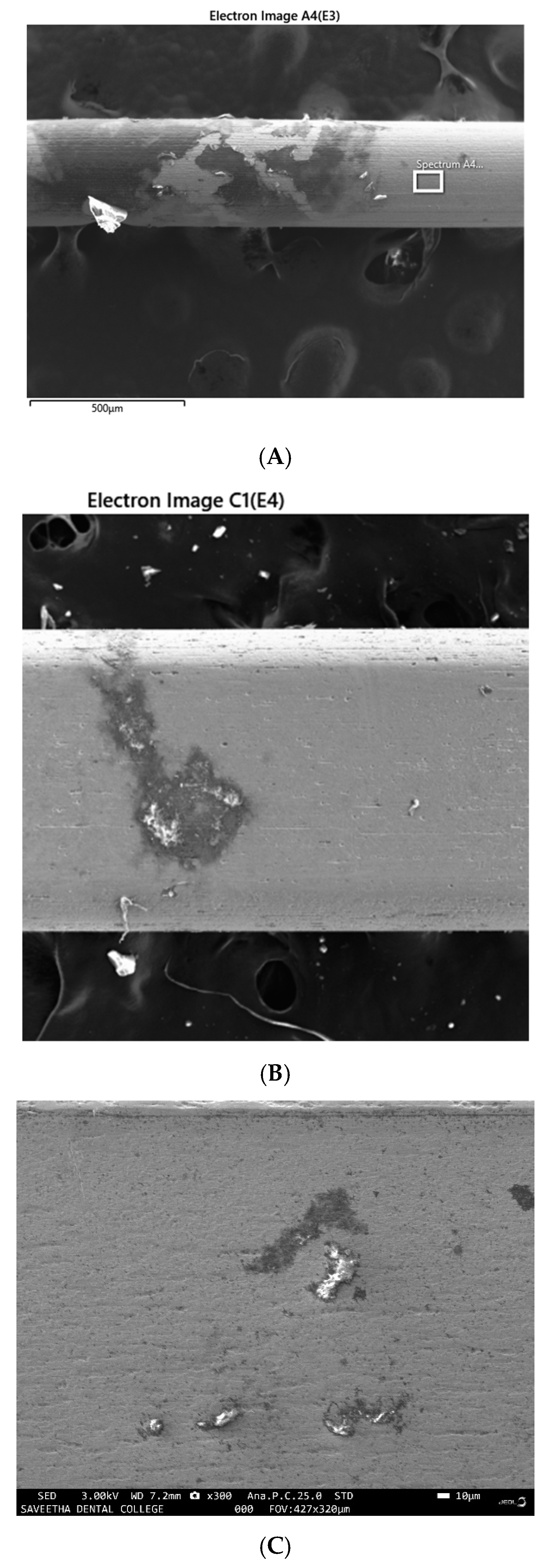
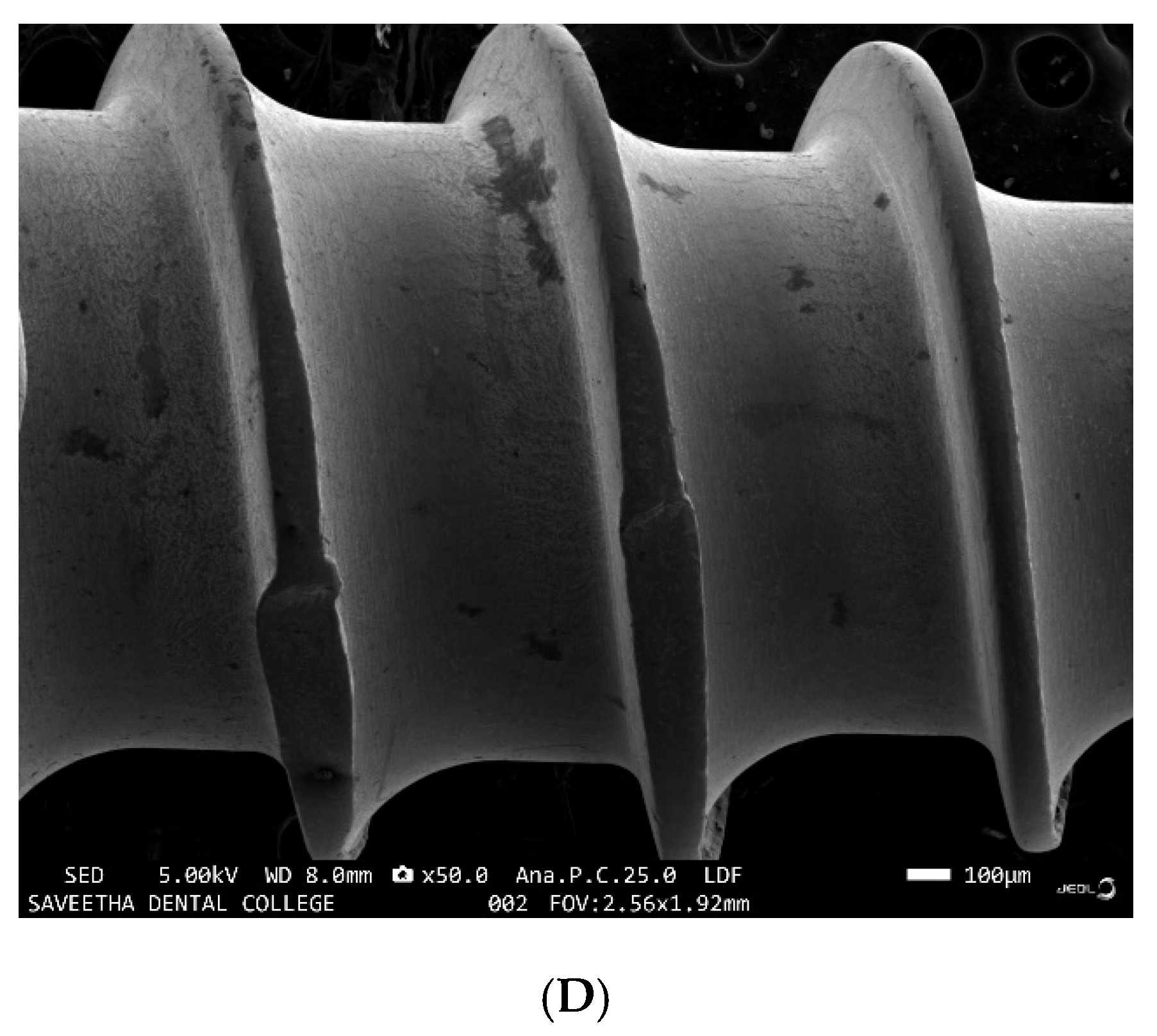
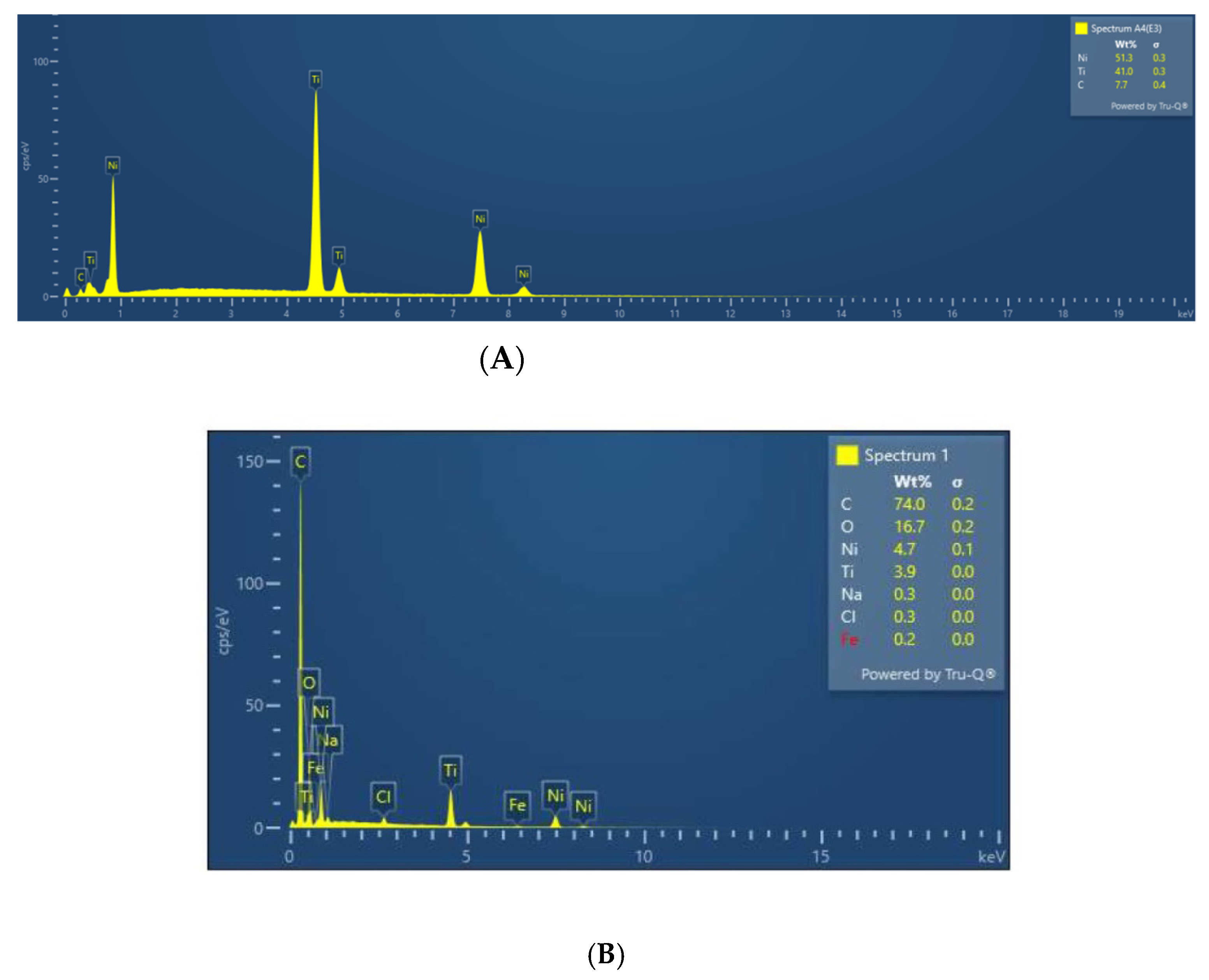
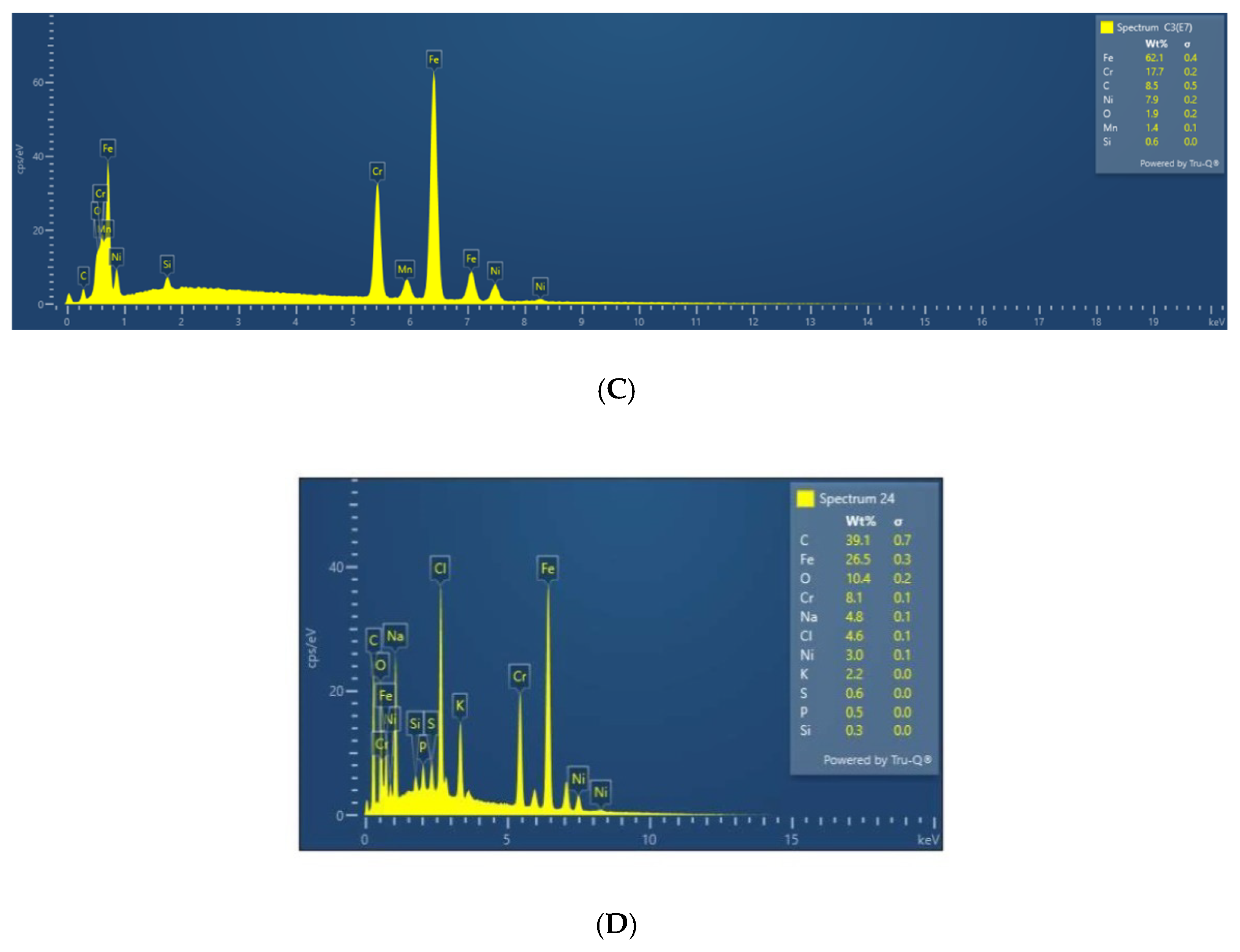
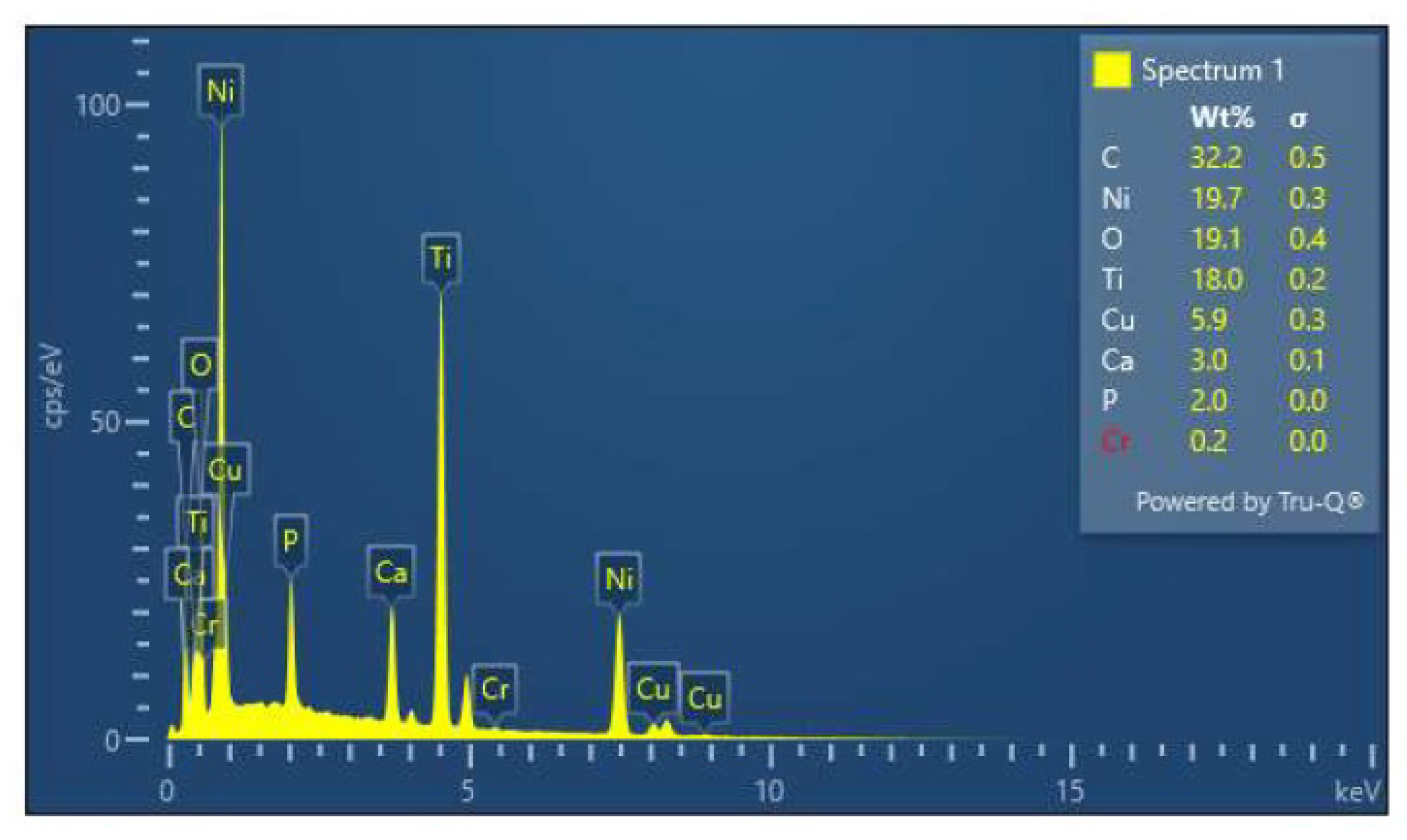
3.3. EDX Analysis of the Samples before and after Immersion in Media with SRB
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stott, J.F.D. What progress in the understanding of microbially induced corrosion has been made in the last 25 years? A personal viewpoint. Corros. Sci. 1993, 35, 667–673. [Google Scholar] [CrossRef]
- Iverson, W.P. Microbial Corrosion of Metals. Adv. Appl. Microbiology. 1987, 32, 1–36. [Google Scholar] [CrossRef]
- Gopalakrishnan, U.; Sudhakar, R.; Felicita, S.; Manikandan, K.; Selvaraj, V. Bibliometric analysis on microbial corrosion in dentistry. Int. J. Orthod. Rehabil. 2022, 13, 22–33. [Google Scholar] [CrossRef]
- Heggendorn, F.L.; Fraga, A.G.M.; Ferreira, D.D.C.; Gonçalves, L.S.; Lione, V.D.O.F.; Lutterbach, M.T.S. Sulfate-Reducing Bacteria: Biofilm Formation and Corrosive Activity in Endodontic Files. Int. J. Dent. 2018, 2018, 8303450. [Google Scholar] [CrossRef] [PubMed]
- Mikulewicz, M.; Chojnacka, K. Trace Metal Release from Orthodontic Appliances by In Vivo Studies: A Systematic Literature Review. Biol. Trace Elem. Res. 2009, 137, 127–138. [Google Scholar] [CrossRef]
- Behroozi, Z.; Danaei, S.M.; Sardarian, A.R.; Moshkelghosha, V.; Sardarian, A.R. Evaluation of the Corrosion of Five Different Bracket-Archwire Combination: An In-Vitro Analysis Using Inductively Coupled Plasma Mass Spectrometry. J. Dent. 2016, 17, 262–267. [Google Scholar]
- Willis, C.L.; Gibson, G.R.; Allison, C.; Macfarlane, S.; Holt, J.S. Growth, incidence and activities of dissimilatory sulfate-reducing bacteria in the human oral cavity. FEMS Microbiol. Lett. 1995, 129, 267–271. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Langendijk, P.S.; Hagemann, J.; Van Der Hoeven, J.S. Sulfate-reducing bacteria in periodontal pockets and in healthy oral sites. J. Clin. Periodontol. 1999, 26, 596–599. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, U.; Murthy, R.T.; Felicita, A.S.; Alshehri, A.; Awadh, W.; Almalki, A.; Vinothkumar, T.S.; Baeshen, H.A.; Bhandi, S.; Kathir, A.; et al. Sulfate-Reducing Bacteria in Patients Undergoing Fixed Orthodontic Treatment. Int. Dent. J. 2022. [Google Scholar] [CrossRef] [PubMed]
- Heggendorn, F.L.; Gonçalves, L.S.; Dias, E.P.; Lione, V.D.O.F.; Lutterbach, M.T.S. Biocorrosion of Endodontic Files through the Action of Two Species of Sulfate-reducing Bacteria: Desulfovibrio desulfuricans and Desulfovibrio fairfieldensis. J. Contemp. Dent. Pract. 2015, 16, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Renita, D.; Rajendran, S.C. Influence of Artificial Saliva on the Corrosion Behavior of Dental Alloys: A Review. Indian J. Adv. Chem. Sci. 2016, 4, 478–483. [Google Scholar]
- Corfield, A.P. Mucins: A biologically relevant glycan barrier in mucosal protection. Biochim. Biophys. Acta 2015, 1850, 236–252. [Google Scholar] [CrossRef] [PubMed]
- Von Wolzogen Kühr, C.A.H.; Van der Vlugt, L.S. Graphitization of cast iron as an electrobiochemical process in anaerobic soils. Water 1934, 18, 147–165. [Google Scholar]
- Hamilton, W.A.; Lee, W.L.L.; Barton, E. Biotechnology Handbooks Sulfate-Reducing Bacteria. Biocorrosion 1995, 8, 243–264. [Google Scholar]
- Enning, D.; Garrelfs, J. Corrosion of iron by sulfate-reducing bacteria: New views of an old problem. Appl. Environ. Microbiol. 2014, 80, 1226–1236. [Google Scholar] [CrossRef] [PubMed]
- Gaylarde, C.; Morton, L.H.G. Deteriogenic biofilms on buildings and their control: A review. Biofouling 1999, 14, 59–74. [Google Scholar] [CrossRef]
- Isa, Z.; Grusenmeyer, S.; Verstraete, W. Sulfate Reduction Relative to Methane Production in High-Rate Anaerobic Digestion: Microbiological Aspects. Appl. Environ. Microbiol. 1986, 51, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Gu, T.; Nesic, S.; Hu, A.; Jhobalia, C. Biochemical Engineering Approaches to MIC; Corrosion, NACE. Int.: Houston, TX, USA, 2005. [Google Scholar]
- Heggendorn, F.L.; Silva, G.C.D.C.; Cardoso, E.A.; Castro, H.C.; Gonçalves, L.S.; Dias, E.P.; Lione, V.D.O.F.; Lutterbach, M.T.S. Initial cytotoxicity assays of media for sulfate-reducing bacteria: An endodontic biopharmaceutical product under development. Dent. Mater. J. 2016, 35, 762–768. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gopalakrishnan, U.; Thiagarajan, K.; Felicita, A.S.; Gosh, P.; Alshehri, A.; Awadh, W.; Alzahrani, K.J.; Alzahrani, F.M.; Alsharif, K.F.; Halawani, I.F.; et al. In-Vitro Assessment of the Corrosion Potential of an Oral Strain of Sulfate-Reducing Bacteria on Metallic Orthodontic Materials. Int. J. Environ. Res. Public Health 2022, 19, 15312. https://doi.org/10.3390/ijerph192215312
Gopalakrishnan U, Thiagarajan K, Felicita AS, Gosh P, Alshehri A, Awadh W, Alzahrani KJ, Alzahrani FM, Alsharif KF, Halawani IF, et al. In-Vitro Assessment of the Corrosion Potential of an Oral Strain of Sulfate-Reducing Bacteria on Metallic Orthodontic Materials. International Journal of Environmental Research and Public Health. 2022; 19(22):15312. https://doi.org/10.3390/ijerph192215312
Chicago/Turabian StyleGopalakrishnan, Umarevathi, Kavitha Thiagarajan, A. Sumathi Felicita, Pallabhi Gosh, Abdulrahman Alshehri, Wael Awadh, Khalid J. Alzahrani, Fuad M. Alzahrani, Khalaf F. Alsharif, Ibrahim F. Halawani, and et al. 2022. "In-Vitro Assessment of the Corrosion Potential of an Oral Strain of Sulfate-Reducing Bacteria on Metallic Orthodontic Materials" International Journal of Environmental Research and Public Health 19, no. 22: 15312. https://doi.org/10.3390/ijerph192215312
APA StyleGopalakrishnan, U., Thiagarajan, K., Felicita, A. S., Gosh, P., Alshehri, A., Awadh, W., Alzahrani, K. J., Alzahrani, F. M., Alsharif, K. F., Halawani, I. F., Alshammeri, S., Alamoudi, A., Albar, D. H., Baeshen, H. A., & Patil, S. (2022). In-Vitro Assessment of the Corrosion Potential of an Oral Strain of Sulfate-Reducing Bacteria on Metallic Orthodontic Materials. International Journal of Environmental Research and Public Health, 19(22), 15312. https://doi.org/10.3390/ijerph192215312







