Poor Agreement between Responses to the International Physical Activity Questionnaire and Objective ActiGraph® Data among Persons with Major Depressive or Bipolar Disorders
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Interviews and Tools for Characterizing the Participants
2.3. Mini International Neuropsychiatric Interview (MINI)
2.4. Montgomery–Asberg Depression Scale (MADRS)
2.5. Young Mania Rating Scale (YMRS)
2.6. International Physical Activity Questionnaire Short Form (IPAQ)
2.7. ActiGraph®
2.8. Statistical Analyses
3. Results
3.1. Comparisons between the ActiGraph® and IPAQ Data
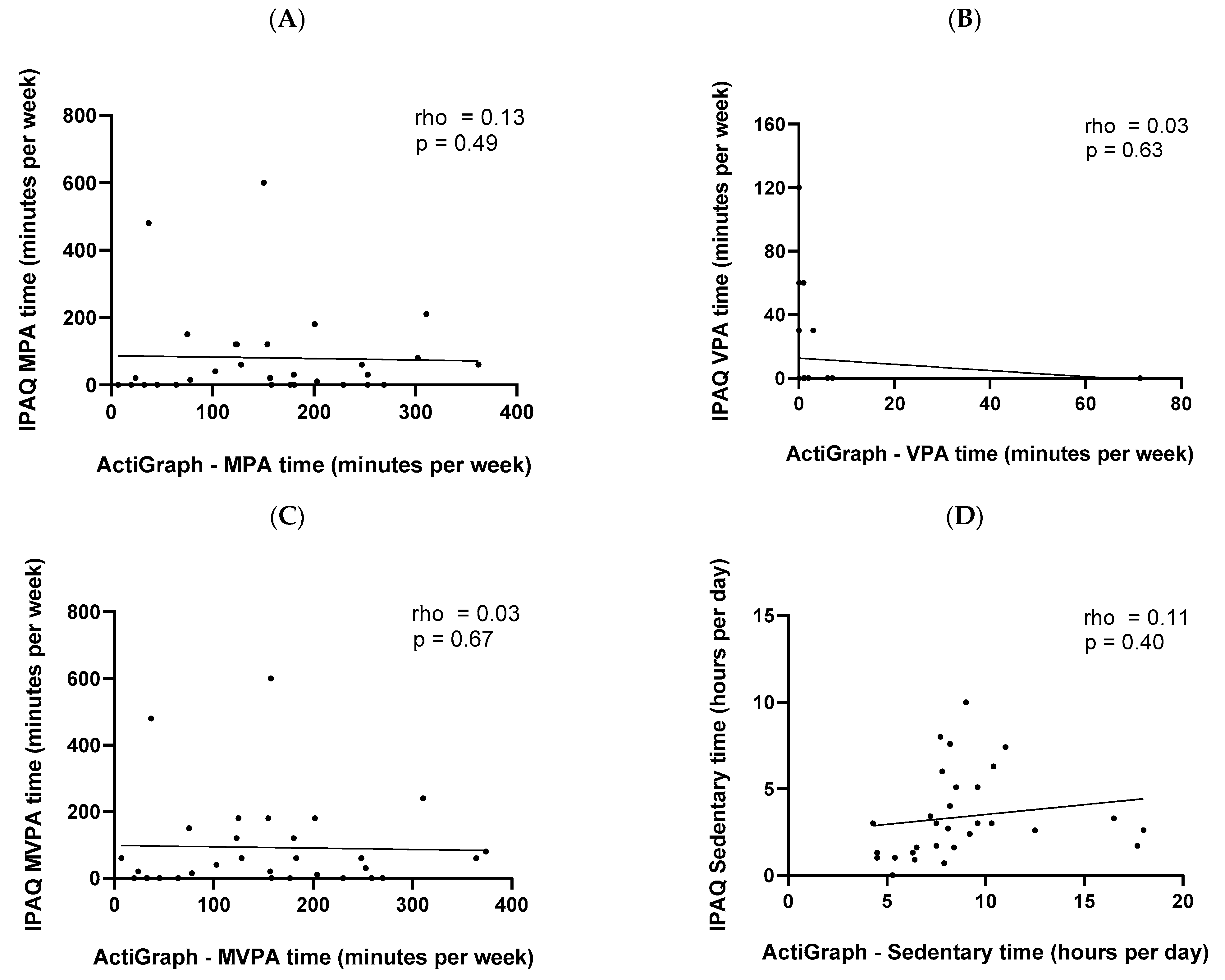
3.2. Associations between Data from the ActiGraph® and IPAQ
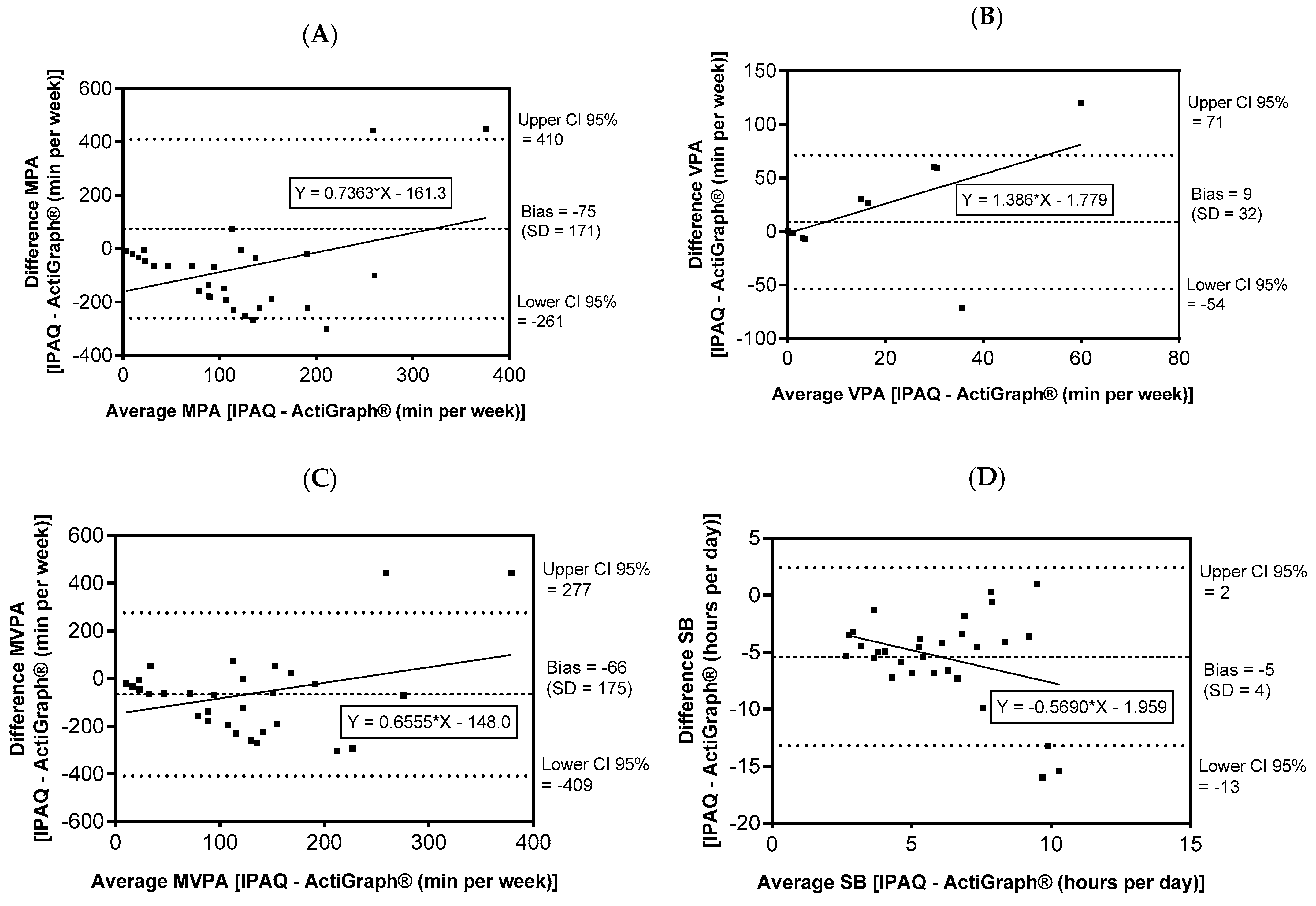
3.3. Bland–Altman Plot
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®); American Psychiatric Pub: Arlington, VA, USA, 2014. [Google Scholar]
- GBD. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Firth, J.; Siddiqi, N.; Koyanagi, A.; Siskind, D.; Rosenbaum, S.; Galletly, C.; Allan, S.; Caneo, C.; Carney, R.; Carvalho, A.F. The Lancet Psychiatry Commission: A blueprint for protecting physical health in people with mental illness. Lancet Psychiatry 2019, 6, 675–712. [Google Scholar] [CrossRef]
- Plana-Ripoll, O.; Pedersen, C.B.; Agerbo, E.; Holtz, Y.; Erlangsen, A.; Canudas-Romo, V.; Andersen, P.K.; Charlson, F.J.; Christensen, M.K.; Erskine, H.E.; et al. A comprehensive analysis of mortality-related health metrics associated with mental disorders: A nationwide, register-based cohort study. Lancet 2019, 394, 1827–1835. [Google Scholar] [CrossRef]
- Caspersen, C.J.; Powell, K.E.; Christenson, G.M. Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Rep. 1985, 100, 126–131. [Google Scholar] [PubMed]
- Schuch, F.B.; Stubbs, B. The Role of Exercise in Preventing and Treating Depression. Curr. Sport. Med. Rep. 2019, 18, 299–304. [Google Scholar] [CrossRef]
- Schuch, F.B.; Vancampfort, D.; Firth, J.; Rosenbaum, S.; Ward, P.B.; Silva, E.S.; Hallgren, M.; Ponce De Leon, A.; Dunn, A.L.; Deslandes, A.C.; et al. Physical Activity and Incident Depression: A Meta-Analysis of Prospective Cohort Studies. Am. J. Psychiatry 2018, 175, 631–648. [Google Scholar] [CrossRef] [PubMed]
- Schuch, F.B.; Vancampfort, D.; Richards, J.; Rosenbaum, S.; Ward, P.B.; Stubbs, B. Exercise as a treatment for depression: A meta-analysis adjusting for publication bias. J. Psychiatr. Res. 2016, 77, 42–51. [Google Scholar] [CrossRef]
- Stubbs, B.; Vancampfort, D.; Firth, J.; Schuch, F.B.; Hallgren, M.; Smith, L.; Gardner, B.; Kahl, K.G.; Veronese, N.; Solmi, M.; et al. Relationship between sedentary behavior and depression: A mediation analysis of influential factors across the lifespan among 42,469 people in low- and middle-income countries. J. Affect. Disord. 2018, 229, 231–238. [Google Scholar]
- Sun, H.L.; Gao, X.; Que, X.M.; Liu, L.; Ma, J.S.; He, S.M.; Gao, Q.; Wang, T. The causal relationships of device-measured physical activity with bipolar disorder and schizophrenia in adults: A 2-Sample mendelian randomization study. J. Affect. Disord. 2020, 263, 598–604. [Google Scholar]
- Duarte, C.; Belizario, G.O.; Mathias, K.; Silva, M.; Roberto, P.; Greve, J.; Lafer, B. Structured physical exercise in bipolar depression: A pilot study. Bipolar Disord. 2020, 22, 85–86. [Google Scholar]
- Melo, M.C.A.; Garcia, R.F.; de Araújo, C.F.C.; Rangel, D.M.; de Bruin, P.F.C.; de Bruin, V.M.S. Physical activity as prognostic factor for bipolar disorder: An 18-month prospective study. J. Affect. Disord. 2019, 251, 100–106. [Google Scholar] [CrossRef]
- Vancampfort, D.; Probst, M.; Wyckaert, S.; De Hert, M.; Stubbs, B.; Rosenbaum, S.; Sienaert, P. Physical activity as a vital sign in patients with bipolar disorder. Psychiatry Res. 2016, 246, 218–222. [Google Scholar] [CrossRef]
- De Sá Filho, A.S.; De Souza Moura, A.M.; Lamego, M.K.; Rocha, N.B.F.; Paes, F.; Oliveira, A.C.; Lattari, E.; Rimes, R.; Manochio, J.; Budde, H.; et al. Potential therapeutic effects of physical exercise for bipolar disorder. CNS Neurol. Disord.-Drug Targets 2015, 14, 1255–1259. [Google Scholar] [CrossRef] [PubMed]
- SBRN. Letter to the editor: Standardized use of the terms “sedentary” and “sedentary behaviours”. Appl. Physiol. Nutr. Metab. = Physiol. Appl. Nutr. Et Metab. 2012, 37, 540–542. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Oh, P.I.; Faulkner, G.E.; Bajaj, R.R.; Silver, M.A.; Mitchell, M.S.; Alter, D.A. Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults: A systematic review and meta-analysis. Ann. Intern. Med. 2015, 162, 123–132. [Google Scholar] [CrossRef]
- Kandola, A.A.; Del Pozo Cruz, B.; Osborn, D.P.J.; Stubbs, B.; Choi, K.W.; Hayes, J.F. Impact of replacing sedentary behaviour with other movement behaviours on depression and anxiety symptoms: A prospective cohort study in the UK Biobank. BMC Med. 2021, 19, 133. [Google Scholar] [CrossRef]
- Werneck, A.O.; Hoare, E.; Stubbs, B.; van Sluijs, E.M.F.; Corder, K. Association of mentally-active and mentally-passive sedentary behaviour with depressive symptoms among adolescents. J. Affect. Disord. 2021, 294, 143–150. [Google Scholar] [CrossRef]
- Vancampfort, D.; Firth, J.; Schuch, F.B.; Rosenbaum, S.; Mugisha, J.; Hallgren, M.; Probst, M.; Ward, P.B.; Gaughran, F.; De Hert, M. Sedentary behavior and physical activity levels in people with schizophrenia, bipolar disorder and major depressive disorder: A global systematic review and meta-analysis. World Psychiatry Off. J. World Psychiatr. Assoc. (WPA) 2017, 16, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sport. Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- Lee, P.H.; Macfarlane, D.J.; Lam, T.H.; Stewart, S.M. Validity of the International Physical Activity Questionnaire Short Form (IPAQ-SF): A systematic review. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 115. [Google Scholar] [CrossRef]
- Bauman, A.; Ainsworth, B.E.; Bull, F.; Craig, C.L.; Hagstromer, M.; Sallis, J.F.; Pratt, M.; Sjostrom, M. Progress and pitfalls in the use of the International Physical Activity Questionnaire (IPAQ) for adult physical activity surveillance. J. Phys. Act. Health 2009, 6 (Suppl. S1), S5–S8. [Google Scholar] [CrossRef]
- Durante, R.; Ainsworth, B.E. The recall of physical activity: Using a cognitive model of the question-answering process. Med. Sci. Sport. Exerc. 1996, 28, 1282–1291. [Google Scholar] [CrossRef]
- Dillon, D.G.; Pizzagalli, D.A. Mechanisms of Memory Disruption in Depression. Trends Neurosci. 2018, 41, 137–149. [Google Scholar] [CrossRef]
- Walia, H.K.; Mehra, R. Practical aspects of actigraphy and approaches in clinical and research domains. Handb. Clin. Neurol. 2019, 160, 371–379. [Google Scholar]
- Trost, S.G.; McIver, K.L.; Pate, R.R. Conducting accelerometer-based activity assessments in field-based research. Med. Sci. Sport. Exerc. 2005, 37 (Suppl. S11), S531–S543. [Google Scholar] [CrossRef]
- Curry, W.B.; Thompson, J.L. Comparability of accelerometer- and IPAQ-derived physical activity and sedentary time in South Asian women: A cross-sectional study. Eur. J. Sport Sci. 2015, 15, 655–662. [Google Scholar] [CrossRef]
- Pinto, A.J.; Roschel, H.; Benatti, F.B.; de Sá Pinto, A.L.; Sallum, A.M.; Silva, C.A.; Gualano, B. Poor agreement of objectively measured and self-reported physical activity in juvenile dermatomyositis and juvenile systemic lupus erythematosus. Clin. Rheumatol. 2016, 35, 1507–1514. [Google Scholar] [CrossRef] [PubMed]
- Firth, J.; Stubbs, B.; Vancampfort, D.; Schuch, F.B.; Rosenbaum, S.; Ward, P.B.; Firth, J.A.; Sarris, J.; Yung, A.R. The Validity and Value of Self-reported Physical Activity and Accelerometry in People With Schizophrenia: A Population-Scale Study of the UK Biobank. Schizophr. Bull. 2018, 44, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Duncan, M.J.; Arbour-Nicitopoulos, K.; Subramanieapillai, M.; Remington, G.; Faulkner, G. Revisiting the International Physical Activity Questionnaire (IPAQ): Assessing physical activity among individuals with schizophrenia. Schizophr. Res. 2017, 179, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Amorim, P. Mini International Neuropsychiatric Interview (MINI): Validação de entrevista breve para diagnóstico de transtornos mentais. Rev. Bras. Psiquiatr. 2000, 22, 106–115. [Google Scholar] [CrossRef]
- Sheehan, D.V.; Lecrubier, Y.; Sheehan, K.H.; Amorim, P.; Janavs, J.; Weiller, E.; Hergueta, T.; Baker, R.; Dunbar, G.C. The Mini-International Neuropsychiatric Interview (MINI): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 1998, 59, 22–33. [Google Scholar] [PubMed]
- Goodyear, M.D.; Krleza-Jeric, K.; Lemmens, T. The declaration of Helsinki. BMJ 2007, 335, 624–625. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, S.A.; Asberg, M. A new depression scale designed to be sensitive to change. Br. J. Psychiatry 1979, 134, 382–389. [Google Scholar] [CrossRef]
- Davidson, J.; Turnbull, C.D.; Strickland, R.; Miller, R.; Graves, K. The Montgomery-Åsberg Depression Scale: Reliability and validity. Acta Psychiatr. Scand. 1986, 73, 544–548. [Google Scholar] [CrossRef]
- Young, R.C.; Biggs, J.T.; Ziegler, V.E.; Meyer, D.A. A rating scale for mania: Reliability, validity and sensitivity. Br. J. Psychiatry J. Ment. Sci. 1978, 133, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Vilela, J.; Crippa, J.A.d.S.; Del-Ben, C.M.; Loureiro, S.R. Reliability and validity of a Portuguese version of the Young Mania Rating Scale. Braz. J. Med. Biol. Res. 2005, 38, 1429–1439. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, J.; Coutinho, A.; Santos, C.; Bertuol, C.; Minatto, G.; Berria, J.; Tonosaki, L.; Lima, L.; Marchesan, M.; Silveira, P. Orientações para utilização de acelerômetros no Brasil. Rev. Bras. De Ativ. Física Saúde 2017, 22, 110–126. [Google Scholar] [CrossRef]
- Choi, L.; Liu, Z.; Matthews, C.E.; Buchowski, M.S. Validation of accelerometer wear and nonwear time classification algorithm. Med. Sci. Sport. Exerc. 2011, 43, 357–364. [Google Scholar] [CrossRef]
- Sasaki, J.E.; John, D.; Freedson, P.S. Validation and comparison of ActiGraph activity monitors. J. Sci. Med. Sport 2011, 14, 411–416. [Google Scholar] [CrossRef]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation coefficients: Appropriate use and interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef]
- Mansournia, M.A.; Waters, R.; Nazemipour, M.; Bland, M.; Altman, D.G. Bland-Altman methods for comparing methods of measurement and response to criticisms. Glob. Epidemiol. 2021, 3, 100045. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Int. J. Nurs. Stud. 2010, 47, 931–936. [Google Scholar] [CrossRef]
- Prince, S.A.; LeBlanc, A.G.; Colley, R.C.; Saunders, T.J. Measurement of sedentary behaviour in population health surveys: A review and recommendations. PeerJ 2017, 5, e4130. [Google Scholar] [CrossRef] [PubMed]
- Healy, G.N.; Clark, B.K.; Winkler, E.A.; Gardiner, P.A.; Brown, W.J.; Matthews, C.E. Measurement of adults’ sedentary time in population-based studies. Am. J. Prev. Med. 2011, 41, 216–227. [Google Scholar] [CrossRef]
- Hallal, P.C.; Gomez, L.F.; Parra, D.C.; Lobelo, F.; Mosquera, J.; Florindo, A.A.; Sarmiento, O.L. Lessons learned after 10 years of IPAQ use in Brazil and Colombia. J. Phys. Act. Health 2010, 7, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, S.; Morell, R.; Abdel-Baki, A.; Ahmadpanah, M.; Anilkumar, T.V.; Baie, L.; Bauman, A.; Bender, S.; Boyan Han, J.; Brand, S.; et al. Assessing physical activity in people with mental illness: 23-country reliability and validity of the simple physical activity questionnaire (SIMPAQ). BMC Psychiatry 2020, 20, 108. [Google Scholar] [CrossRef]
- Cleland, C.; Ferguson, S.; Ellis, G.; Hunter, R.F. Validity of the International Physical Activity Questionnaire (IPAQ) for assessing moderate-to-vigorous physical activity and sedentary behaviour of older adults in the United Kingdom. BMC Med. Res. Methodol. 2018, 18, 176. [Google Scholar] [CrossRef]
- Soundy, A.; Roskell, C.; Stubbs, B.; Vancampfort, D. Selection, use and psychometric properties of physical activity measures to assess individuals with severe mental illness: A narrative synthesis. Arch. Psychiatr. Nurs. 2014, 28, 135–151. [Google Scholar] [CrossRef]
- Ravindran, A.V.; Balneaves, L.G.; Faulkner, G.; Ortiz, A.; McIntosh, D.; Morehouse, R.L.; Ravindran, L.; Yatham, L.N.; Kennedy, S.H.; Lam, R.W.; et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder: Section 5. Complementary and Alternative Medicine Treatments. Canadian journal of psychiatry. Rev. Can. De Psychiatr. 2016, 61, 576–587. [Google Scholar] [CrossRef]
- Stubbs, B.; Vancampfort, D.; Hallgren, M.; Firth, J.; Veronese, N.; Solmi, M.; Brand, S.; Cordes, J.; Malchow, B.; Gerber, M.; et al. EPA guidance on physical activity as a treatment for severe mental illness: A meta-review of the evidence and Position Statement from the European Psychiatric Association (EPA), supported by the International Organization of Physical Therapists in Mental Health (IOPTMH). Eur. Psychiatry 2018, 54, 124–144. [Google Scholar]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef] [PubMed]
- Valkenet, K.; Veenhof, C. Validity of three accelerometers to investigate lying, sitting, standing and walking. PLoS ONE 2019, 14, e0217545. [Google Scholar] [CrossRef] [PubMed]
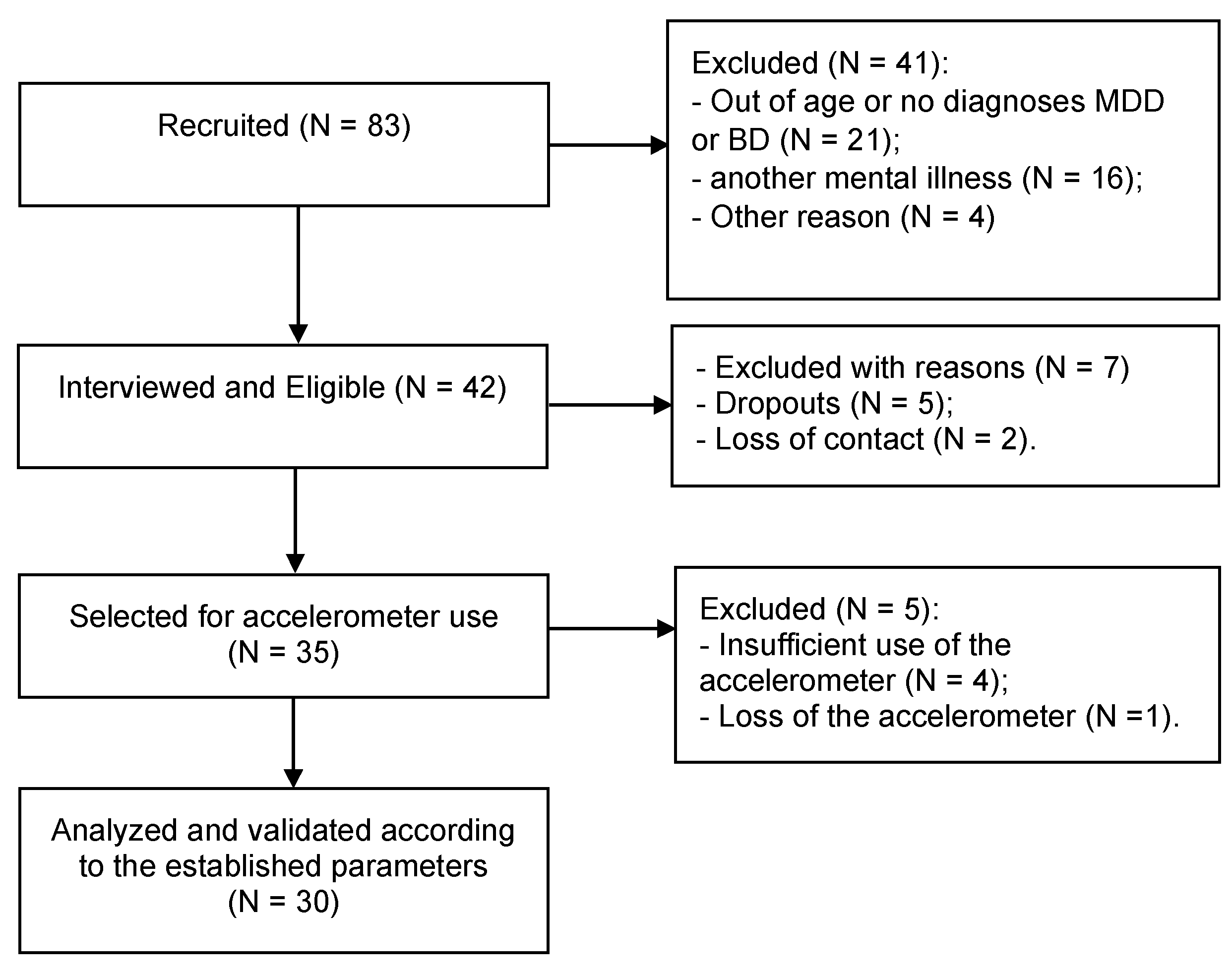
| Variable | MDD People (n = 18) | BD People (n = 12) |
|---|---|---|
| Female | 83.3% | 91.7% |
| Age (years) | 42.5 ± 10.8 | 40.8 ± 14.2 |
| Weight (kilograms) | 82.8 ± 12.0 | 69.0 ± 16.0 |
| Height (centimeters) | 164 ± 11 | 162 ± 14 |
| Body Mass Index (kg/m2) | 30.6 ± 4.1 | 26.7 ± 5.6 |
| Symptoms of disease | ||
| Depression—MADRS (points) | 14.7 ± 8.6 | 15.6 ± 9.1 |
| Mania—YMRS (points) | --- | 7.2 ± 3.6 |
| Marital status | ||
| Single | 50.0% | 50.0% |
| Married | 38.9% | 33.3% |
| Divorced/Widowed | 11.1% | 16.7% |
| Schooling | ||
| Elementary school | 16.7% | 33.3% |
| High school | 38.9% | 33.3% |
| University education | 44.4% | 33.3% |
| Profession or occupation | ||
| Health and education | 27.8% | 8.3% |
| Housemaid | 11.1% | 8.3% |
| Unemployed and at home | 33.3% | 50.0% |
| Other areas | 27.8% | 33.3% |
| Comorbidities | ||
| Hypertension | 22.2% | 25.0% |
| Diabetes | 11.1% | 8.3% |
| Obesity | 61.1% | 25.0% |
| Others | 5.6% | 8.3% |
| Number of drugs used for treating psychiatric disorders | ||
| 1 drug | 33.3% | 16.7% |
| 2 drugs | 50.0% | 25.0% |
| >3 drugs | 16.7% | 58.3% |
| Subjective health perception | ||
| Do you usually sleep well? (Yes) | 38.9% | 41.7% |
| Do you have a balanced diet? (Yes) | 16.7% | 16.7% |
| Do you smoke (tobacco/cigarette)? (Yes) | 5.6% | 33.3% |
| Do you use excessive alcohol? (Yes) | 0% | 0% |
| Do you perform systematic exercise? (Yes) | 0% | 16.7% |
| Variable | MDD (n = 18) | BD (n = 12) | Pooled (n = 30) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ActiGraph® | IPAQ | p | ActiGraph® | IPAQ | p | ActiGraph® | IPAQ | p | |
| Walking (Minutes per week) | -- | 39 (0–93) | -- | -- | 73 (0–25) | -- | -- | 40 (0–100) | |
| LPA (Minutes per week) | 2221 (1637–2848) | -- | -- | 2063 (1417–2368) | -- | -- | 2193 (1568–2706) | -- | -- |
| MPA (Minutes per week) | 167 (130–243) | 25 (0–105) | 0.003 | 113 (68–186) | 28 (0–90) | <0.001 | 155 (76–222) | 25 (0–110) | <0.001 |
| VPA (Minutes per week) | 0 (0–1) | 0 (0–0) | ns | 0 (0–1) | 0 (0–0) | ns | 0 (0–1) | 0 (0–0) | ns |
| MVPA (Minutes per week) | 167 (131–244) | 45 (0–105) | 0.012 | 114 (68–186) | 50 (8–128) | ns | 157 (76–223) | 50 (0–120) | 0.005 |
| SB (Hours per day) | 8 (6–9) | 3 (2–6) | <0.001 | 8 (7–11) | 3 (2–6) | 0.002 | 8 (7–10) | 3 (1–5) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
do Nascimento, R.B.; Santos, R.P.G.; Gomes, T.H.S.; França, C.N.; Rossi, F.E.; Natrielli-Filho, D.G.; Jambassi-Filho, J.C.; Gil, S.; Stubbs, B.; Lafer, B.; et al. Poor Agreement between Responses to the International Physical Activity Questionnaire and Objective ActiGraph® Data among Persons with Major Depressive or Bipolar Disorders. Int. J. Environ. Res. Public Health 2022, 19, 14913. https://doi.org/10.3390/ijerph192214913
do Nascimento RB, Santos RPG, Gomes THS, França CN, Rossi FE, Natrielli-Filho DG, Jambassi-Filho JC, Gil S, Stubbs B, Lafer B, et al. Poor Agreement between Responses to the International Physical Activity Questionnaire and Objective ActiGraph® Data among Persons with Major Depressive or Bipolar Disorders. International Journal of Environmental Research and Public Health. 2022; 19(22):14913. https://doi.org/10.3390/ijerph192214913
Chicago/Turabian Styledo Nascimento, Rafael Bonfim, Rafael Pereira Guimarães Santos, Tabatah Hellen Santos Gomes, Carolina Nunes França, Fabricio Eduardo Rossi, Decio Gilberto Natrielli-Filho, José Claudio Jambassi-Filho, Saulo Gil, Brendon Stubbs, Beny Lafer, and et al. 2022. "Poor Agreement between Responses to the International Physical Activity Questionnaire and Objective ActiGraph® Data among Persons with Major Depressive or Bipolar Disorders" International Journal of Environmental Research and Public Health 19, no. 22: 14913. https://doi.org/10.3390/ijerph192214913
APA Styledo Nascimento, R. B., Santos, R. P. G., Gomes, T. H. S., França, C. N., Rossi, F. E., Natrielli-Filho, D. G., Jambassi-Filho, J. C., Gil, S., Stubbs, B., Lafer, B., & Neves, L. M. (2022). Poor Agreement between Responses to the International Physical Activity Questionnaire and Objective ActiGraph® Data among Persons with Major Depressive or Bipolar Disorders. International Journal of Environmental Research and Public Health, 19(22), 14913. https://doi.org/10.3390/ijerph192214913








