Classification and Prediction on Hypertension with Blood Pressure Determinants in a Deep Learning Algorithm
Abstract
1. Introduction
2. Materials and Methods
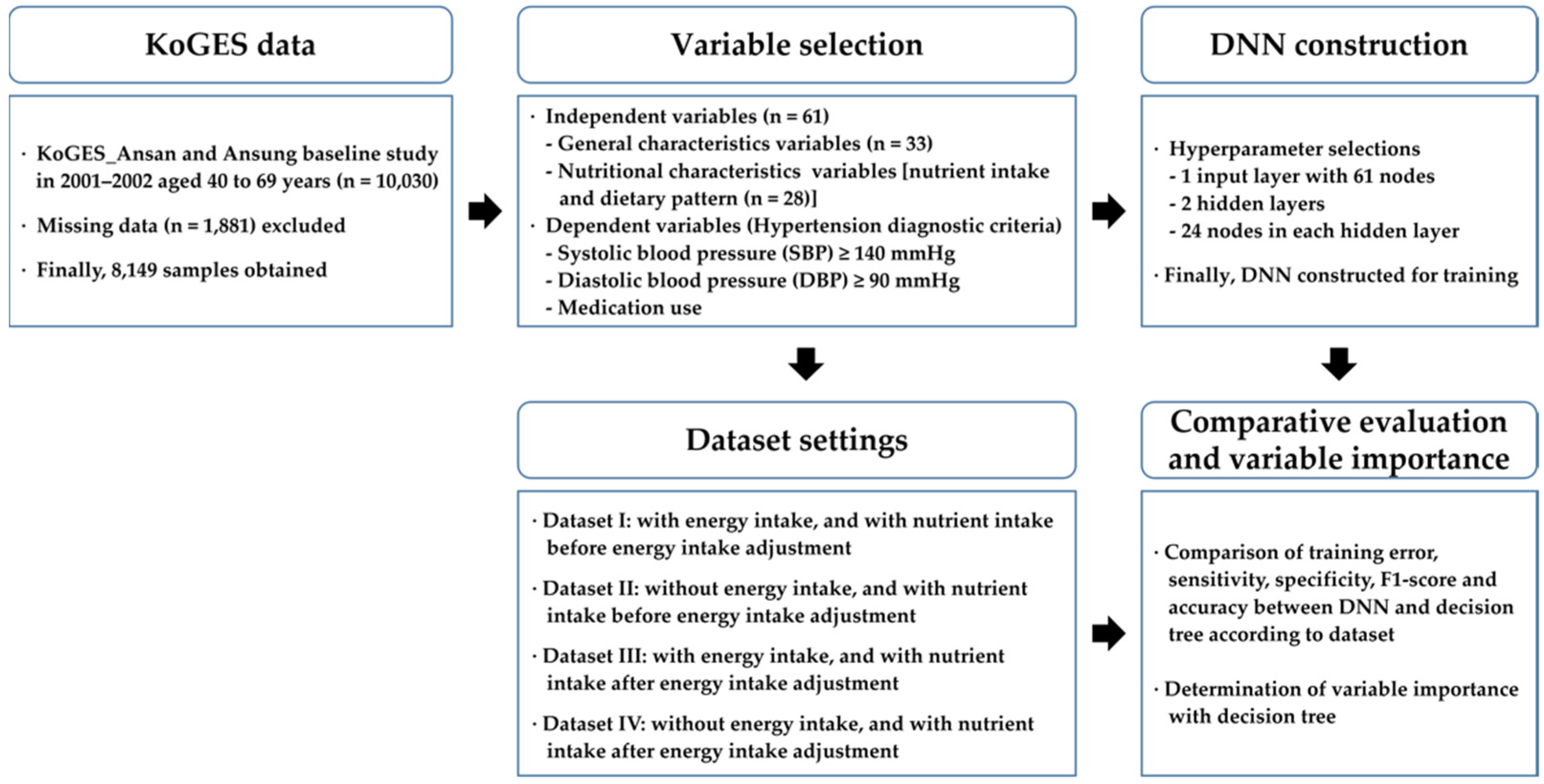
2.1. Data and Subjects
2.2. Variable Selection
2.3. Statistical Analysis
2.4. Development Environment
2.5. Data Pre-Processing
2.6. Dataset Setting
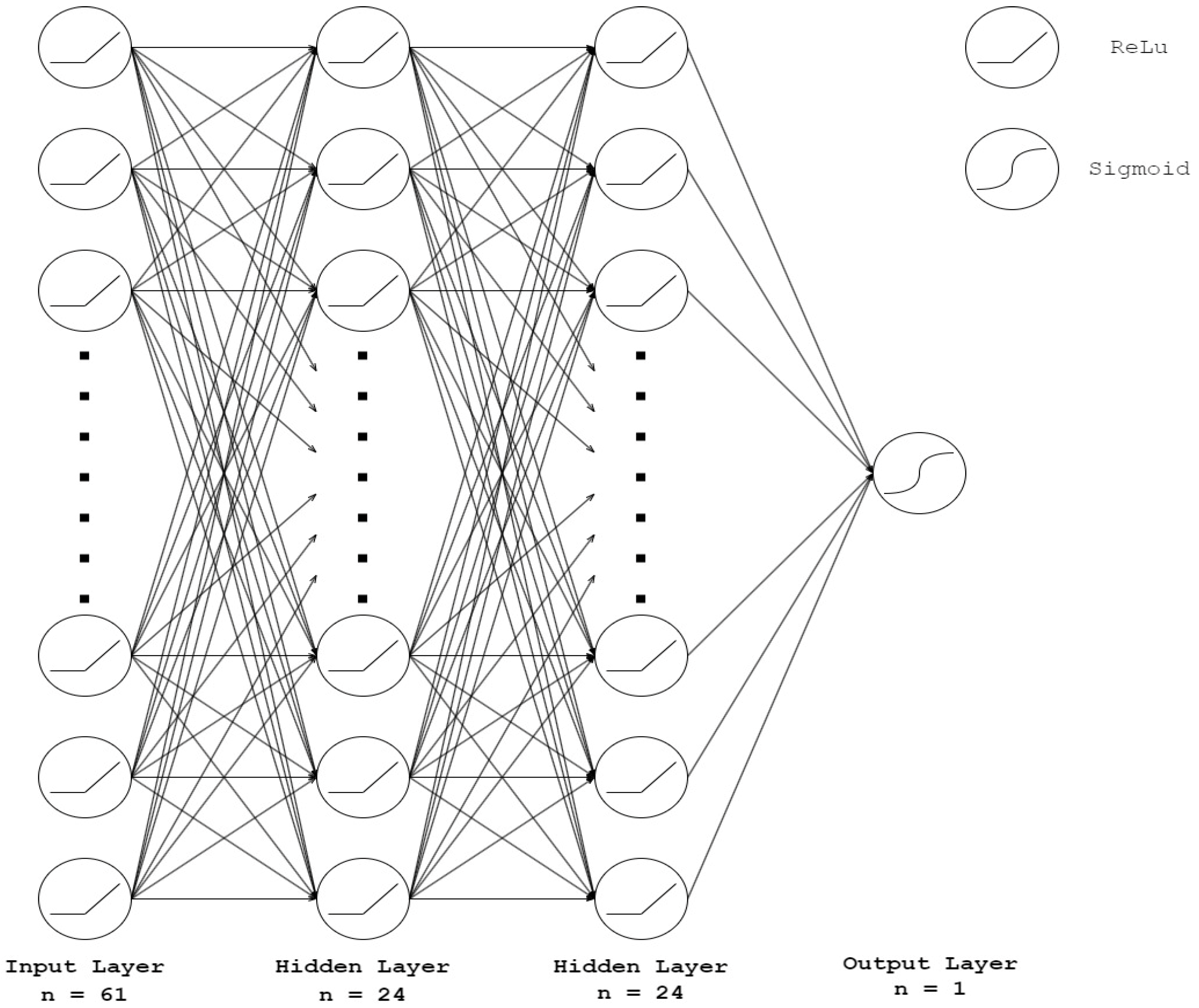
2.7. Algorithm Construction
2.8. Decision Tree Processing for Variable Importance
3. Analysis
3.1. Baseline Characteristics of Independent Variables
3.2. Factor Analysis for Food Group Determination
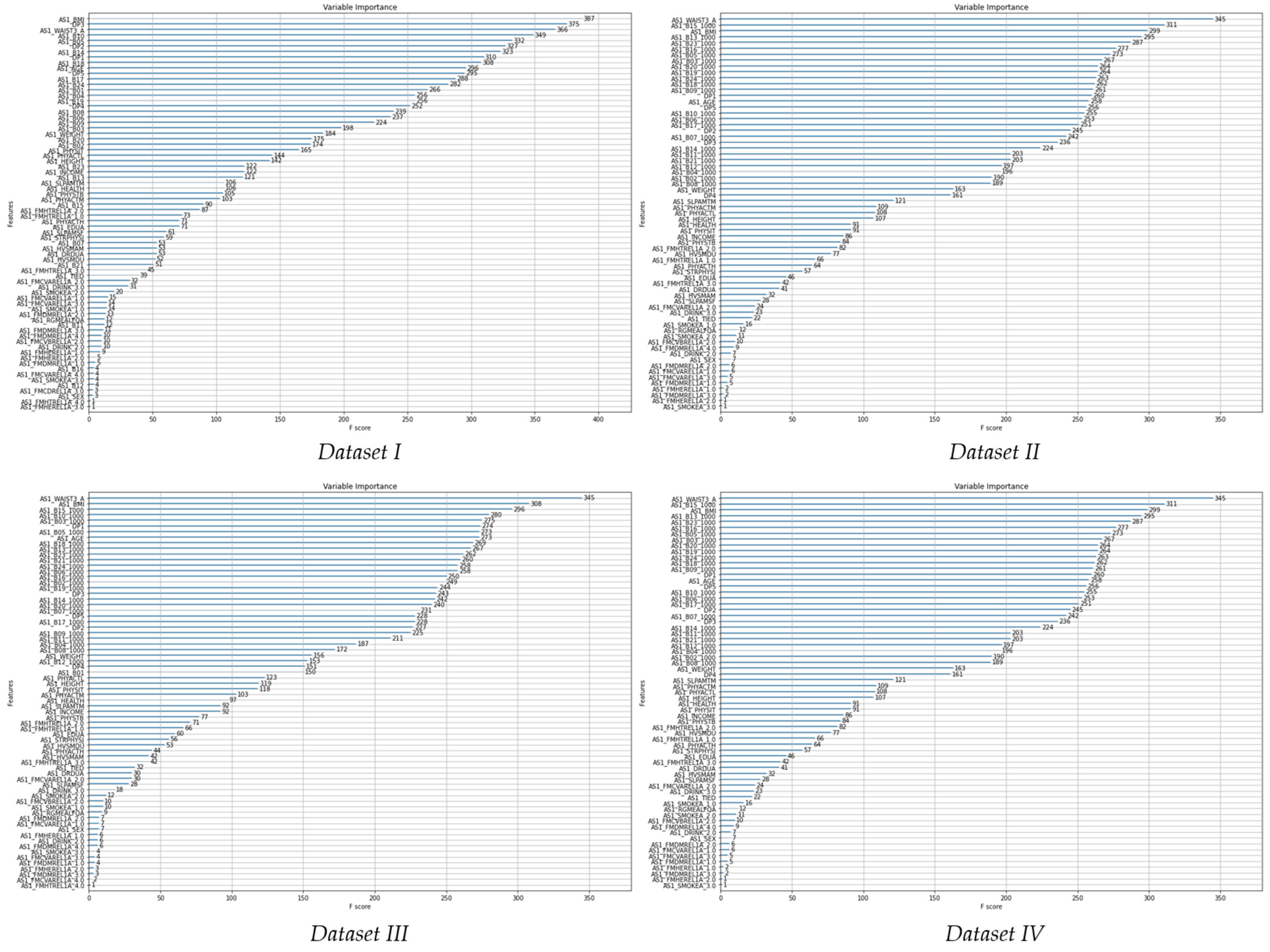
3.3. Decision Tree for Variable Importance
4. Experimental Results
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- NCD Risk Factor Collaboration (NCD-RisC). Long-term and recent trends in hypertension awareness, treatment, and control in 12 high-income countries: An analysis of 123 nationally representative surveys. Lancet 2019, 394, 639–651. [Google Scholar] [CrossRef]
- Kim, H.C.; Cho, M.C. Korea hypertension fact sheet 2018. Clin. Hypertens. 2018, 24, 13. [Google Scholar] [CrossRef] [PubMed]
- GBD 2017 Risk Factor Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1923–1994. [Google Scholar] [CrossRef]
- GBD 2015 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1659–1724. [Google Scholar] [CrossRef]
- Global Burden of Metabolic Risk Factors for Chronic Diseases Collaboration. Cardiovascular disease, chronic kidney disease, and diabetes mortality burden of cardiometabolic risk factors from 1980 to 2010: A comparative risk assessment. Lancet. Diabetes Endocrinol. 2014, 2, 634–647. [Google Scholar] [CrossRef]
- Lewington, S.; Clarke, R.; Qizilbash, N.; Peto, R.; Collins, R. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002, 360, 1903–1913. [Google Scholar] [CrossRef]
- Yusuf, S.; Joseph, P.; Rangarajan, S.; Islam, S.; Mente, A.; Hystad, P.; Brauer, M.; Kutty, V.R.; Gupta, R.; Wielgosz, A.; et al. Modifiable risk factors, cardiovascular disease, and mortality in 155,722 individuals from 21 high-income, middle-income, and low-income countries (PURE): A prospective cohort study. Lancet 2020, 395, 795–808. [Google Scholar] [CrossRef]
- World Health Organization. A Global Brief on Hypertension: Silent Killer, Global Public Health Crisis 2013. Available online: https://www.who.int/publications/i/item/a-global-brief-on-hypertension-silent-killer-global-public-health-crisis-world-health-day-2013 (accessed on 26 August 2021).
- World Health Organization. Hypertension. Available online: https://www.who.int/news-room/fact-sheets/detail/hypertension (accessed on 26 August 2021).
- Al-Makki, A.; DiPette, D.; Whelton, P.K.; Murad, M.H.; Mustafa, R.A.; Acharya, S.; Beheiry, H.M.; Champagne, B.; Connell, K.; Cooney, M.T. Hypertension pharmacological treatment in adults: A World Health Organization guideline executive summary. Hypertension 2022, 79, 293–301. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J. Hypertens. 2018, 36, 1953–2041. [Google Scholar] [CrossRef]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E., Jr.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018, 71, e127–e248. [Google Scholar] [CrossRef]
- Fodor, J.G.; Whitmore, B.; Leenen, F.; Larochelle, P. Lifestyle modifications to prevent and control hypertension. 5. Recommendations on dietary salt. Canadian Hypertension Society, Canadian Coalition for High Blood Pressure Prevention and Control, Laboratory Centre for Disease Control at Health Canada, Heart and Stroke Foundation of Canada. CMAJ Can. Med. Assoc. J. 1999, 160, S29–S34. [Google Scholar]
- Filippou, C.D.; Tsioufis, C.P.; Thomopoulos, C.G.; Mihas, C.C.; Dimitriadis, K.S.; Sotiropoulou, L.I.; Chrysochoou, C.A.; Nihoyannopoulos, P.I.; Tousoulis, D.M. Dietary Approaches to Stop Hypertension (DASH) Diet and Blood Pressure Reduction in Adults with and without Hypertension: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2020, 11, 1150–1160. [Google Scholar] [CrossRef] [PubMed]
- Saneei, P.; Salehi-Abargouei, A.; Esmaillzadeh, A.; Azadbakht, L. Influence of Dietary Approaches to Stop Hypertension (DASH) diet on blood pressure: A systematic review and meta-analysis on randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. NMCD 2014, 24, 1253–1261. [Google Scholar] [CrossRef]
- Sacks, F.M.; Svetkey, L.P.; Vollmer, W.M.; Appel, L.J.; Bray, G.A.; Harsha, D.; Obarzanek, E.; Conlin, P.R.; Miller, E.R., 3rd; Simons-Morton, D.G.; et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N. Engl. J. Med. 2001, 344, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Appel, L.J. The Effects of Dietary Factors on Blood Pressure. Cardiol. Clin. 2017, 35, 197–212. [Google Scholar] [CrossRef]
- Jennings, A.; Berendsen, A.M.; de Groot, L.; Feskens, E.J.M.; Brzozowska, A.; Sicinska, E.; Pietruszka, B.; Meunier, N.; Caumon, E.; Malpuech-Brugère, C.; et al. Mediterranean-Style Diet Improves Systolic Blood Pressure and Arterial Stiffness in Older Adults. Hypertension 2019, 73, 578–586. [Google Scholar] [CrossRef]
- Sukhato, K.; Akksilp, K.; Dellow, A.; Vathesatogkit, P.; Anothaisintawee, T. Efficacy of different dietary patterns on lowering of blood pressure level: An umbrella review. Am. J. Clin. Nutr. 2020, 112, 1584–1598. [Google Scholar] [CrossRef]
- Santhanam, P.; Ahima, R.S. Machine learning and blood pressure. J. Clin. Hypertens. 2019, 21, 1735–1737. [Google Scholar] [CrossRef]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef]
- Schmidhuber, J. Deep learning in neural networks: An overview. Neural Netw. 2015, 61, 85–117. [Google Scholar] [CrossRef]
- Guttag, J.V. Introduction to Computation and Programming Using Python: With Application to Understanding Data; MIT Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Valavanis, I.K.; Mougiakakou, S.G.; Grimaldi, K.A.; Nikita, K.S. A multifactorial analysis of obesity as CVD risk factor: Use of neural network based methods in a nutrigenetics context. BMC Bioinform. 2010, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Panaretos, D.; Koloverou, E.; Dimopoulos, A.C.; Kouli, G.-M.; Vamvakari, M.; Tzavelas, G.; Pitsavos, C.; Panagiotakos, D.B. A comparison of statistical and machine-learning techniques in evaluating the association between dietary patterns and 10-year cardiometabolic risk (2002–2012): The ATTICA study. Br. J. Nutr. 2018, 120, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Rigdon, J.; Basu, S. Machine learning with sparse nutrition data to improve cardiovascular mortality risk prediction in the USA using nationally randomly sampled data. BMJ Open 2019, 9, e032703. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Li, Y.; Xu, H.; Zhang, Q.; Liu, A.; Du, S.; Guo, H.; Ma, G. Leading dietary determinants identified using machine learning techniques and a healthy diet score for changes in cardiometabolic risk factors in children: A longitudinal analysis. Nutr. J. 2020, 19, 1–16. [Google Scholar] [CrossRef]
- Zeng, J.; Zhang, J.; Li, Z.; Li, T.; Li, G. Prediction model of artificial neural network for the risk of hyperuricemia incorporating dietary risk factors in a Chinese adult study. Food Nutr. Res. 2020, 64, e2020. [Google Scholar] [CrossRef]
- Lee, T.; Kim, J.; Uh, Y.; Lee, H. Deep neural network for estimating low density lipoprotein cholesterol. Clin. Chim. Acta 2019, 489, 35–40. [Google Scholar] [CrossRef]
- Faruqui, S.H.A.; Du, Y.; Meka, R.; Alaeddini, A.; Li, C.; Shirinkam, S.; Wang, J. Development of a deep learning model for dynamic forecasting of blood glucose level for type 2 diabetes mellitus: Secondary analysis of a randomized controlled trial. JMIR Mhealth Uhealth 2019, 7, e14452. [Google Scholar] [CrossRef]
- Choe, E.K.; Rhee, H.; Lee, S.; Shin, E.; Oh, S.-W.; Lee, J.-E.; Choi, S.H. Metabolic syndrome prediction using machine learning models with genetic and clinical information from a nonobese healthy population. Genom. Inform. 2018, 16, e31. [Google Scholar] [CrossRef]
- Kim, H.; Lim, D.H.; Kim, Y. Classification and prediction on the effects of nutritional intake on overweight/obesity, dyslipidemia, hypertension and type 2 diabetes mellitus using deep learning model: 4–7th Korea national health and nutrition examination survey. Int. J. Environ. Res. Public Health 2021, 18, 5597. [Google Scholar] [CrossRef]
- Kim, Y.; Han, B.G. Cohort Profile: The Korean Genome and Epidemiology Study (KoGES) Consortium. Int. J. Epidemiol. 2017, 46, e20. [Google Scholar] [CrossRef]
- National Institute of Health Korean Centers for Disease Control and Prevention. Examination and Survey Quality Control of the Korean Genome and Epidemiology Study. Available online: https://www.kdca.go.kr/contents.es?mid=a40504010000 (accessed on 26 August 2021).
- Cho, N.H.; Kim, K.M.; Choi, S.H.; Park, K.S.; Jang, H.C.; Kim, S.S.; Sattar, N.; Lim, S. High blood pressure and its association with incident diabetes over 10 years in the Korean Genome and Epidemiology Study (KoGES). Diabetes Care 2015, 38, 1333–1338. [Google Scholar] [PubMed]
- Korea Disease Control and Prevention Agency. Findings from Korea National Health and Nutrition Examination Survey. Available online: https://knhanes.cdc.go.kr/knhanes/sub01/sub01_05.do#s5_02 (accessed on 1 June 2021).
- Younjhin, A.; Lee, J.E.; Paik, H.Y.; Lee, H.K.; Inho, J. Development of a semi-quantitative food frequency questionnaire based on dietary data from the Korea national health and nutrition examination survey. Nutr. Sci. 2003, 6, 172–184. [Google Scholar]
- Kim, J.; Kim, Y.; Ahn, Y.O.; Paik, H.Y.; Ahn, Y.; Tokudome, Y.; Hamajima, N.; Inoue, M.; Tajima, K. Development of a food frequency questionnaire in Koreans. Asia Pac. J. Clin. Nutr. 2003, 12, 243–250. [Google Scholar] [PubMed]
- Ahn, Y.; Kwon, E.; Shim, J.E.; Park, M.K.; Joo, Y.; Kimm, K.; Park, C.; Kim, D.H. Validation and reproducibility of food frequency questionnaire for Korean genome epidemiologic study. Eur. J. Clin. Nutr. 2007, 61, 1435–1441. [Google Scholar] [CrossRef] [PubMed]
- AlKaabi, L.A.; Ahmed, L.S.; Al Attiyah, M.F.; Abdel-Rahman, M.E. Predicting hypertension using machine learning: Findings from Qatar Biobank Study. PLoS ONE 2020, 15, e0240370. [Google Scholar] [CrossRef]
- González-Aguilera, J.; Maceo-Gómez, L.; Suárez-Quesada, A. Predictive model for the development of hypertensive cardiopathy: A prospective cohort study. Medwave 2017, 17, e6954. [Google Scholar]
- Huang, S.; Xu, Y.; Yue, L.; Wei, S.; Liu, L.; Gan, X.; Zhou, S.; Nie, S. Evaluating the risk of hypertension using an artificial neural network method in rural residents over the age of 35 years in a Chinese area. Hypertens. Res. 2010, 33, 722–726. [Google Scholar] [CrossRef]
- Zhu, R.; Lv, Y.; Wang, Z.; Chen, X. Prediction of the hypertension risk of the elderly in built environments based on the LSTM deep learning and bayesian fitting method. Sustainability 2021, 13, 5724. [Google Scholar] [CrossRef]
- Ahn, Y.J.; Park, Y.J.; Park, S.J.; Min, H.S.; Kwak, H.K.; Oh, K.S.; Park, C. Dietary Patterns and Prevalence Odds Ratio in Middle-aged Adults of Rural and Mid-size City in Korean Genome Epidemiology Study. Korean J. Nutr. 2007, 40, 259–269. [Google Scholar]
- Kingma, D.P.; Ba, J. Adam: A method for stochastic optimization. arXiv 2014, arXiv:1412.6980. [Google Scholar]
- Duchi, J.; Hazan, E.; Singer, Y. Adaptive subgradient methods for online learning and stochastic optimization. J. Mach. Learn. Res. 2011, 12, 2121–2159. [Google Scholar]
- Tieleman, T.; Hinton, G. Lecture 6.5-rmsprop: Divide the gradient by a running average of its recent magnitude. COURSERA Neural Netw. Mach. Learn. 2012, 4, 26–31. [Google Scholar]
- Tayefi, M.; Esmaeili, H.; Saberi Karimian, M.; Amirabadi Zadeh, A.; Ebrahimi, M.; Safarian, M.; Nematy, M.; Parizadeh, S.M.R.; Ferns, G.A.; Ghayour-Mobarhan, M. The application of a decision tree to establish the parameters associated with hypertension. Comput. Methods Programs Biomed. 2017, 139, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Neter, J.E.; Stam, B.E.; Kok, F.J.; Grobbee, D.E.; Geleijnse, J.M. Influence of weight reduction on blood pressure: A meta-analysis of randomized controlled trials. Hypertension 2003, 42, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Howard, A.G.; Adair, L.S.; Wang, H.; Avery, C.L.; Gordon-Larsen, P. Waist Circumference Change is Associated with Blood Pressure Change Independent of BMI Change. Obesity 2020, 28, 146–153. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, X.; Xu, Y.; Gao, L.; Ma, Z.; Sun, Y.; Wang, W. Predicting the risk of hypertension based on several easy-to-collect risk factors: A machine learning method. Front. Public Health 2021, 9, 619429. [Google Scholar] [CrossRef]
- Shah, M.; Adams-Huet, B.; Garg, A. Effect of high-carbohydrate or high-cis-monounsaturated fat diets on blood pressure: A meta-analysis of intervention trials. Am. J. Clin. Nutr. 2007, 85, 1251–1256. [Google Scholar] [CrossRef]
- Kim, J.; Jo, I.; Joung, H. A rice-based traditional dietary pattern is associated with obesity in Korean adults. J. Acad. Nutr. Diet. 2012, 112, 246–253. [Google Scholar] [CrossRef]
- Iida, H.; Kurita, N.; Takahashi, S.; Sasaki, S.; Nishiwaki, H.; Omae, K.; Yajima, N.; Fukuma, S.; Hasegawa, T.; Fukuhara, S. Salt intake and body weight correlate with higher blood pressure in the very elderly population: The Sukagawa study. J. Clin. Hypertens. 2019, 21, 942–949. [Google Scholar] [CrossRef]
- Stern, N.; Buch, A.; Goldsmith, R.; Nitsan, L.; Margaliot, M.; Endevelt, R.; Marcus, Y.; Shefer, G.; Grotto, I. The role of caloric intake in the association of high salt intake with high blood pressure. Sci. Rep. 2021, 11, 15803. [Google Scholar] [CrossRef]
- Huang, L.; Trieu, K.; Yoshimura, S.; Neal, B.; Woodward, M.; Campbell, N.R.C.; Li, Q.; Lackland, D.T.; Leung, A.A.; Anderson, C.A.M.; et al. Effect of dose and duration of reduction in dietary sodium on blood pressure levels: Systematic review and meta-analysis of randomised trials. BMJ 2020, 368, m315. [Google Scholar] [CrossRef] [PubMed]
- Juraschek, S.P.; Miller, E.R., 3rd; Weaver, C.M.; Appel, L.J. Effects of Sodium Reduction and the DASH Diet in Relation to Baseline Blood Pressure. J. Am. Coll. Cardiol. 2017, 70, 2841–2848. [Google Scholar] [CrossRef] [PubMed]
- He, F.J.; Li, J.; Macgregor, G.A. Effect of longer term modest salt reduction on blood pressure: Cochrane systematic review and meta-analysis of randomised trials. BMJ 2013, 346, f1325. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; He, J.; Hamm, L.; Batuman, V.; Whelton, P.K. Serum antioxidant vitamins and blood pressure in the United States population. Hypertension 2002, 40, 810–816. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, M.; Zhou, C.; Zhang, Z.; He, P.; Li, Q.; Liu, C.; Qin, X. Inverse association between dietary vitamin A intake and new-onset hypertension. Clin. Nutr. 2021, 40, 2868–2875. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, H.; Song, Y.; Lin, T.; Zhou, Z.; Guo, H.; Liu, L.; Wang, B.; Liu, C.; Li, J.; et al. Plasma retinol and the risk of first stroke in hypertensive adults: A nested case-control study. Am. J. Clin. Nutr. 2019, 109, 449–456. [Google Scholar] [CrossRef]
- Li, H.; He, P.; Lin, T.; Guo, H.; Li, Y.; Song, Y.; Wang, B.; Liu, C.; Liu, L.; Li, J.; et al. Association between plasma retinol levels and the risk of all-cause mortality in general hypertensive patients: A nested case-control study. J. Clin. Hypertens. 2020, 22, 906–913. [Google Scholar] [CrossRef]
- Zemel, M.B. Calcium modulation of hypertension and obesity: Mechanisms and implications. J. Am. Coll. Nutr. 2001, 20, 428S–435S. [Google Scholar] [CrossRef]
| Food Category (n) | Food Items (n = 103) |
|---|---|
| Cereal-rice (4) | steamed rice, cooked rice with barely, cooked rice with other cereals, mixed multi-grain powder (misugaru/mixed grain powder). |
| Cereal-oriental (8) | ramen, noodle, black bean sauce noodle(chajangmyun), Korean cold noodle/buckwheat noodle, dumpling, white rice cake/rice-cake soup(tteoggug), sticky white rice cake(injeolmi). |
| Cereal-western (6) | bread, bread with red bean/steam bun, other breads (cream bread, castella etc.), pizza/hamburger, cornflake, butter/margarine. |
| Vegetables (17) | green pepper, red pepper leaves, spinach, lettuce, perilla leaf, leek/water dropwort, other vegetables (shepherd’s purse, crown daisy, curled mallow, radish leave etc.), radish (radish soup, boiled radish)/pickled radish, balloon flower/lance asiabell (deodeok), onion, cabbage/cabbage soup, cucumber, bean sprouts, carrot/carrot juice, pumpkin (pumpkin gruel)/pumpkin juice, green pumpkin, vegetable juice/green vegetable juice, bracken/sweet potato stalk, Korean style pickles. |
| Fruits (14) | persimmon/dried persimmon, tangerine, oriental melon/muskmelon, banana, pear, apple/apple juice, orange/orange juice, watermelon, peach/plum, strawberry, grape/grape juice, tomato/tomato juice. |
| Potatoes (3) | potato, sweet potato, starch vermicelli (japchae). |
| Legumes (3) | beans/cooked beans in soy sauce, tofu, stew with soybean paste. |
| Kimchi (4) | kimchi, small radish kimchi(kkakduki), kimchi with liquid, other kimchi (green onion kimchi, leaf mustard kimchi, korean lettuce kimchi etc.) |
| Mushrooms (2) | oyster mushroom, other mushrooms (button mushroom, enokitake, frarant mushroom). |
| Seaweeds (2) | laver, dried kelp/sea mustard. |
| Seeds and nuts (2) | starch jelly, peanut/almond/pine nut. |
| Meats (8) | dog meat, chicken/chicken leg/chicken wing, roast pork (spare rib, pork sirloin etc.), pork belly, steamed pork (soy sauce braised pork, pig trotter, Korean sausage), ham/sausage, roast beef, beef soup/Korean-style braised short rips organ meats. |
| Fishes and seafoods (15) | sashimi (halibut, flat fish, tuna, rockfish etc.), hairtail(cutlassfish), eel, yellow cracker, alaska pollack/frozen pollack/dried pollack, external blue colored fish (mackerel, pacific saury, Spanish mackerel), dried anchovy/stir-fried anchovies, cuttlefish//octopus, tuna/canned tuna, fish paste/crab meat, crab, clam (cockle/short neck clam/clam meat), oyster, shrimp, salt-fermented fish. |
| Milk and dairy products (4) | milk, yogurt, ice cream, cheese. |
| Eggs (2) | egg/quail egg. |
| Snack (3) | marshmallow choco pie(chocopie)/cakes, snacks, candy/chocolate. |
| Beverages (7) | soybean milk, carbonated drinks (cola, sprite), coffee, coffee sugar, coffee cream, green tea, other drinks (sweet rice drink, Korean citron tea[yujacha]). |
| Food Groups | Dietary Patterns Factors | ||||
|---|---|---|---|---|---|
| Dietary Pattern 1 | Dietary Pattern 2 | Dietary Pattern 3 | Dietary Pattern 4 | Dietary Pattern 5 | |
| Cereal-oriental | 0.714 | ||||
| potatoes | 0.621 | ||||
| Cereal-western | 0.578 | 0.383 | |||
| Seeds and nuts | 0.563 | ||||
| Meats | 0.807 | ||||
| Fishes and seafoods | 0.671 | ||||
| Fruits | 0.533 | ||||
| Milk and dairy products | 0.612 | ||||
| Snacks | 0.573 | −0.306 | |||
| Eggs | 0.429 | ||||
| Beverages | 0.424 | ||||
| Seaweeds | 0.377 | ||||
| Mushrooms | 0.758 | ||||
| Vegetables | 0.623 | ||||
| Cereal-rice | 0.737 | ||||
| Kimchi | 0.611 | ||||
| Legumes | 0.409 | ||||
| Variable Name | Variable Explanation | Variable Description | Training Dataset | Testing Dataset |
|---|---|---|---|---|
| AS1_DrugHtCu | Blood pressure medicine use | 1 = No | 5802 | 1466 |
| 2 = Yes | 717 | 164 | ||
| AS1_BPLIE2S_A | Mean value of 2–time systolic blood pressure measurements (lying position) | mmHg | 117.45 (69–211) | 117.18 (73–204) |
| AS1_BPLIE2D_A | Mean value of 2–time diastolic blood pressure measurements (lying position) | mmHg | 75.18 (40–125) | 75.04 (40–132) |
| Hypertension | Hypertension | 0 = No 1 = Yes | 5008 1511 | 1258 372 |
| Dataset | Energy Intake Adjustment Method |
|---|---|
| Dataset I | with energy intake, and nutrient intake before energy intake adjustment |
| Dataset II | without energy intake, and with nutrient intake before energy intake adjustment |
| Dataset III | with energy intake, and with nutrient intake after energy intake adjustment |
| Dataset IV | without energy intake, and with nutrient intake after energy intake adjustment |
| Independent Variables | ||||||
|---|---|---|---|---|---|---|
| Variable Name | Hypertension (n = 1883) (%) | Non-hypertension (n = 6266) (%) | p-Value | Training Dataset | Testing Dataset | |
| AS1_Sex | 0.096 | |||||
| Male | 858 (45.6) | 2992 (47.7) | 3075 | 775 | ||
| Female | 1025 (54.4) | 3274 (52.3) | 3444 | 855 | ||
| AS1_Age | <0.001 | |||||
| 40~49 years | 481 (25.5) | 3435 (54.8) | 3145 | 771 | ||
| 50~59 years | 579 (30.8) | 1533 (24.5) | 1684 | 428 | ||
| 60~69 years | 823 (43.7) | 1298 (20.7) | 1690 | 431 | ||
| AS1_BMI † | BMI(Kg/m2) | 25.1, 23.6 | 24.6, 23.1 | <0.001 | 24.67 (14.85–42.00) | 24.50 (15.13–36.40) |
| AS1_Weight † | Weight (Kg) | 62.86, 57.40 | 62.0, 58.0 | 0.088 | 63.30 (34–103) | 62.84 (36–105) |
| AS1_WAIST3A † | Waist circumference average value of 3 measurement | 86.0, 80.3 | 81.0, 75.0 | <0.001 | 82.46 (55.66–122.66) | 82.21 (57–110) |
| AS1_B01 † | Energy (Kcal) | 1771.0, 1464.0 | 1849.0, 1545.0 | <0.001 | 1937.29 (127–9985) | 1937.00 (230–7034) |
| AS1_B02 † | Protein (g) | 59.0, 45.0 | 62.0, 48.0 | <0.001 | 66.08 (7–558) | 66.30 (7–333) |
| AS1_B03 † | Fat (g) | 25.0, 16.0 | 29.0, 20.0 | <0.001 | 32.19 (2–357) | 32.81 (1–199) |
| AS1_B04 † | Sugar (carbohydrate, g) | 316.0, 272.0 | 323.0, 279.0 | 0.014 | 340.87 (18–1615) | 339.35 (35–1184) |
| AS1_B05 † | Ca (calcium, mg) | 397.0, 267.0 | 433.0, 300.0 | <0.001 | 473.34 (18–2694) | 489.25 (51–3226) |
| AS1_B06 † | P (phosphorus, mg) | 930.0, 719.0 | 971.0, 762.0 | <0.001 | 1019.59 (99–6526) | 1028.03 (120–4238) |
| AS1_B07 † | Fe (iron; mg) | 10.0, 7.0 | 10.0, 8.0 | <0.001 | 10.86 (1–71) | 10.90 (1–78) |
| AS1_B08 † | K (potassium, mg) | 2270.0, 1678.0 | 2351.0, 1770.8 | <0.001 | 2515.75 (193–15,818) | 2545.48 (357–13,269) |
| AS1_B09 † | Vitamin A (retinoids, R.E) | 408.0, 263.0 | 441.0, 293.0 | <0.001 | 530.23 (0–5948) | 545.46 (12–6392) |
| AS1_B10 † | Na (sodium, mg) | 2884.0, 2000.0 | 2917.0, 2092.0 | 0.142 | 3163.06 (158–16,760) | 3195.08 (160–16,623) |
| AS1_B11 † | Vitamin B1 (thiamine, mg) | 1.0, 1.0 | 1.0, 1.0 | 0.016 | 1.25 (0–10) | 1.26 (0–6) |
| AS1_B12 † | Vitamin B2 (riboflavin, mg) | 1.0, 1.0 | 1.0, 1.0 | <0.001 | 1.03 (0–8 | 1.05 (0–6) |
| AS1_B13 † | Vitamin B3 (niacin, nicotinic acid, mg) | 14.0, 10.0 | 15.0, 11.0 | <0.001 | 15.57 (2–170) | 15.55 (3–79) |
| AS1_B14 † | Vitamin C (ascorbic acid, mg) | 99.0, 67.0 | 102.0, 68.0 | 0.318 | 125.83 (1–1378) | 126.82 (11–987) |
| AS1_B15 † | Zinc (mg) | 8.0, 6.0 | 8.0, 6.0 | <0.001 | 8.74 (1–112) | 8.79 (1–58) |
| AS1_B16 † | Vitamin B6 (pyridoaxamin, µg) | 2.0, 1.0 | 2.0, 1.0 | 0.001 | 1.78 (0–12) | 1.78 (0–10) |
| AS1_B17 † | Folate (µg) | 216.0, 156.0 | 221.0, 165.0 | 0.010 | 244.74 (18–1455) | 246.50 (23–1835) |
| AS1_B18 † | Retinol (µg) | 44.0, 19.0 | 58.0, 29.0 | <0.001 | 67.94 (0–742) | 70.10 (0–695) |
| AS1_B19 † | Carotene (µg) | 1991.0, 1299.0 | 2116.0, 1383.0 | 0.005 | 2712.82 (3–39,944) | 2795.23 (49–40,069) |
| AS1_B20 † | Ash content (mg) | 17.0, 12.0 | 17.0, 12.0 | 0.353 | 21.37 (2–132) | 21.71 (3–122) |
| AS1_B21 † | Fiber (g) | 7.0, 5.0 | 6.0, 5.0 | 0.203 | 6.97 (1–38) | 7.04 (1–46) |
| AS1_B23 † | Vitamin E (tocotrienol, µg) | 8.0, 5.0 | 8.0, 6.0 | <0.001 | 9.30 (1–96) | 9.38 (1–71) |
| AS1_B24 † | Cholesterol (mg) | 124.0, 64.0 | 150.0, 87.0 | <0.001 | 175.71 (0–1857) | 174.15 (0–1328) |
| DP1 † | Dietary pattern 1 (cereal-oriental, cereal-western, potatoes, seeds and nuts) | 21.0, 8.0 | 25.0, 10.0 | <0.001 | 45.87 (0–3845) | 45.31 (0–1160) |
| DP2 † | Dietary pattern 2 (fruits, meats, fishes and seafoods) | 37.0, 16.0 | 35.0, 16.0 | 0.395 | 136.91 (0–5460) | 132.69 (0–2620) |
| DP3 † | Dietary pattern 3 (snacks, eggs, seaweeds, milk and dairy products, beverages) | 74.0, 13.0 | 114.0, 20.0 | <0.001 | 160.26 (0–1887) | 169.05 (0–1971) |
| DP4 † | Dietary pattern 4 (vegetables, mushrooms) | 17.0, 1.0 | 20.0, 2.0 | 0.107 | 44.28 (0–1725) | 48.15 (0–1815) |
| DP5 † | Dietary pattern 5 (cereal-rice, legumes, kimchi) | 819.0, 735.0 | 815.0, 725.8 | 0.002 | 846.71 (0–3750) | 840.24 (0–2344) |
| Decision Tree for Variable Importance | |||
|---|---|---|---|
| Dataset I | Dataset II | Dataset III | Dataset IV |
| waist circumference | waist circumference | waist circumference | waist circumference |
| body mass index | DP3 | niacin, nicotinic acid | zinc |
| age | body mass index | age | body mass index |
| DP3 | age | DP1 | niacin, nicotinic acid |
| retinol | sodium | body mass index | tocotrienol |
| DP5 | sugar (carbohydrate) | zinc | pyridoaxamine |
| DP1 | retinol | retinol | calcium |
| sodium | DP2 | pyridoaxamine | fat |
| ascorbic acid | DP1 | DP3 | ash content |
| DP2 | calcium | DP5 | carotene |
| Accuracy | Hidden Layers | ||||
|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | ||
| Nodes | 8 | 0.732 | 0.735 | 0.735 | 0.732 |
| 16 | 0.718 | 0.731 | 0.735 | 0.729 | |
| 24 | 0.750 | 0.737 | 0.734 | 0.740 | |
| 32 | 0.740 | 0.728 | 0.735 | 0.734 | |
| 40 | 0.726 | 0.732 | 0.732 | 0.732 | |
| 48 | 0.740 | 0.731 | 0.731 | 0.732 | |
| 56 | 0.741 | 0.737 | 0.732 | 0.730 | |
| 64 | 0.740 | 0.742 | 0.734 | 0.733 | |
| Dataset I | Dataset II | Dataset III | Dataset IV | ||
|---|---|---|---|---|---|
| DNN | Training error | 0.249 | 0.260 | 0.256 | 0.256 |
| TP | 189 | 166 | 172 | 1031 | |
| TN | 1034 | 1040 | 1040 | 181 | |
| FP | 224 | 218 | 218 | 227 | |
| FN | 183 | 206 | 200 | 191 | |
| Sensitivity | 0.508 | 0.446 | 0.462 | 0.487 | |
| Specificity | 0.822 | 0.827 | 0.827 | 0.820 | |
| F1-score | 0.482 | 0.439 | 0.451 | 0.464 | |
| Accuracy | 0.750 | 0.739 | 0.743 | 0.743 | |
| Decision tree | Training error | 0.323 | 0.315 | 0.309 | 0.319 |
| TP | 139 | 148 | 168 | 146 | |
| TN | 963 | 967 | 957 | 963 | |
| FP | 295 | 291 | 301 | 295 | |
| FN | 233 | 224 | 204 | 226 | |
| Sensitivity | 0.374 | 0.398 | 0.452 | 0.392 | |
| Specificity | 0.766 | 0.769 | 0.761 | 0.766 | |
| F1-score | 0.345 | 0.365 | 0.400 | 0.359 | |
| Accuracy | 0.676 | 0.684 | 0.690 | 0.680 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Hwang, S.; Lee, S.; Kim, Y. Classification and Prediction on Hypertension with Blood Pressure Determinants in a Deep Learning Algorithm. Int. J. Environ. Res. Public Health 2022, 19, 15301. https://doi.org/10.3390/ijerph192215301
Kim H, Hwang S, Lee S, Kim Y. Classification and Prediction on Hypertension with Blood Pressure Determinants in a Deep Learning Algorithm. International Journal of Environmental Research and Public Health. 2022; 19(22):15301. https://doi.org/10.3390/ijerph192215301
Chicago/Turabian StyleKim, Hyerim, Seunghyeon Hwang, Suwon Lee, and Yoona Kim. 2022. "Classification and Prediction on Hypertension with Blood Pressure Determinants in a Deep Learning Algorithm" International Journal of Environmental Research and Public Health 19, no. 22: 15301. https://doi.org/10.3390/ijerph192215301
APA StyleKim, H., Hwang, S., Lee, S., & Kim, Y. (2022). Classification and Prediction on Hypertension with Blood Pressure Determinants in a Deep Learning Algorithm. International Journal of Environmental Research and Public Health, 19(22), 15301. https://doi.org/10.3390/ijerph192215301







