Effect of a Child-Owned Poultry Intervention Providing Eggs on Nutrition Status and Motor Skills of Young Children in Southern Ethiopia: A Cluster Randomized and Controlled Community Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Participants and Sample Size
2.2. Intervention
2.2.1. Chicken and Caging Gift Ceremony Model
2.2.2. Nutrition and Poultry Promotion
2.3. Data Collection and Measurements
2.4. Statistical Method
2.5. Ethics
3. Results
3.1. Baseline Characteristics
3.2. Intestinal Helminthiasis and Malaria Infection
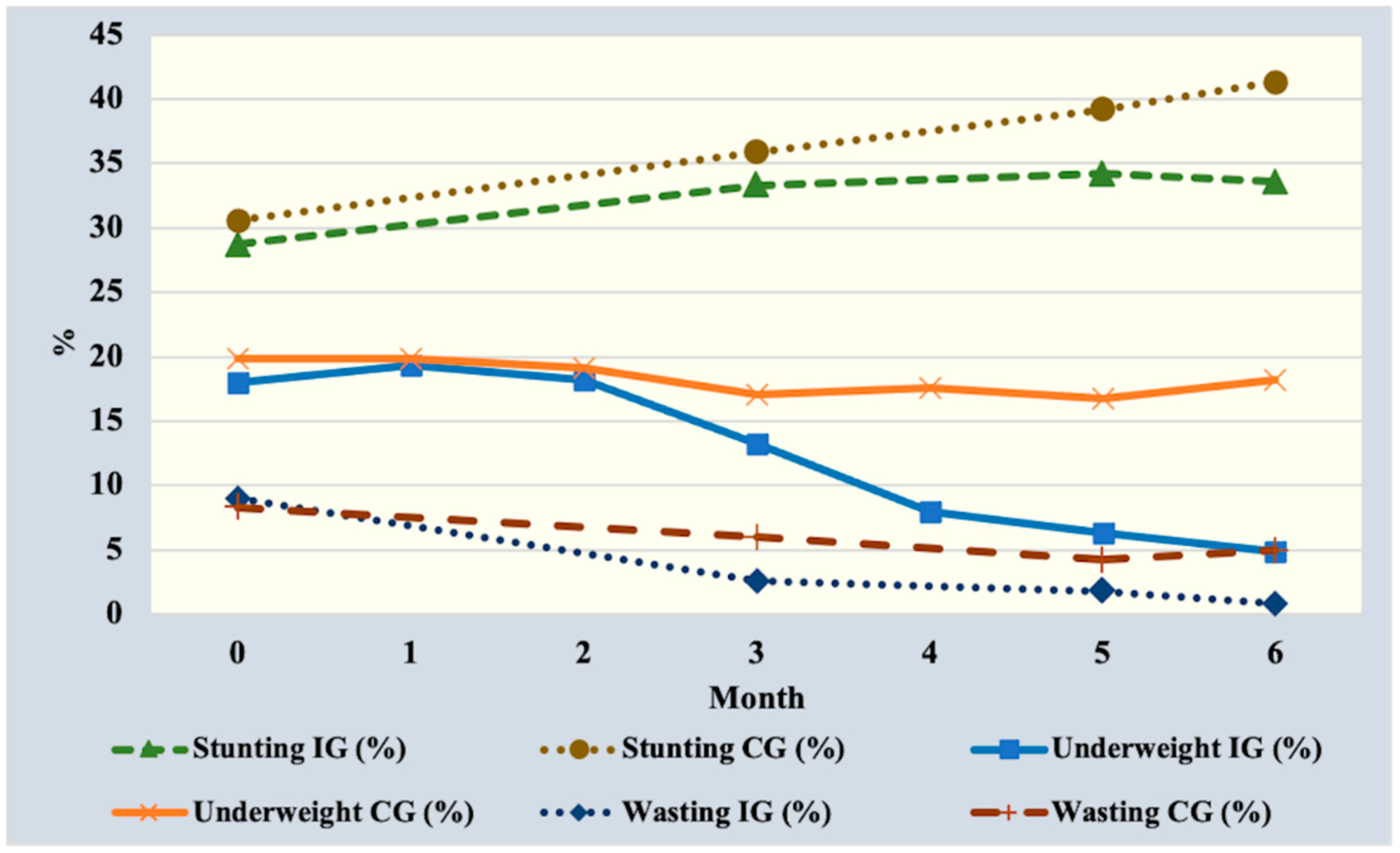
3.3. Anthropometric Indicators
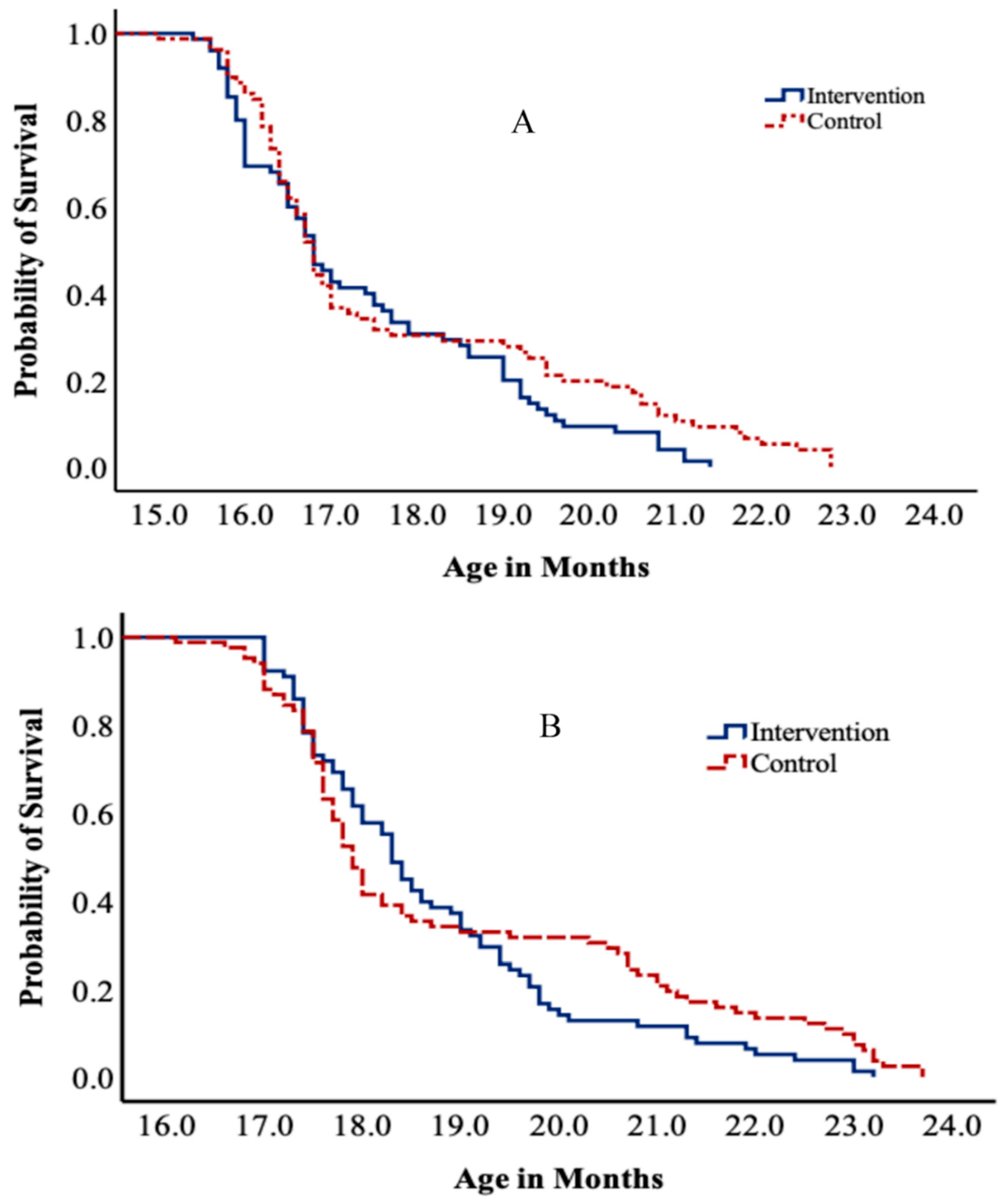
3.4. Gross Motor Milestones
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- United Nations Children’s Fund (UNICEF); World Health Organization; International Bank for Reconstruction and Development/World Bank. Levels and Trends in Child Malnutrition: Key Findings of the 2021 Edition of the Joint Child Malnutrition Estimates; World Health Organization: Geneva, Switzerland, 2021; ISBN 978-92-4-002525-7. [Google Scholar]
- Ethiopian Public Health Institute (EPHI); ICF. Ethiopia Mini Demographic and Health Survey 2019: Key Indicators; EPHI; ICF: Rockville, MD, USA, 2021. [Google Scholar]
- United Nations Children’s Fund (UNICEF); World Health Organization; International Bank for Reconstruction and Development/The World Bank. Levels and Trends in Child Malnutrition: Key Findings of the 2020 Edition of the Joint Child Malnutrition Estimates; World Health Organization: Geneva, Switzerland, 2020; ISBN 978-92-4-000357-6. [Google Scholar]
- Amaha, N.D. Ethiopian Progress towards Achieving the Global Nutrition Targets of 2025: Analysis of Sub-National Trends and Progress Inequalities. BMC Res. Notes 2020, 13, 559. [Google Scholar] [CrossRef] [PubMed]
- Global Nutrition Report Ethiopia Nutrition Profile. Available online: https://globalnutritionreport.org/resources/nutrition-profiles/africa/eastern-africa/ethiopia/ (accessed on 15 July 2022).
- Ethiopian Public Health Institute. Ethiopian National Food Consumption Survey; Ethiopian Public Health Institute: Addis Ababa, Ethiopia, 2013. [Google Scholar]
- ICF Central Statistical Agency (CSA); ICF. Ethiopia Demographic and Health Survey 2016; CSA: Addis Ababa, Ethiopia; ICF: Rockville, MD, USA, 2016. [Google Scholar]
- Réhault-Godbert, S.; Guyot, N.; Nys, Y. The Golden Egg: Nutritional Value, Bioactivities, and Emerging Benefits for Human Health. Nutrients 2019, 11, 684. [Google Scholar] [CrossRef] [PubMed]
- Bragg, M.; Caswell, B.; Maleta, K.; Stewart, C. Choline Intake in Malawian Children Aged 6–9 and 12–15 Months in an Egg Intervention Trial. Curr. Dev. Nutr. 2020, 4, 816. [Google Scholar] [CrossRef]
- Caswell, B.; Arnold, C.; Lutter, C.; Maleta, K.; Stewart, C. An Egg Feeding Intervention Increased Protein Quantity and Quality Among Young Malawian Children. Curr. Dev. Nutr. 2020, 4, 955. [Google Scholar] [CrossRef]
- Iannotti, L.L.; Lutter, C.K.; Waters, W.F.; Gallegos Riofrío, C.A.; Malo, C.; Reinhart, G.; Palacios, A.; Karp, C.; Chapnick, M.; Cox, K.; et al. Eggs Early in Complementary Feeding Increase Choline Pathway Biomarkers and DHA: A Randomized Controlled Trial in Ecuador. Am. J. Clin. Nutr. 2017, 106, 1482–1489. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, Y.; Fulgoni, V.L. Increasing Egg Consumption at Breakfast Is Associated with Increased Usual Nutrient Intakes: A Modeling Analysis Using NHANES and the USDA Child and Adult Care Food Program School Breakfast Guidelines. Nutrients 2021, 13, 1379. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, Y.; Fulgoni, V.L. Egg Consumption in U.S. Children Is Associated with Greater Daily Nutrient Intakes, Including Protein, Lutein + Zeaxanthin, Choline, α-Linolenic Acid, and Docosahexanoic Acid. Nutrients 2019, 11, 1137. [Google Scholar] [CrossRef] [PubMed]
- Iannotti, L.L.; Lutter, C.K.; Stewart, C.P.; Gallegos Riofrío, C.A.; Malo, C.; Reinhart, G.; Palacios, A.; Karp, C.; Chapnick, M.; Cox, K.; et al. Eggs in Early Complementary Feeding and Child Growth: A Randomized Controlled Trial. Pediatrics 2017, 140, e20163459. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.P.; Caswell, B.; Iannotti, L.; Lutter, C.; Arnold, C.D.; Chipatala, R.; Prado, E.L.; Maleta, K. The Effect of Eggs on Early Child Growth in Rural Malawi: The Mazira Project Randomized Controlled Trial. Am. J. Clin. Nutr. 2019, 110, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Omer, A.; Mulualem, D.; Classen, H.; Vatanparast, H.; Whiting, S.J. Promotion of Egg and Eggshell Powder Consumption on the Nutritional Status of Young Children in Ethiopia. Int. J. Food Sci. Nutr. Res. 2019, 1, 1004. [Google Scholar] [CrossRef]
- McKune, S.L.; Stark, H.; Sapp, A.C.; Yang, Y.; Slanzi, C.M.; Moore, E.V.; Omer, A.; Wereme N’Diaye, A. Behavior Change, Egg Consumption, and Child Nutrition: A Cluster Randomized Controlled Trial. Pediatrics 2020, 146, e2020007930. [Google Scholar] [CrossRef] [PubMed]
- Prado, E.L.; Maleta, K.; Caswell, B.L.; George, M.; Oakes, L.M.; DeBolt, M.C.; Bragg, M.G.; Arnold, C.D.; Iannotti, L.L.; Lutter, C.K.; et al. Early Child Development Outcomes of a Randomized Trial Providing 1 Egg Per Day to Children Age 6 to 15 Months in Malawi. J. Nutr. 2020, 150, 1933–1942. [Google Scholar] [CrossRef]
- Alderman, H.; Gilligan, D.O.; Leight, J.; Mulford, M.; Tambet, H. The Role of Poultry Transfers in Diet Diversity: A Cluster Randomized Intent to Treat Analysis. Food Policy 2022, 107, 102212. [Google Scholar] [CrossRef]
- Broaddus-Shea, E.T.; Manohar, S.; Thorne-Lyman, A.L.; Bhandari, S.; Nonyane, B.A.S.; Winch, P.J.; West, K.P. Small-Scale Livestock Production in Nepal Is Directly Associated with Children’s Increased Intakes of Eggs and Dairy, But Not Meat. Nutrients 2020, 12, 252. [Google Scholar] [CrossRef] [PubMed]
- Mosites, E.; Aol, G.; Otiang, E.; Bigogo, G.; Munyua, P.; Montgomery, J.M.; Neuhouser, M.L.; Palmer, G.H.; Thumbi, S.M. Child Height Gain Is Associated with Consumption of Animal-Source Foods in Livestock-Owning Households in Western Kenya. Public Health Nutr. 2017, 20, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Bardosh, K.L.; Hussein, J.W.; Sadik, E.A.; Hassen, J.Y.; Ketema, M.; Ibrahim, A.M.; McKune, S.L.; Havelaar, A.H. Chicken Eggs, Childhood Stunting and Environmental Hygiene: An Ethnographic Study from the Campylobacter Genomics and Environmental Enteric Dysfunction (CAGED) Project in Ethiopia. One Health Outlook 2020, 2, 5. [Google Scholar] [CrossRef]
- Headey, D.; Nguyen, P.; Kim, S.; Rawat, R.; Ruel, M.; Menon, P. Is Exposure to Animal Feces Harmful to Child Nutrition and Health Outcomes? A Multicountry Observational Analysis. Am. J. Trop. Med. Hyg. 2017, 96, 961–969. [Google Scholar] [CrossRef]
- Headey, D.; Hirvonen, K. Is Exposure to Poultry Harmful to Child Nutrition? An Observational Analysis for Rural Ethiopia. PLoS ONE 2016, 11, e0160590. [Google Scholar] [CrossRef]
- Syed, S.; Ali, A.; Duggan, C. Environmental Enteric Dysfunction in Children: A Review. J. Pediatr. Gastroenterol. Nutr. 2016, 63, 6–14. [Google Scholar] [CrossRef]
- Passarelli, S.; Ambikapathi, R.; Gunaratna, N.S.; Madzorera, I.; Canavan, C.R.; Noor, R.A.; Tewahido, D.; Berhane, Y.; Sibanda, S.; Sibanda, L.M.; et al. The Role of Chicken Management Practices in Children’s Exposure to Environmental Contamination: A Mixed-Methods Analysis. BMC Public Health 2021, 21, 1097. [Google Scholar] [CrossRef]
- Omer, A.; Mulualem, D.; Classen, H.; Vatanparast, H.; Whiting, S.J. A Community Poultry Intervention to Promote Egg and Eggshell Powder Consumption by Young Children in Halaba Special Woreda, SNNPR, Ethiopia. J. Agric. Sci. 2018, 10, 1. [Google Scholar] [CrossRef]
- Omer, A.; Hailu, D.; Whiting, S.J. Egg Consumption of Children under Two Years of Age through a Child-Owned Poultry and Nutrition Intervention in Rural Ethiopia: A Community-Based Randomized Controlled Trial. J. Agric. Food Res. 2022, 9, 100354. [Google Scholar] [CrossRef]
- World Health Organization; United Nations Children’s Fund (UNICEF). Recommendations for Data Collection, Analysis and Reporting on Anthropometric Indicators in Children under 5 Years Old; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- WHO. Multicentre Growth Reference Study Group WHO Motor Development Study: Windows of Achievement for Six Gross Motor Development Milestones. Acta Paediatr. Suppl. 2006, 450, 86–95. [Google Scholar] [CrossRef]
- Wijnhoven, T.M.; de Onis, M.; Onyango, A.W.; Wang, T.; Bjoerneboe, G.-E.A.; Bhandari, N.; Lartey, A.; al Rashidi, B. Assessment of Gross Motor Development in the WHO Multicentre Growth Reference Study. Food Nutr. Bull. 2004, 25, S37–S45. [Google Scholar] [CrossRef] [PubMed]
- Frankenburg, W.K.; Dodds, J.; Archer, P.; Shapiro, H.; Bresnick, B. The Denver II: A Major Revision and Restandardization of the Denver Developmental Screening Test. Pediatrics 1992, 89, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Cheesbrough, M. District Laboratory Practice in Tropical Countries. Part 1; Cambridge University Press: Cambridge, UK, 2005; ISBN 978-0-511-34935-5. [Google Scholar]
- World Health Organization. WHO Anthro Survey Analyser: Software for Analysing Survey Anthropometric Data for Children under 5 Years of Age. Built-in Software Edition; Version 1.0; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Pasqualino, M.; Shaikh, S.; Hossain, M.I.; Islam, M.T.; Ali, H.; Haque, R.; Ayesha, K.; Wu, L.; Schuh, H.; Dyer, B.; et al. The Effect of Eggs on Growth Among Infants 6–12 Months of Age in Rural Bangladesh: A Cluster Randomized Controlled Trial. Curr. Dev. Nutr. 2020, 4, 885. [Google Scholar] [CrossRef]
- Mekonnen, T.C.; Tadesse, S.E.; Dawed, Y.A.; Cherie, N.; Abebe, H.; Shumye, G.; Mohammed, F.; Hussien, A. The Role of Nutrition-sensitive Agriculture Combined with Behavioral Interventions in Childhood Growth in Ethiopia: An Adequacy Evaluation Study. Health Sci. Rep. 2022, 5, e524. [Google Scholar] [CrossRef] [PubMed]
- Dumas, S.E.; Lewis, D.; Travis, A.J. Small-Scale Egg Production Centres Increase Children’s Egg Consumption in Rural Zambia. Matern. Child Nutr. 2018, 14, e12662. [Google Scholar] [CrossRef]
- Gupta, A.; Kalaivani, M.; Gupta, S.K.; Rai, S.K.; Nongkynrih, B. The Study on Achievement of Motor Milestones and Associated Factors among Children in Rural North India. J. Family Med. Prim. Care 2016, 5, 378–382. [Google Scholar] [CrossRef]
- Kariger, P.K.; Stoltzfus, R.J.; Olney, D.; Sazawal, S.; Black, R.; Tielsch, J.M.; Frongillo, E.A.; Khalfan, S.S.; Pollitt, E. Iron Deficiency and Physical Growth Predict Attainment of Walking but Not Crawling in Poorly Nourished Zanzibari Infants. J. Nutr. 2005, 135, 814–819. [Google Scholar] [CrossRef]
- Siegel, E.H.; Stoltzfus, R.J.; Kariger, P.K.; Katz, J.; Khatry, S.K.; LeClerq, S.C.; Pollitt, E.; Tielsch, J.M. Growth Indices, Anemia, and Diet Independently Predict Motor Milestone Acquisition of Infants in South Central Nepal. J. Nutr. 2005, 135, 2840–2844. [Google Scholar] [CrossRef] [PubMed]
- Vaida, N. Development of Children during First 2 Years of Life. Stud. Home Community Sci. 2013, 7, 13–19. [Google Scholar] [CrossRef]
- Stewart, C.P.; Kariger, P.; Fernald, L.; Pickering, A.J.; Arnold, C.D.; Arnold, B.F.; Hubbard, A.E.; Dentz, H.N.; Lin, A.; Meerkerk, T.J.; et al. Effects of Water Quality, Sanitation, Handwashing, and Nutritional Interventions on Child Development in Rural Kenya (WASH Benefits Kenya): A Cluster-Randomised Controlled Trial. Lancet Child Adolesc. Health 2018, 2, 269–280. [Google Scholar] [CrossRef]
- Tofail, F.; Fernald, L.C.; Das, K.K.; Rahman, M.; Ahmed, T.; Jannat, K.K.; Unicomb, L.; Arnold, B.F.; Ashraf, S.; Winch, P.J.; et al. Effect of Water Quality, Sanitation, Hand Washing, and Nutritional Interventions on Child Development in Rural Bangladesh (WASH Benefits Bangladesh): A Cluster-Randomised Controlled Trial. Lancet Child Adolesc. Health 2018, 2, 255–268. [Google Scholar] [CrossRef]
- Frongillo, E.A.; Nguyen, P.H.; Saha, K.K.; Sanghvi, T.; Afsana, K.; Haque, R.; Baker, J.; Ruel, M.T.; Rawat, R.; Menon, P. Large-Scale Behavior-Change Initiative for Infant and Young Child Feeding Advanced Language and Motor Development in a Cluster-Randomized Program Evaluation in Bangladesh. J. Nutr. 2017, 147, 256–263. [Google Scholar] [CrossRef]
- Prado, E.L.; Adu-Afarwuah, S.; Lartey, A.; Ocansey, M.; Ashorn, P.; Vosti, S.A.; Dewey, K.G. Effects of Pre- and Post-Natal Lipid-Based Nutrient Supplements on Infant Development in a Randomized Trial in Ghana. Early Hum. Dev. 2016, 99, 43–51. [Google Scholar] [CrossRef]
- Prado, E.L.; Abbeddou, S.; Yakes Jimenez, E.; Somé, J.W.; Ouédraogo, Z.P.; Vosti, S.A.; Dewey, K.G.; Brown, K.H.; Hess, S.Y.; Ouédraogo, J.-B. Lipid-Based Nutrient Supplements Plus Malaria and Diarrhea Treatment Increase Infant Development Scores in a Cluster-Randomized Trial in Burkina Faso. J. Nutr. 2015, 146, 814–822. [Google Scholar] [CrossRef]
- Matias, S.L.; Mridha, M.K.; Tofail, F.; Arnold, C.D.; Khan, M.S.A.; Siddiqui, Z.; Ullah, M.B.; Dewey, K.G. Home Fortification during the First 1000 d Improves Child Development in Bangladesh: A Cluster-Randomized Effectiveness Trial. Am. J. Clin. Nutr. 2017, 105, 958–969. [Google Scholar] [CrossRef]
- Prado, E.L.; Maleta, K.; Ashorn, P.; Ashorn, U.; Vosti, S.A.; Sadalaki, J.; Dewey, K.G. Effects of Maternal and Child Lipid-Based Nutrient Supplements on Infant Development: A Randomized Trial in Malawi. Am. J. Clin. Nutr. 2016, 103, 784–793. [Google Scholar] [CrossRef]
- Zulkarnaen, Z. The Influence of Nutritional Status on Gross and Fine Motor Skills Development in Early Childhood. Asian Soc. Sci. 2019, 15, 75. [Google Scholar] [CrossRef]
- Workie, S.B.; Mekonen, T.; Mekonen, T.C.; Fekadu, W. Child Development and Nutritional Status in 12–59 Months of Age in Resource Limited Setting of Ethiopia. J. Health Popul. Nutr. 2020, 39, 6. [Google Scholar] [CrossRef] [PubMed]
- Iannotti, L.; Jean Louis Dulience, S.; Wolff, P.; Cox, K.; Lesorogol, C.; Kohl, P. Nutrition Factors Predict Earlier Acquisition of Motor and Language Milestones among Young Children in Haiti. Acta Paediatr. 2016, 105, e406–e411. [Google Scholar] [CrossRef] [PubMed]
- Faber, M.; Malan, L.; Kruger, H.S.; Asare, H.; Visser, M.; Mukwevho, T.; Ricci, C.; Smuts, C.M. Potential of Egg as Complementary Food to Improve Nutrient Intake and Dietary Diversity. Nutrients 2022, 14, 3396. [Google Scholar] [CrossRef] [PubMed]
- Caswell, B.L.; Arnold, C.D.; Lutter, C.K.; Iannotti, L.L.; Chipatala, R.; Werner, E.R.; Maleta, K.M.; Stewart, C.P. Impacts of an Egg Intervention on Nutrient Adequacy among Young Malawian Children. Matern. Child Nutr. 2021, 17, e13196. [Google Scholar] [CrossRef]
- Bragg, M.G.; Prado, E.L.; Stewart, C.P. Choline and Docosahexaenoic Acid during the First 1000 Days and Children’s Health and Development in Low- and Middle-Income Countries. Nutr. Rev. 2022, 80, 656–676. [Google Scholar] [CrossRef] [PubMed]
- Cohen Kadosh, K.; Muhardi, L.; Parikh, P.; Basso, M.; Jan Mohamed, H.J.; Prawitasari, T.; Samuel, F.; Ma, G.; Geurts, J.M.W. Nutritional Support of Neurodevelopment and Cognitive Function in Infants and Young Children—An Update and Novel Insights. Nutrients 2021, 13, 199. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenberg, S.J.; Georgieff, M.K.; Daniels, S.; Corkins, M.; Golden, N.H.; Kim, J.H.; Lindsey, C.W.; Magge, S.N. Advocacy for Improving Nutrition in the First 1000 Days to Support Childhood Development and Adult Health. Pediatrics 2018, 141, e20173716. [Google Scholar] [CrossRef]
- Georgieff, M.K. Long-Term Brain and Behavioral Consequences of Early Iron Deficiency. Nutr. Rev. 2011, 69 (Suppl. 1), S43–S48. [Google Scholar] [CrossRef]
- Haas, J.D.; Rivera-Dommarco, J. The Effects of Improved Nutrition in Early Childhood on Adolescent and Early Adulthood Body Size, Composition, Maturity, and Function: Results from the First INCAP Follow-Up Study. Food Nutr. Bull. 2020, 41, S23–S30. [Google Scholar] [CrossRef]
- Wozniak, J.R.; Fink, B.A.; Fuglestad, A.J.; Eckerle, J.K.; Boys, C.J.; Sandness, K.E.; Radke, J.P.; Miller, N.C.; Lindgren, C.; Brearley, A.M.; et al. Four-Year Follow-up of a Randomized Controlled Trial of Choline for Neurodevelopment in Fetal Alcohol Spectrum Disorder. J. Neurodev. Disord. 2020, 12, 9. [Google Scholar] [CrossRef]
- Oberhelman, R.A.; Gilman, R.H.; Sheen, P.; Cordova, J.; Zimic, M.; Cabrera, L.; Meza, R.; Perez, J. An Intervention-Control Study of Corralling of Free-Ranging Chickens to Control Campylobacter Infections Among Children in a Peruvian Periurban Shantytown. Am. J. Trop. Med. Hyg. 2006, 74, 1054–1059. [Google Scholar] [CrossRef] [PubMed]
- Omer, A. Poultry Interventions and Child Nutrition Status in Low-Income Countries. Afr. J. Food Agric. Nutr. Dev. 2020, 20, 16013–16028. [Google Scholar] [CrossRef]
- Iannotti, L.L.; Chapnick, M.; Nicholas, J.; Gallegos-Riofrio, C.A.; Moreno, P.; Douglas, K.; Habif, D.; Cui, Y.; Stewart, C.; Lutter, C.K.; et al. Egg Intervention Effect on Linear Growth No Longer Present after Two Years. Matern. Child Nutr. 2020, 16, e12925. [Google Scholar] [CrossRef] [PubMed]

| Description | Intervention (N = 127) | Control (N = 126) | |
|---|---|---|---|
| % | % | ||
| Household characteristics | |||
| Main source of income | Farming | 89.8 | 84.1 |
| Main source of drinking water | Community tap water | 100 | 97.6 |
| Livestock production | |||
| Poultry production | Chicken | 20.5 | 26.2 |
| Chicken care | Day cage/separated place | 11.5 | 6.1 |
| Night shelter/cage | 19.2 | 15.2 | |
| Maternal Characteristics | |||
| Age in years | Mean age (SD) | 27.3 (4.68) | 27.5 (4.18) |
| Educational Status | Illiterate | 66.1 | 54 |
| Read and write | 26.8 | 34.1 | |
| Formal education | 7.1 | 11.9 | |
| Education on feeding eggs | Received | 40.2 | 41.3 |
| Awareness on disease risk of chicken feces | Aware | 23.6 | 31.7 |
| Child Characteristics | |||
| Sex | Female * | 36.2 | 54.8 |
| Age (month) | Mean (SD) | 10.9 (3.18) | 11.4 (4.28) |
| IYCF | |||
| Breastfeeding | Currently fed on breastmilk | 98.4 | 96.8 |
| Complementary food | Currently on complementary food | 94.5 | 92.9 |
| Mean age of introduction: months (SD) | 6.13 (0.59) | 6.2 (0.69) | |
| Egg intake history | Ever fed | 51.2 | 45.2 |
| % children fed with egg | 24 h before survey | 7.9 | 9.5 |
| The week before survey | 23.6 | 27.8 | |
| Eggs consumed (per week per child) | Mean (SD) | 0.23 (0.42) | 0.29 (0.51) |
| Minimum dietary diversity (MDD) | Children fulfilling MDD | 4.7 | 6.3 |
| Mean Dietary Diversity score (SD) | 2.34 (0.97) | 2.45 (0.94) | |
| Baseline | End Line | Significance Testing | ||||
|---|---|---|---|---|---|---|
| Intervention (N = 122) | Control (N = 121) | Intervention (N = 122) | Control (N = 121) | |||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | β (95% CI) a | p | |
| WAZ | −1.12 (1.00) | −1.02 (1.17) | −0.20 (0.86) | −1.09 (1.05) | 0.38 (0.13, 0.63) | 0.003 |
| LAZ | −1.32 (1.19) | −1.10 (1.48) | −1.04 (1.04) | −1.58 (1.25) | 0.15 (−0.15, 0.44) | 0.325 |
| WHZ | −0.65 (0.97) | −0.63 (1.03) | 0.43 (0.79) | −0.40 (0.89) | 0.43 (0.21, 0.64) | <0.001 |
| N (%) | N (%) | N (%) | N (%) | OR (95% CI) b | p | |
| Underweight | 22 (18) | 24 (19.8) | 6 (4.9) | 22 (18.2) | 0.46 (0.26, 0.84) | 0.011 |
| Stunting | 35 (28.7) | 37 (30.6) | 41 (33.6) | 50 (41.3) | 0.58 (0.37, 0.91) | 0.017 |
| Wasting | 11 (9) | 10 (8.3) | 1 (0.8) | 6 (5) | 0.52 (0.26, 1.05) | 0.067 |
| Gross Motor Milestone | Number of Children | Age of Attainment Mean (SD) Months | p1 | CHR (95% CI) | p | AHR (95% CI) 2 | p | AHR (95% CI) 3 | p |
|---|---|---|---|---|---|---|---|---|---|
| Hands and knees crawling | IG = 6 | 10.93 (0.06) | 0.753 * | 1.22 (0.30–4.97) | 0.784 | 1.03 (0.24–4.41) a | 0.970 | 0.94 (0.22–3.92) | 0.928 |
| CG = 12 | 10.85 (0.41) | ||||||||
| Walking with assistance | IG = 28 | 12.24 (0.71) | 0.721 ** | 0.90 (0.49–1.67) | 0.740 | 0.90 (0.48–1.70) a | 0.751 | 0.85 (0.45–1.59) | 0.606 |
| CG = 34 | 11.98 (0.67) | ||||||||
| Standing alone | IG = 58 | 13.54 (1.07) | 0.943 * | 0.99 (0.65–1.49) | 0.945 | 0.98 (0.65–1.49) a | 0.922 | 1.02 (0.67–1.55) | 0.944 |
| CG = 62 | 13.39 (1.12) | ||||||||
| Walking alone | IG = 89 | 15.15 (1.81) | 0.317 * | 1.09 (0.77–1.53) | 0.623 | 1.18 (0.83–1.67) | 0.369 | 1.19 (0.83–1.69) | 0.342 |
| CG = 89 | 15.0 (1.97) | ||||||||
| Running | IG = 117 | 17.53 (1.70) | 0.021 * | 1.34 (0.99–1.82) | 0.061 | 1.43 (1.05–1.95) a | 0.022 | 1.41 (1.04–1.93) | 0.028 |
| CG = 110 | 17.96 (2.26) | ||||||||
| Kicking ball forward | IG = 124 | 18.96 (1.73) | 0.027 * | 1.32 (0.99–1.77) | 0.061 | 1.37 (1.02–1.84) | 0.036 | 1.39 (1.04–1.87) | 0.027 |
| CG = 122 | 19.41 (2.38) | ||||||||
| Throwing ball overhead | IG = 113 | 20.90 (1.31) | 0.046 ** | 1.29 (0.95–1.75) | 0.099 | 1.34 (0.98– 1.82) | 0.064 | 1.37 (1.01–1.86) | 0.045 |
| CG = 96 | 21.18 (1.64) | ||||||||
| Jumping up | IG = 67 | 23.42 (0.70) | 0.061 ** | 1.33 (0.91–1.94) | 0.135 | 1.37 (0.94– 2.00) | 0.106 | 1.38 (0.94–2.02) | 0.099 |
| CG = 63 | 23.50 (0.83) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omer, A.; Hailu, D.; Whiting, S.J. Effect of a Child-Owned Poultry Intervention Providing Eggs on Nutrition Status and Motor Skills of Young Children in Southern Ethiopia: A Cluster Randomized and Controlled Community Trial. Int. J. Environ. Res. Public Health 2022, 19, 15305. https://doi.org/10.3390/ijerph192215305
Omer A, Hailu D, Whiting SJ. Effect of a Child-Owned Poultry Intervention Providing Eggs on Nutrition Status and Motor Skills of Young Children in Southern Ethiopia: A Cluster Randomized and Controlled Community Trial. International Journal of Environmental Research and Public Health. 2022; 19(22):15305. https://doi.org/10.3390/ijerph192215305
Chicago/Turabian StyleOmer, Anteneh, Dejene Hailu, and Susan J. Whiting. 2022. "Effect of a Child-Owned Poultry Intervention Providing Eggs on Nutrition Status and Motor Skills of Young Children in Southern Ethiopia: A Cluster Randomized and Controlled Community Trial" International Journal of Environmental Research and Public Health 19, no. 22: 15305. https://doi.org/10.3390/ijerph192215305
APA StyleOmer, A., Hailu, D., & Whiting, S. J. (2022). Effect of a Child-Owned Poultry Intervention Providing Eggs on Nutrition Status and Motor Skills of Young Children in Southern Ethiopia: A Cluster Randomized and Controlled Community Trial. International Journal of Environmental Research and Public Health, 19(22), 15305. https://doi.org/10.3390/ijerph192215305










