A Novel Index in the Prediction of Pneumonia Following Acute Ischemic Stroke
Abstract
1. Introduction
2. Material and Methods
2.1. Study Population
2.2. Data Collection
2.3. CRP and Hemoglobin
2.4. Diagnosis of Pneumonia
2.5. Ethical Statement
2.6. Statistical Analysis
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, B.D.; Smith, C.J.; Cloud, G.C.; Enderby, P.; James, M.; Paley, L.; Tyrrell, P.J.; Wolfe, C.D.A.; Rudd, A.G.; SSNAP Collaboration. The association between delays in screening for and assessing dysphagia after acute stroke, and the risk of stroke-associated pneumonia. J. Neurol. Neurosurg. Psychiatry 2017, 88, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Al-Khaled, M.; Matthis, C.; Binder, A.; Mudter, J.; Schattschneider, J.; Pulkowski, U.; Strohmaier, T.; Niehoff, T.; Zybur, R.; Eggers, J.; et al. Dysphagia in patients with acute ischemic stroke: Early dysphagia screening may reduce stroke-related Pneumonia and Improve Stroke Outcomes. Cerebrovasc. Dis. 2016, 42, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Cugy, E.; Sibon, I. Stroke-associated pneumonia risk score: Validity in a French stroke unit. J. Stroke Cerebrovasc. Dis. 2017, 26, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Kishore, A.K.; Vail, A.; Chamorro, A.; Garau, J.; Hopkins, S.J.; di Napoli, M.; Kalra, L.; Langhorne, P.; Montaner, J.; Roffe, C.; et al. How is pneumonia diagnosed in clinical stroke research? A systematic review and meta-analysis. Stroke 2015, 46, 1202–1209. [Google Scholar] [CrossRef]
- Szylińska, A.; Kotfis, K.; Bott-Olejnik, M.; Wańkowicz, P.; Rotter, I. Post-Stroke Outcomes of Patients with Chronic Obstructive Pulmonary Disease. Brain Sci. 2022, 12, 106. [Google Scholar] [CrossRef]
- Finlayson, O.; Kapral, M.; Hall, R.; Asllani, E.; Selchen, D.; Saposnik, G. Risk factors, inpatient care, and outcomes of pneumonia after ischemic stroke. Neurology 2011, 77, 1338–1345. [Google Scholar] [CrossRef]
- Westendorp, W.F.; Nederkoorn, P.J.; Vermeij, J.-D.; Dijkgraaf, M.G.; Beek, D. Post-stroke infection: A systematic review and meta-analysis. BMC Neurol. 2011, 11, 110. [Google Scholar] [CrossRef]
- Kwon, H.M.; Jeong, S.W.; Lee, S.H.; Yoon, B.W. The pneumonia score: A simple grading scale for prediction of pneumonia after acute stroke. Am. J. Infect. Control. 2006, 34, 64–68. [Google Scholar] [CrossRef]
- Ovbiagele, B.; Hills, N.K.; Saver, J.L.; Johnston, S.C. Frequency and determinants of pneumonia and urinary tract infection during stroke hospitalization. J. Stroke Cerebrovasc. Dis. 2006, 15, 209–213. [Google Scholar] [CrossRef]
- Bang, O.Y.; Ovbiagele, B.; Kim, J.S. Nontraditional Risk Factors for Ischemic Stroke: An Update. Stroke 2015, 46, 3571–3578. [Google Scholar] [CrossRef]
- Li, L.; Yiin, G.S.; Geraghty, O.C.; Schulz, U.G.; Kuker, W.; Mehta, Z. Incidence, outcome, risk factors, and long-term prognosis of cryptogenic transient ischaemic attack and ischaemic stroke: A population-based study. Lancet Neurol. 2015, 14, 903–913. [Google Scholar] [CrossRef]
- Fabjan, T.H.; Penko, M.; Hojs, R. Anemia on admission and long-term mortality risk in patients with acute ischemic stroke. Adv. Clin. Exp. Med. 2019, 28, 1419–1424. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhou, T.; Li, Y.; Chen, P.; Chen, L. Anemia increases the mortality risk in patients with stroke: A meta-analysis of cohort studies. Sci. Rep. 2016, 23, 26636. [Google Scholar] [CrossRef] [PubMed]
- Ziv-Baran, T.; Wasserman, A.; Goldiner, I.; Stark, M.; Shenhar-Tsarfaty, S.; Shapira, I.; Zeltser, D.; Mailis, I.; Berliner, S.; Rogowski, O. The association between C-reactive protein and common blood tests in apparently healthy individuals undergoing a routine health examination. Clin. Chim. Acta. 2020, 501, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Santos-Silva, M.A.; Sousa, N.; Sousa, J.C. Correlation Analysis between Hemoglobin and C-Reactive Protein in Patients Admitted to an Emergency Unit. J. Clin. Med. 2021, 10, 5411. [Google Scholar] [CrossRef]
- Ji, R.; Shen, H.; Pan, Y.; Wang, P.; Liu, G.; Wang, Y.; Li, H.; Wang, Y. Novel risk score to predict pneumonia after acute ischemic stroke. Stroke 2013, 44, 1303–1309. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.J.; Bray, B.D.; Hoffman, A.; Meisel, A.; Heuschmann, P.U.; Wolfe, C.D.A.; Tyrrell, P.J.; Rudd, A.G.; the Intercollegiate Stroke Working Party Group. Can a novel clinical risk score improve pneumonia prediction in acute stroke care? A UK multicenter cohort study. J. Am. Heart Assoc. 2015, 4, e001307. [Google Scholar] [CrossRef]
- Harms, H.; Grittner, U.; Dröge, H.; Meisel, A. Predicting post-stroke pneumonia: The PANTHERIS score. Acta Neurol. Scand. 2013, 128, 178–184. [Google Scholar] [CrossRef]
- Huang, G.-Q.; Lin, Y.-T.; Wu, Y.-M.; Cheng, Q.-Q.; Cheng, H.-R.; Wang, Z. Individualized prediction of stroke associated pneumonia for patients with acute ischemic stroke. Clin. Interv. Aging 2019, 14, 1951–1962. [Google Scholar] [CrossRef]
- Badrick, T. Evidence-Based Laboratory Medicine. Clin. Biochem. Rev. 2013, 34, 43–46. [Google Scholar]
- Hallworth, M.J. The ‘70% claim’: What is the evidence base? Ann. Clin. Biochem. 2011, 48, 487–488. [Google Scholar] [CrossRef] [PubMed]
- Kalil, A.C.; Metersky, M.L.; Klompas, M.; Muscedere, J.; Sweeney, D.A.; Palmer, L.B.; Napolitano, L.M.; O’Grady, N.P.; Bartlett, J.G.; Carratala, J.; et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. Dis. 2016, 63, e61–e111. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Y.; Wei, L.; Ye, R.C.; Chen, J.; Nie, D.; Zhang, G.; Zhang, X.P. Reducing the incidence of stroke-associated pneumonia: An evidence-based practice. BMC Neurol. 2022, 22, 297. [Google Scholar] [CrossRef] [PubMed]
- Alon, D.; Stein, G.Y.; Korenfeld, R.; Fuchs, S. Predictors and outcomes of infection-related hospital admissions of heart failure patients. PLoS ONE 2013, 8, e72476. [Google Scholar] [CrossRef]
- Jobs, A.; Simon, R.; de Waha, S.; Rogacev, K.; Katalinic, A.; Babaev, V.; Thiele, H. Pneumonia and inflammation in acute decompensated heart failure: A registry-based analysis of 1939 patients. Eur. Heart J. Acute Cardiovasc. Care 2018, 7, 362–370. [Google Scholar] [CrossRef]
- Shen, L.; Jhund, P.S.; Anand, I.S.; Bhatt, A.S.; Desai, A.S.; Maggioni, A.P.; Martinez, F.A.; Pfeffer, M.A.; Rizkala, A.R.; Rouleau, J.L.; et al. Incidence and Outcomes of Pneumonia in Patients With Heart Failure. J. Am. Coll. Cardiol. 2021, 77, 1961–1973. [Google Scholar] [CrossRef]
- Almirall, J.; Bolíbar, I.; Balanzó, X.; González, C.A. Risk factors for community-acquired pneumonia in adults: A population-based case-control study. Eur. Respir. J. 1999, 13, 349–355. [Google Scholar] [CrossRef]
- García-Ordóñez, M.A.; García-Jiménez, J.M.; Páez, F.; Álvarez, F.; Poyato, B.; Franquelo, M.; Colmenero, J.D.; Juárez, C. Clinical aspects and prognostic factors in elderly patients hospitalised for community-acquired pneumonia. Eur. J. Clin. Microbiol. Infect. Dis. 2001, 20, 14–19. [Google Scholar] [CrossRef]
- Lim, W.S.; Lewis, S.; Macfarlane, J.T. Severity prediction rules in community acquired pneumonia: A validation study. Thorax 2000, 55, 219–223. [Google Scholar] [CrossRef]
- Lim, W.S.; van der Eerden, M.M.; Laing, R.; Boersma, W.G.; Karalus, N.; I Town, G.; A Lewis, S.; Macfarlane, J.T. Defining community acquired pneumonia severity on presentation to hospital: An international derivation and validation study. Thorax 2003, 58, 377–382. [Google Scholar] [CrossRef]
- Ramirez, J.A.; Wiemken, T.L.; Peyrani, P.; Arnold, F.W.; Kelley, R.; A Mattingly, W.; Nakamatsu, R.; Pena, S.; E Guinn, B.; Furmanek, S.P.; et al. Adults Hospitalized with Pneumonia in the United States: Incidence, Epidemiology, and Mortality. Clin. Infect. Dis. 2017, 65, 1806–1812. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Stewart, P.; Dales, R.; Johansen, H.; Bryan, S.; Taylor, G. In a retrospective study of chronic obstructive pulmonary disease inpatients, respiratory comorbidities were significantly associated with prognosis. J. Clin. Epidemiol. 2005, 58, 1199–1205. [Google Scholar] [CrossRef] [PubMed]
- Restrepo, M.I.; Mortensen, E.M.; Pugh, J.A.; Anzueto, A. COPD is associated with increased mortality in patients with community-acquired pneumonia. Eur. Respir. J. 2006, 28, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Rello, J.; Rodriguez, A.; Torres, A.; Roig, J.; Sole-Violan, J.; Garnacho-Montero, J.; De La Torree, M.V.; Sirvent, J.M.; Bodí, M. Implications of COPD in patients admitted to the intensive care unit by community-acquired pneumonia. Eur. Respir. J. 2006, 27, 1210–1216. [Google Scholar] [CrossRef]
- Soriano, J.B.; Visick, G.T.; Muellerova, H.; Payvandi, N.; Hansell, A.L. Patterns of comorbidities in newly diagnosed COPD and asthma in primary care. Chest 2005, 128, 2099–2107. [Google Scholar] [CrossRef]
- Müllerova, H.; Chigbo, C.; Hagan, G.W.; Woodhead, M.A.; Miravitlles, M.; Davis, K.J.; Wedzicha, J.A. The natural history of community-acquired pneumonia in COPD patients: A population database analysis. Respir. Med. 2012, 106, 1124–1133. [Google Scholar] [CrossRef]
- Chumbler, N.R.; Williams, L.S.; Wells, C.K.; Lo, A.C.; Nadeau, S.; Peixoto, A.J.; Gorman, M.; Boice, J.L.; Concato, J.; Bravata, D.M. Derivation and validation of a clinical system for predicting pneumonia in acute stroke. Neuroepidemiology 2010, 34, 193–199. [Google Scholar] [CrossRef]
- Hoffmann, S.; Malzahn, U.; Harms, H.; Koennecke, H.; Berger, K.; Kalic, M.; Walter, G.; Meisel, A.; Heuschmann, P.U.; Berlin Stroke Register and the Stroke Register of Northwest Germany. Development of a clinical score (A2DS2) to predict pneumonia in acute ischemic stroke. Stroke 2012, 43, 2617–2623. [Google Scholar] [CrossRef]
- Kumar, S.; Marchina, S.; Massaro, J.; Feng, W.; Lahoti, S.; Selim, M.; Herzig, S.J. ACDD4score: A simple tool for assessing risk of pneumonia after stroke. J. Neurol. Sci. 2017, 372, 399–402. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, L.; Niu, L.; Tian, Y.; Chen, K. Comparison of six risk scores for stroke-associated pneumonia in patients with acute ischemic stroke: A systematic review and Bayesian network meta-analysis. Front. Med. 2022, 9, 964616. [Google Scholar] [CrossRef]
| No Pneumonia (n = 774) | Pneumonia (n = 227) | p | |
|---|---|---|---|
| Demographic data | |||
| Age [years], mean ± SD; Me | 70.55 ± 12.29; 70.0 | 77.09 ± 10.35; 79.0 | <0.001 |
| Gender [male], n (%) | 405 (52.33%) | 118 (51.98%) | 0.927 |
| BMI [kg/m2], mean ± SD; Me | 26.88 ± 4.74; 25.9 | 26.56 ± 4.58; 26.3 | 0.786 |
| Smoking, n (%) | 320 (41.34%) | 97 (42.73%) | 0.709 |
| Comorbidities | |||
| Arterial hypertension, n (%) | 664 (85.79%) | 205 (90.31%) | 0.077 |
| Ischemic heart diseases, n (%) | 167 (21.58%) | 91 (40.09%) | <0.001 |
| Myocardial infarction, n (%) | 74 (9.56%) | 33 (14.54%) | 0.033 |
| NYHA 3 & 4, n (%) | 16 (2.07%) | 26 (11.45%) | <0.001 |
| TIA, n (%) | 146 (18.86%) | 53 (23.35%) | 0.136 |
| History of ischemic stroke, n (%) | 154 (19.90%) | 68 (29.96%) | 0.001 |
| History of hemorrhagic stroke, n (%) | 19 (2.45%) | 5 (2.20%) | 0.827 |
| Acute renal failure on admission, n (%) | 5 (0.65%) | 4 (1.76%) | 0.117 |
| Chronic renal failure, n (%) | 90 (11.63%) | 53 (23.35%) | <0.001 |
| Chronic dialysis, n (%) | 1 (0.13%) | 1 (0.44%) | 0.354 |
| Impaired insulin tolerance, n (%) | 31 (4.01%) | 17 (7.49%) | 0.031 |
| Diabetes on oral medications, n (%) | 151 (19.51%) | 54 (23.79%) | 0.160 |
| Diabetes on insulin, n (%) | 86 (11.13%) | 43 (18.94%) | 0.002 |
| Gout, n (%) | 45 (5.81%) | 20 (8.81%) | 0.107 |
| Extracardiac arteriopathy, n (%) | 290 (37.47%) | 164 (72.25%) | <0.001 |
| COPD, n (%) | 53 (6.85%) | 42 (18.50%) | <0.001 |
| Atrial fibrillation, n (%) | 192 (24.84%) | 101 (44.49%) | <0.001 |
| Carotid artery stenosis, n (%) | 61 (36.53%) | 26 (52.00%) | 0.051 |
| Scale at admission | |||
| Rankin score, mean ± SD; Me | 9.20 ± 6.52; 7.0 | 18.74 ± 8.05; 20.0 | <0.001 |
| NIHSS, mean ± SD; Me | 2.73 ± 1.52; 2.0 | 4.43 ± 1.08; 5.0 | <0.001 |
| Variable | No Pneumonia (n = 774) | Pneumonia (n = 227) | p | ||||
|---|---|---|---|---|---|---|---|
| Mean | Me | ±SD | Mean | Me | ±SD | ||
| Glycemia (mg/dl) | 141.56 | 123.00 | 61.85 | 147.99 | 135.00 | 56.94 | 0.004 |
| Leucocyte count (×109/L) | 9.45 | 8.89 | 3.37 | 11.02 | 9.71 | 5.38 | <0.001 |
| Neutrophil count (×109/L) | 6.61 | 5.82 | 4.35 | 8.26 | 7.18 | 4.93 | <0.001 |
| Lymphocyte count (×109/L) | 2.10 | 1.89 | 2.48 | 1.85 | 1.58 | 1.62 | <0.001 |
| Platelet count (×109/L) | 239.92 | 228.00 | 81.93 | 239.83 | 225.00 | 104.37 | 0.649 |
| Hemoglobin | 13.95 | 14.00 | 1.75 | 13.39 | 13.60 | 2.01 | <0.001 |
| Creatinine | 1.04 | 0.91 | 0.60 | 1.20 | 1.01 | 0.71 | <0.001 |
| C-reactive protein | 10.56 | 2.60 | 25.05 | 46.34 | 16.67 | 71.12 | <0.001 |
| Aspartate aminotransferase | 24.26 | 20.00 | 14.42 | 36.96 | 21.00 | 108.93 | 0.701 |
| Alanine aminotransferase | 22.70 | 18.00 | 14.01 | 29.21 | 17.00 | 72.94 | 0.133 |
| Cholesterol | 199.48 | 195.00 | 53.48 | 171.02 | 167.00 | 49.79 | <0.001 |
| Triglycerides | 146.26 | 122.00 | 91.21 | 121.54 | 105.00 | 63.92 | <0.001 |
| Troponin T | 27.52 | 10.00 | 89.47 | 58.09 | 21.98 | 137.19 | <0.001 |
| CRP/Hgb | 0.83 | 0.19 | 2.07 | 3.73 | 1.15 | 5.98 | <0.001 |
| Variables | No Pneumonia (n = 774) | Pneumonia (n = 227) | p | ||||
|---|---|---|---|---|---|---|---|
| mean ± SD; Me/n (%) | mean ± SD; Me/n (%) | OR | CI − 95% | CI + 95% | |||
| Hospitalization time (days) | 9.34 ± 4.05; 8.0 | 15.43 ± 11.56; 13.0 | 1.168 | 1.131 | 1.206 | <0.001 | |
| NIHSS at discharge | 7.21 ± 10.02; 3.0 | 23.61 ± 13.75; 20.0 | 1.096 | 1.082 | 1.111 | <0.001 | |
| Rankin score at discharge | 1.99 ± 2.02; 1.0 | 4.79 ± 1.39; 5.0 | 2.090 | 1.884 | 2.317 | <0.001 | |
| Mortality until day 7 | 36 (4.65%) | 32 (14.10%) | 3.364 | 2.037 | 5.556 | <0.001 | |
| Mortality until day 30 | 63 (8.14%) | 104 (45.81%) | 9.542 | 6.612 | 13.771 | <0.001 | |
| Mortality until day 90 | 91 (11.76%) | 151 (66.52%) | 14.912 | 10.489 | 21.202 | <0.001 | |
| Mortality until year 1 | 135 (17.44%) | 169 (74.45%) | 13.792 | 9.706 | 19.598 | <0.001 | |
| Outcome | In-hospital death | 38 (4.91%) | 66 (29.07%) | 7.940 | 5.144 | 12.255 | <0.001 |
| Discharged home | 510 (65.89%) | 74 (32.60%) | 0.250 | 0.183 | 0.343 | <0.001 | |
| Nursing home | 44 (5.68%) | 48 (21.15%) | 4.449 | 2.864 | 6.912 | <0.001 | |
| Rehabilitation facility | 172 (22.22%) | 33 (14.54%) | 0.595 | 0.397 | 0.894 | 0.012 | |
| Another ward | 10 (1.29%) | 6 (2.64%) | 2.074 | 0.746 | 5.770 | 0.162 | |
| Variables | p | OR | CI − 95% | CI + 95% |
|---|---|---|---|---|
| Demographic data | ||||
| Age [years] | <0.001 | 1.051 | 1.036 | 1.066 |
| Gender [male] | 0.927 | 0.986 | 0.734 | 1.326 |
| BMI [kg/m2] | 0.367 | 0.985 | 0.954 | 1.018 |
| Smoking | 0.709 | 1.059 | 0.785 | 1.428 |
| Co-morbidities | ||||
| Arterial hypertension | 0.079 | 1.544 | 0.952 | 2.504 |
| Ischemic heart diseases | <0.001 | 2.432 | 1.773 | 3.336 |
| Myocardial infarction | 0.034 | 1.609 | 1.036 | 2.498 |
| Heart failure III and IV NYHA | <0.001 | 6.128 | 3.225 | 11.644 |
| TIA | 0.137 | 1.310 | 0.917 | 1.871 |
| History of ischemic stroke | 0.001 | 1.722 | 1.232 | 2.405 |
| History of hemorrhagic stroke | 0.827 | 0.895 | 0.330 | 2.424 |
| Acute renal failure on admission | 0.133 | 2.759 | 0.735 | 10.361 |
| Chronic renal failure | <0.001 | 2.315 | 1.586 | 3.378 |
| Chronic dialysis | 0.384 | 3.436 | 0.214 | 55.147 |
| Impaired insulin tolerance | 0.033 | 1.940 | 1.053 | 3.575 |
| Diabetes on oral medications | 0.161 | 1.288 | 0.904 | 1.834 |
| Diabetes on insulin | 0.002 | 1.867 | 1.251 | 2.787 |
| Gout | 0.110 | 1.565 | 0.904 | 2.710 |
| Extracardiac arteriopathy | <0.001 | 4.345 | 3.139 | 6.013 |
| Atrial fibrillation | <0.001 | 2.426 | 1.782 | 3.302 |
| COPD | <0.001 | 3.088 | 1.997 | 4.776 |
| Carotid artery stenosis | 0.052 | 1.883 | 0.995 | 3.563 |
| Scale at admission | ||||
| Rankin score | <0.001 | 1.175 | 1.148 | 1.203 |
| NIHSS | <0.001 | 2.436 | 2.112 | 2.809 |
| Laboratory data | ||||
| Glycemia 0 (mg/dl) | 0.164 | 1.002 | 0.999 | 1.004 |
| Leucocyte count (×109/L) | <0.001 | 1.094 | 1.055 | 1.135 |
| Neutrophil count (×109/L) | <0.001 | 1.088 | 1.047 | 1.131 |
| Lymphocyte count (×109/L) | 0.064 | 0.854 | 0.724 | 1.009 |
| Platelet count (×109/L) | 0.990 | 1.000 | 0.998 | 1.002 |
| Hemoglobin | <0.001 | 0.848 | 0.783 | 0.919 |
| Creatinine | 0.003 | 1.444 | 1.134 | 1.838 |
| C-reactive protein | <0.001 | 1.020 | 1.015 | 1.025 |
| Aspartate aminotransferase | 0.295 | 1.004 | 0.996 | 1.013 |
| Alanine aminotransferase | 0.281 | 1.004 | 0.997 | 1.011 |
| Cholesterol | <0.001 | 0.989 | 0.985 | 0.992 |
| Triglycerides | <0.001 | 0.996 | 0.993 | 0.998 |
| Troponin T | 0.002 | 1.002 | 1.001 | 1.004 |
| CRP/HGB | <0.001 | 1.266 | 1.198 | 1.339 |
| p | OR | CI − 95% | CI + 95% | |
|---|---|---|---|---|
| NYHA III & IV | <0.001 | 4.555 | 1.979 | 10.487 |
| COPD | <0.001 | 2.659 | 1.540 | 4.593 |
| Extracardiac atherosclerosis | <0.001 | 3.055 | 2.060 | 4.530 |
| NIHHS score (points) | <0.001 | 1.152 | 1.122 | 1.181 |
| CRP/HGB | <0.001 | 1.166 | 1.101 | 1.234 |
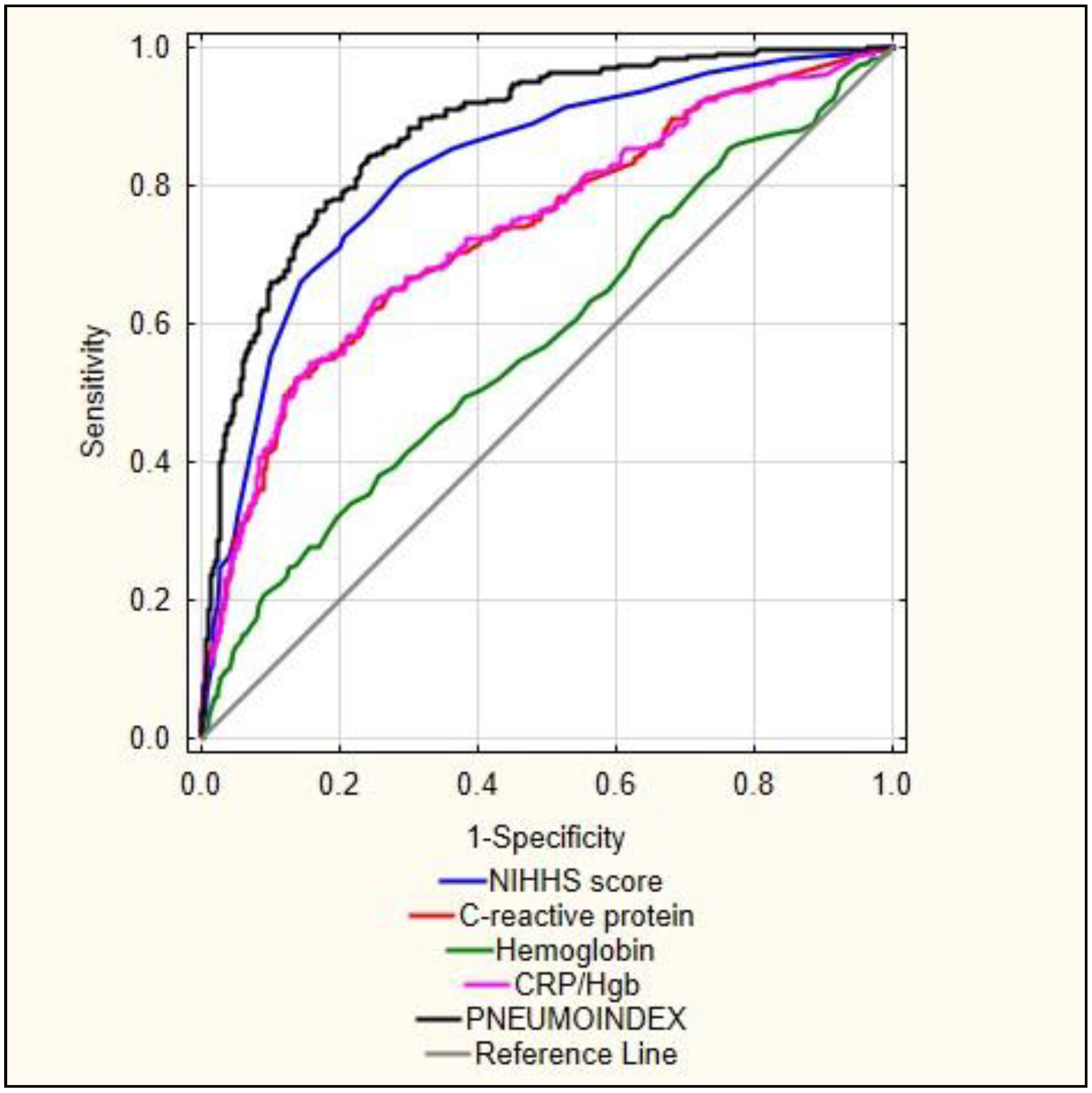
| AUC | AUC − 95% | AUC + 95% | p | |
|---|---|---|---|---|
| PNEUMOINDEX | 0.876 | 0.851 | 0.902 | <0.001 |
| NIHHS score | 0.825 | 0.794 | 0.856 | <0.001 |
| CRP/Hgb | 0.735 | 0.696 | 0.775 | <0.001 |
| C-reactive protein | 0.734 | 0.695 | 0.773 | <0.001 |
| Haemoglobin | 0.577 | 0.534 | 0.621 | <0.001 |
| p | OR | CI − 95% | CI + 95% | |
|---|---|---|---|---|
| PNEUMOINDEX | <0.001 | 2.738 | 2.381 | 3.149 |
| PNEUMOINDEX * | <0.001 | 2.639 | 2.292 | 3.037 |
| PNEUMOINDEX ** | <0.001 | 2.636 | 2.281 | 3.047 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szylińska, A.; Bott-Olejnik, M.; Wańkowicz, P.; Karoń, D.; Rotter, I.; Kotfis, K. A Novel Index in the Prediction of Pneumonia Following Acute Ischemic Stroke. Int. J. Environ. Res. Public Health 2022, 19, 15306. https://doi.org/10.3390/ijerph192215306
Szylińska A, Bott-Olejnik M, Wańkowicz P, Karoń D, Rotter I, Kotfis K. A Novel Index in the Prediction of Pneumonia Following Acute Ischemic Stroke. International Journal of Environmental Research and Public Health. 2022; 19(22):15306. https://doi.org/10.3390/ijerph192215306
Chicago/Turabian StyleSzylińska, Aleksandra, Marta Bott-Olejnik, Paweł Wańkowicz, Dariusz Karoń, Iwona Rotter, and Katarzyna Kotfis. 2022. "A Novel Index in the Prediction of Pneumonia Following Acute Ischemic Stroke" International Journal of Environmental Research and Public Health 19, no. 22: 15306. https://doi.org/10.3390/ijerph192215306
APA StyleSzylińska, A., Bott-Olejnik, M., Wańkowicz, P., Karoń, D., Rotter, I., & Kotfis, K. (2022). A Novel Index in the Prediction of Pneumonia Following Acute Ischemic Stroke. International Journal of Environmental Research and Public Health, 19(22), 15306. https://doi.org/10.3390/ijerph192215306







