The Mediating Role of Physical Activity and Physical Function in the Association between Body Mass Index and Health-Related Quality of Life: A Population-Based Study with Older Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Project and Participants
2.2. Data Collection
2.2.1. Demographics and Health Profile
2.2.2. Anthropometry
2.2.3. Physical Activity
2.2.4. Physical Function
2.2.5. Health-Related Quality of Life
2.2.6. Covariates
2.3. Statistical Analysis
3. Results
3.1. Main Characteristics of Participants
3.2. Correlations between the Main Variables of Interest in the Study
3.3. Associations between BMI and HRQoL
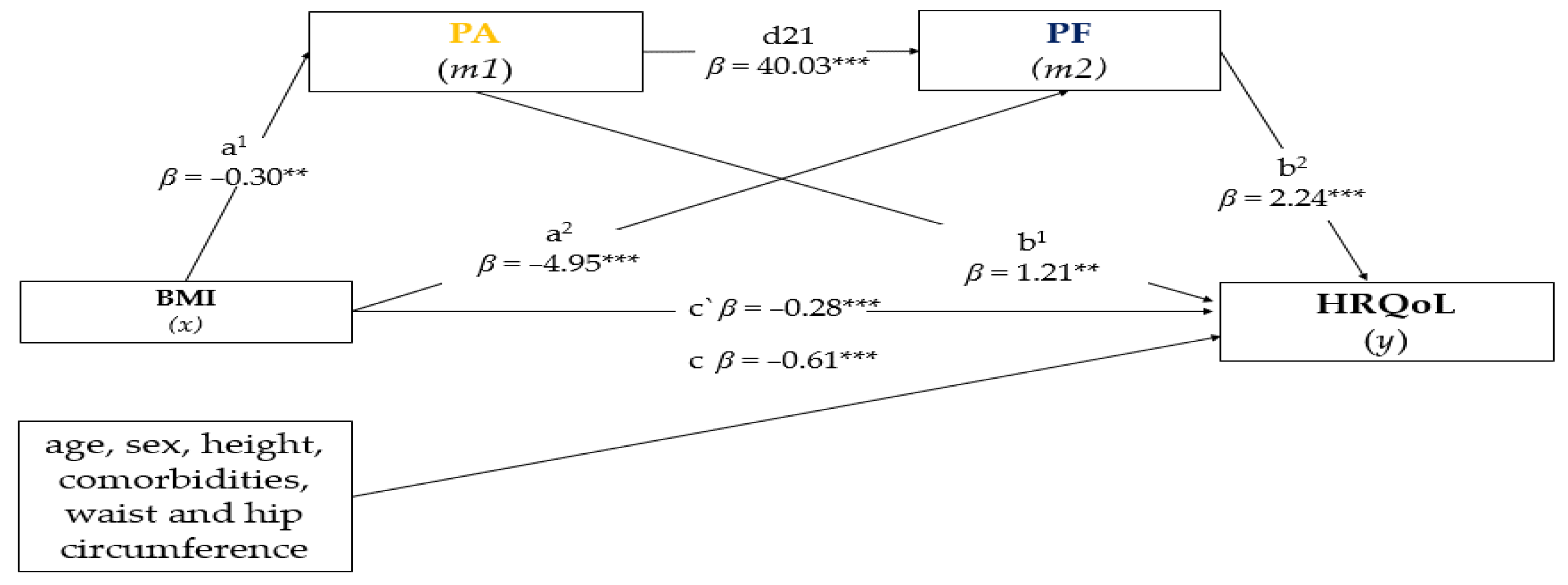
3.4. Mediation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flegal, K.M.; Kruszon-Moran, D.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Trends in Obesity among Adults in the United States, 2005 to 2014. JAMA 2016, 315, 2284. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.C.; Gilman, A.P.; Odland, J.Ø. Is thermogenesis a significant causal factor in preventing the “globesity” epidemic? Med. Hypotheses 2010, 75, 250–256. [Google Scholar] [CrossRef]
- WHO-World Health Organization Controlling the Global Obesity Epidemic. Available online: https://www.who.int/activities/controlling-the-global-obesity-epidemic (accessed on 25 July 2022).
- Corica, F.; Corsonello, A.; Apolone, G.; Mannucci, E.; Lucchetti, M.; Bonfiglio, C.; Melchionda, N.; Marchesini, G. Metabolic syndrome, psychological status and quality of life in obesity: The QUOVADIS Study. Int. J. Obes. 2008, 32, 185–191. [Google Scholar] [CrossRef]
- Giuli, C.; Papa, R.; Bevilacqua, R.; Felici, E.; Gagliardi, C.; Marcellini, F.; Boscaro, M.; De Robertis, M.; Mocchegiani, E.; Faloia, E.; et al. Correlates of perceived health related quality of life in obese, overweight and normal weight older adults: An observational study. BMC Public Health 2014, 14, 35. [Google Scholar] [CrossRef]
- Kelly, M.E.; Loughrey, D.; Lawlor, B.A.; Robertson, I.H.; Walsh, C.; Brennan, S. The impact of exercise on the cognitive functioning of healthy older adults: A systematic review and meta-analysis. Ageing Res. Rev. 2014, 16, 12–31. [Google Scholar] [CrossRef] [PubMed]
- Elagizi, A.; Kachur, S.; Carbone, S.; Lavie, C.J.; Blair, S.N. A Review of Obesity, Physical Activity, and Cardiovascular Disease. Curr. Obes. Rep. 2020, 9, 571–581. [Google Scholar] [CrossRef]
- Jia, H.; Lubetkin, E.I. The impact of obesity on health-related quality-of-life in the general adult US population. J. Public Health 2005, 27, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Latner, J.D.; Barile, J.P.; Durso, L.E.; O’Brien, K.S. Weight and health-related quality of life: The moderating role of weight discrimination and internalized weight bias. Eat. Behav. 2014, 15, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, D.; Sharma, S. Abdominal Obesity, Adipokines and Non-communicable Diseases. J. Steroid Biochem. Mol. Biol. 2020, 203, 105737. [Google Scholar] [CrossRef] [PubMed]
- Kilpi, F.; Webber, L.; Musaigner, A.; Aitsi-Selmi, A.; Marsh, T.; Rtveladze, K.; McPherson, K.; Brown, M. Alarming predictions for obesity and non-communicable diseases in the Middle East. Public Health Nutr. 2014, 17, 1078–1086. [Google Scholar] [CrossRef]
- Lau, D.C.W.; Douketis, D.J.; Morrison, K.M.; Hramiak, I.M.; Sharma, A.M.; Ur, E. Canadian Guidelines for obesity. Can. Med. Assoc. J. 2007, 176, S1–S13. [Google Scholar] [CrossRef] [PubMed]
- Rothman, K.J. BMI-related errors in the measurement of obesity. Int. J. Obes. 2008, 32, S56–S59. [Google Scholar] [CrossRef] [PubMed]
- Andreacchi, A.T.; Griffith, L.E.; Guindon, G.E.; Mayhew, A.; Bassim, C.; Pigeyre, M.; Stranges, S.; Anderson, L.N. Body mass index, waist circumference, waist-to-hip ratio, and body fat in relation to health care use in the Canadian Longitudinal Study on Aging. Int. J. Obes. 2021, 45, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Guh, D.P.; Zhang, W.; Bansback, N.; Amarsi, Z.; Birmingham, C.L.; Anis, A.H. The incidence of co-morbidities related to obesity and overweight: A systematic review and meta-analysis. BMC Public Health 2009, 9, 88. [Google Scholar] [CrossRef]
- Piché, M.-E.; Tchernof, A.; Després, J.-P. Obesity Phenotypes, Diabetes, and Cardiovascular Diseases. Circ. Res. 2020, 126, 1477–1500. [Google Scholar] [CrossRef]
- Mathieu, P.; Poirier, P.; Pibarot, P.; Lemieux, I.; Després, J.-P. Visceral Obesity: The Link among Inflammation, Hypertension, and Cardiovascular Disease. Hypertension 2009, 53, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Katta, N.; Loethen, T.; Lavie, C.J.; Alpert, M.A. Obesity and Coronary Heart Disease: Epidemiology, Pathology, and Coronary Artery Imaging. Curr. Probl. Cardiol. 2021, 46, 100655. [Google Scholar] [CrossRef]
- Avgerinos, K.I.; Spyrou, N.; Mantzoros, C.S.; Dalamaga, M. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism 2019, 92, 121–135. [Google Scholar] [CrossRef]
- Wearing, S.C.; Hennig, E.M.; Byrne, N.M.; Steele, J.R.; Hills, A.P. Musculoskeletal disorders associated with obesity: A biomechanical perspective. Obes. Rev. 2006, 7, 239–250. [Google Scholar] [CrossRef]
- DeCaria, J.E.; Sharp, C.; Petrella, R.J. Scoping review report: Obesity in older adults. Int. J. Obes. 2012, 36, 1141–1150. [Google Scholar] [CrossRef]
- Vincent, H.K.; Vincent, K.R.; Lamb, K.M. Obesity and mobility disability in the older adult. Obes. Rev. 2010, 11, 568–579. [Google Scholar] [CrossRef] [PubMed]
- Rillamas-Sun, E.; LaCroix, A.Z.; Waring, M.E.; Kroenke, C.H.; LaMonte, M.J.; Vitolins, M.Z.; Seguin, R.; Bell, C.L.; Gass, M.; Manini, T.M.; et al. Obesity and Late-Age Survival without Major Disease or Disability in Older Women. JAMA Intern. Med. 2014, 174, 98. [Google Scholar] [CrossRef] [PubMed]
- Mathus-Vliegen, E.M.H. Obesity and the Elderly. J. Clin. Gastroenterol. 2012, 46, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Sardinha, L.B.; Santos, D.A.; Silva, A.M.; Baptista, F.; Owen, N. Breaking-up Sedentary Time Is Associated with Physical Function in Older Adults. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2015, 70, 119–124. [Google Scholar] [CrossRef] [PubMed]
- WHO-World Health Organization. WHO Guidelines on Physical Activity and Sedentary Behaviour; World Health Organization: Geneva, Switzerland, 2020; ISBN 9781134470006. [Google Scholar]
- St-Onge, M.-P.; Gallagher, D. Body composition changes with aging: The cause or the result of alterations in metabolic rate and macronutrient oxidation? Nutrition 2010, 26, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Daskalopoulou, C.; Stubbs, B.; Kralj, C.; Koukounari, A.; Prince, M.; Prina, A.M. Physical activity and healthy ageing: A systematic review and meta-analysis of longitudinal cohort studies. Ageing Res. Rev. 2017, 38, 6–17. [Google Scholar] [CrossRef]
- Garcia Meneguci, C.A.; Meneguci, J.; Sasaki, J.E.; Tribess, S.; Júnior, J.S.V. Physical activity, sedentary behavior and functionality in older adults: A cross-sectional path analysis. PLoS ONE 2021, 16, e0246275. [Google Scholar] [CrossRef]
- Chen, H.; Guo, X. Obesity and Functional Disability in Elderly Americans. J. Am. Geriatr. Soc. 2008, 56, 689–694. [Google Scholar] [CrossRef]
- Park, S. Pathways linking obesity to health-related quality of life. Qual. Life Res. 2017, 26, 2209–2218. [Google Scholar] [CrossRef]
- Lopez-Garcia, E.; Guallar-Castillón, P.; Garcia-Esquinas, E.; Rodríguez-Artalejo, F. Metabolically healthy obesity and health-related quality of life: A prospective cohort study. Clin. Nutr. 2017, 36, 853–860. [Google Scholar] [CrossRef]
- Henning, R.J. Obesity and obesity-induced inflammatory disease contribute to atherosclerosis: A review of the pathophysiology and treatment of obesity. Am. J. Cardiovasc. Dis. 2021, 11, 504–529. [Google Scholar] [PubMed]
- Truthmann, J.; Mensink, G.B.M.; Bosy-Westphal, A.; Hapke, U.; Scheidt-Nave, C.; Schienkiewitz, A. Physical health-related quality of life in relation to metabolic health and obesity among men and women in Germany. Health Qual. Life Outcomes 2017, 15, 122. [Google Scholar] [CrossRef] [PubMed]
- He, X.Z.; Baker, D.W. Body mass index, physical activity, and the risk of decline in overall health and physical functioning in late middle age. Am. J. Public Health 2004, 94, 1567–1573. [Google Scholar] [CrossRef] [PubMed]
- Shaw, K.A.; Gennat, H.C.; O’Rourke, P.; Del Mar, C. Exercise for overweight or obesity. Cochrane Database Syst. Rev. 2006, 2006, CD003817. [Google Scholar] [CrossRef]
- Manini, T.M.; Pahor, M. Physical activity and maintaining physical function in older adults. Br. J. Sports Med. 2008, 43, 28–31. [Google Scholar] [CrossRef]
- Hörder, H.; Skoog, I.; Frändin, K. Health-related quality of life in relation to walking habits and fitness: A population-based study of 75-year-olds. Qual. Life Res. 2013, 22, 1213–1223. [Google Scholar] [CrossRef]
- Song, H.J.; Lee, E.K.; Kwon, J.W. Gender Differences in the Impact of Obesity on Health-Related Quality of Life. Asia-Pacific J. Public Health 2016, 28, 146–156. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, L.; Li, J.; Sun, L.; Qin, W.; Ding, G.; Wang, Q.; Zhu, J.; Yu, Z.; Xie, S.; et al. Gender differences in the association between body mass index and health-related quality of life among adults:a cross-sectional study in Shandong, China. BMC Public Health 2019, 19, 1021. [Google Scholar] [CrossRef]
- Søltoft, F.; Hammer, M.; Kragh, N. The association of body mass index and health-related quality of life in the general population: Data from the 2003 Health Survey of England. Qual. Life Res. 2009, 18, 1293–1299. [Google Scholar] [CrossRef]
- Fradelos, E.; Papathanasiou, I.; Mitsi, D.; Tsaras, K.; Kleisiaris, C.; Kourkouta, L. Health Based Geographic Information Systems (GIS) and their Applications. Acta Inform. Medica 2014, 22, 402. [Google Scholar] [CrossRef]
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription, 10th ed.; Lippincott Williams Wilkins: Philadelphia, PA, USA, 2018. [Google Scholar]
- Marfell-Jones, M.; Olds, T.; Stew, A.; Carter, L. International Standards for Anthropometric Assessment; International Society for the Advancement of Kinanthropometry: Wellington, New Zealand, 2012. [Google Scholar]
- Lipschitz, D.A. Screening for nutritional status in the elderly. Prim Care 1994, 21, 55–67. [Google Scholar] [CrossRef]
- WHO-World Health Organization. Obesity: Preventing and Managing the Global Epidemic. Report of a WHO Consultation; World Health Organization: Geneva, Switzerland, 2000; Volume 894. [Google Scholar]
- World Health Organisation (WHO). WHO: Waist Circumference and Waist–Hip Ratio. Report of a WHO Expert Consultation. Geneva, 8–11 December 2008.; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Baecke, J.A.H.; Burema, J.; Frijters, J.E.R. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am. J. Clin. Nutr. 1982, 36, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Ono, R.; Hirata, S.; Yamada, M.; Nishiyama, T.; Kurosaka, M.; Tamura, Y. Reliability and validity of the Baecke physical activity questionnaire in adult women with hip disorders. BMC Musculoskelet. Disord. 2007, 8, 61. [Google Scholar] [CrossRef] [PubMed]
- Rikli, R.E.; Jones, C.J. Development and Validation of Criterion-Referenced Clinically Relevant Fitness Standards for Maintaining Physical Independence in Later Years. Gerontologist 2013, 53, 255–267. [Google Scholar] [CrossRef]
- Buchman, A.S.; Leurgans, S.E.; Boyle, P.A.; Schneider, J.A.; Arnold, S.E.; Bennett, D.A. Combinations of motor measures more strongly predict adverse health outcomes in old age: The rush memory and aging project, a community-based cohort study. BMC Med. 2011, 9, 42. [Google Scholar] [CrossRef]
- Ribeiro, J.L.P. O Importante é a Saúde: Estudo de Adaptação de Uma Técnica de Técnica de Avaliação do Estado de Saúde SF-36; Fundação Merck Sharp & Dohme: Lisboa, Portugal, 2005. [Google Scholar]
- Ware, J.E.; Sherbourne, C.D. The MOS 36-ltem Short-Form Health Survey (SF-36). Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef] [PubMed]
- VanderWeele, T.J. Mediation Analysis: A Practitioner’s Guide. Annu. Rev. Public Health 2016, 37, 17–32. [Google Scholar] [CrossRef] [PubMed]
- MacKinnon, D.P.; Fairchild, A.J.; Fritz, M.S. Mediation Analysis. Annu. Rev. Psychol. 2007, 58, 593–614. [Google Scholar] [CrossRef] [PubMed]
- Preacher, K.J.; Rucker, D.D.; Hayes, A.F. Addressing Moderated Mediation Hypotheses: Theory, Methods, and Prescriptions. Multivariate Behav. Res. 2007, 42, 185–227. [Google Scholar] [CrossRef]
- Hayes, A.F.; Rockwood, N.J. Regression-based statistical mediation and moderation analysis in clinical research: Observations, recommendations, and implementation. Behav. Res. Ther. 2017, 98, 39–57. [Google Scholar] [CrossRef]
- Gomez-Bruton, A.; Navarrete-Villanueva, D.; Pérez-Gómez, J.; Vila-Maldonado, S.; Gesteiro, E.; Gusi, N.; Villa-Vicente, J.G.; Espino, L.; Gonzalez-Gross, M.; Casajus, J.A.; et al. The Effects of Age, Organized Physical Activity and Sedentarism on Fitness in Older Adults: An 8-Year Longitudinal Study. Int. J. Environ. Res. Public Health 2020, 17, 4312. [Google Scholar] [CrossRef] [PubMed]
- Yatsugi, H.; Chen, T.; Chen, S.; Liu, X.; Kishimoto, H. The Associations between Objectively Measured Physical Activity and Physical Function in Community-Dwelling Older Japanese Men and Women. Int. J. Environ. Res. Public Health 2021, 19, 369. [Google Scholar] [CrossRef] [PubMed]
- Mayhew, A.J.; Griffith, L.E.; Gilsing, A.; Beauchamp, M.K.; Kuspinar, A.; Raina, P. The Association between Self-Reported and Performance-Based Physical Function with Activities of Daily Living Disability in the Canadian Longitudinal Study on Aging. J. Gerontol. Ser. A 2020, 75, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Resnick, B.; Boltz, M.; Galik, E.; Holmes, S.; Fix, S.; Zhu, S. Gender Differences in Function, Physical Activity, Falls, Medication Use, and Life Satisfaction Among Residents in Assisted Living Settings. Res. Gerontol. Nurs. 2020, 13, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.; Varghese, M.; Singer, K. Gender and Sex Differences in Adipose Tissue. Curr. Diab. Rep. 2018, 18, 69. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.L.; Daviglus, M.L.; Liu, K.; Pirzada, A.; Garside, D.B.; Schiffer, L.; Dyer, A.R.; Greenland, P. BMI and Health-Related Quality of Life in Adults 65 Years and Older. Obes. Res. 2004, 12, 69–76. [Google Scholar] [CrossRef]
- Stephenson, J.; Smith, C.M.; Kearns, B.; Haywood, A.; Bissell, P. The association between obesity and quality of life: A retrospective analysis of a large-scale population-based cohort study. BMC Public Health 2021, 21, 1990. [Google Scholar] [CrossRef]
- Cunningham, C.; O’ Sullivan, R.; Caserotti, P.; Tully, M.A. Consequences of physical inactivity in older adults: A systematic review of reviews and meta-analyses. Scand. J. Med. Sci. Sports 2020, 30, 816–827. [Google Scholar] [CrossRef]
- Li, P.-S.; Hsieh, C.-J.; Miao, N.-F. A Study of Physical Activity, Frailty, and Health-Related Quality of Life among Community-Dwelling Older Adults in Taiwan. J. Nurs. Res. 2020, 28, e124. [Google Scholar] [CrossRef]
- Zhou, Z.; Macpherson, J.; Gray, S.R.; Gill, J.M.R.; Welsh, P.; Celis-Morales, C.; Sattar, N.; Pell, J.P.; Ho, F.K. Are people with metabolically healthy obesity really healthy? A prospective cohort study of 381,363 UK Biobank participants. Diabetologia 2021, 64, 1963–1972. [Google Scholar] [CrossRef]
- Smith, G.I.; Mittendorfer, B.; Klein, S. Metabolically healthy obesity: Facts and fantasies. J. Clin. Investig. 2019, 129, 3978–3989. [Google Scholar] [CrossRef] [PubMed]
- Stavropoulos-Kalinoglou, A.; Metsios, G.S.; Koutedakis, Y.; Kitas, G.D. Obesity in rheumatoid arthritis. Rheumatology 2011, 50, 450–462. [Google Scholar] [CrossRef]
- Garber, C.E.; Greaney, M.L.; Riebe, D.; Nigg, C.R.; Burbank, P.A.; Clark, P.G. Physical and mental health-related correlates of physical function in community dwelling older adults: A cross sectional study. BMC Geriatr. 2010, 10, 6. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.M. Sarcopenia and sarcopenic obesity. Korean J. Intern. Med. 2016, 31, 1054–1060. [Google Scholar] [CrossRef] [PubMed]
- Batsis, J.A.; Villareal, D.T. Sarcopenic obesity in older adults: Aetiology, epidemiology and treatment strategies. Nat. Rev. Endocrinol. 2018, 14, 513–537. [Google Scholar] [CrossRef]
- Baumgartner, R.N.; Wayne, S.J.; Waters, D.L.; Janssen, I.; Gallagher, D.; Morley, J.E. Sarcopenic Obesity Predicts Instrumental Activities of Daily Living Disability in the Elderly. Obes. Res. 2004, 12, 1995–2004. [Google Scholar] [CrossRef]
- Pietiläinen, K.H.; Kaprio, J.; Borg, P.; Plasqui, G.; Yki-Järvinen, H.; Kujala, U.M.; Rose, R.J.; Westerterp, K.R.; Rissanen, A. Physical Inactivity and Obesity: A Vicious Circle. Obesity 2008, 16, 409–414. [Google Scholar] [CrossRef]
- Dugan, S.A.; Gabriel, K.P.; Lange-Maia, B.S.; Karvonen-Gutierrez, C. Physical Activity and Physical Function. Obstet. Gynecol. Clin. North Am. 2018, 45, 723–736. [Google Scholar] [CrossRef]
- Kadariya, S.; Gautam, R.; Aro, A.R. Physical Activity, Mental Health, and Wellbeing among Older Adults in South and Southeast Asia: A Scoping Review. Biomed Res. Int. 2019, 2019, 6752182. [Google Scholar] [CrossRef]
- Swift, D.L.; McGee, J.E.; Earnest, C.P.; Carlisle, E.; Nygard, M.; Johannsen, N.M. The Effects of Exercise and Physical Activity on Weight Loss and Maintenance. Prog. Cardiovasc. Dis. 2018, 61, 206–213. [Google Scholar] [CrossRef]
- Ferreira, M.L.; Sherrington, C.; Smith, K.; Carswell, P.; Bell, R.; Bell, M.; Nascimento, D.P.; Máximo Pereira, L.S.; Vardon, P. Physical activity improves strength, balance and endurance in adults aged 40–65 years: A systematic review. J. Physiother. 2012, 58, 145–156. [Google Scholar] [CrossRef]
- Amaral Gomes, E.S.; Ramsey, K.A.; Rojer, A.G.M.; Reijnierse, E.M.; Maier, A.B. The Association of Objectively Measured Physical Activity and Sedentary Behavior with (Instrumental) Activities of Daily Living in Community-Dwelling Older Adults: A Systematic Review. Clin. Interv. Aging 2021, 16, 1877–1915. [Google Scholar] [CrossRef] [PubMed]
- Ling, C.H.Y.; Meskers, C.G.M.; Maier, A.B. Can anthropometric measures be used as proxies for body composition and physical function in geriatric outpatients? Arch. Gerontol. Geriatr. 2021, 94, 104379. [Google Scholar]
| Variable | Full Sample (n = 802) | Underweight (n = 17) | Normal Weight (n = 213) | Overweight (n = 572) | p-Value |
|---|---|---|---|---|---|
| Age (years) | 69.81 ± 5.60 | 71.64 ± 6.00 | 69.80 ± 5.90 | 69.76 ± 5.46 | 0.396 |
| 60–69 | 364 (45.4) | 8 (47.1) | 102 (47.9) | 254 (44.4) | |
| 70–79 | 408 (50.9) | 8 (47.1) | 101 (47.4) | 299 (52.3) | |
| ≥80 | 30 (3.7) | 1 (5.9) | 10 (4.7) | 19 (3.3) | |
| Sex n (%) | 0.011 | ||||
| Male | 401 (100.0) | 11 (64.7) | 123 (57.7) | 267 (46.7), | |
| Female | 401 (100.0) | 6 (35.3) | 90 (42.3) | 305 (53.3) | |
| Falls (n) | 1.74 ± 0.80 | 1.65 ± 0.86 | 1.71 ± 0.81 | 1.75 ± 0.80 | 0.735 |
| Medication (n) | 4.66 ± 0.98 | 5.35 ± 0.86 | 4.89 ± 0.94 | 4.55 ± 0.98 | 0.018 * |
| Comorbidities (%) | |||||
| Hypertension | 408 (50.9) | 13 (76.4) b,c | 136 (23.7) a,c | 259 (45.2) a,b | 0.038 |
| Diabetes | 188 (23.4) | 6 (35.2) b,c | 72 (33.8) a,c | 110 (19.2) a,b | 0.028 |
| Visual impairment | 489 (61.0) | 17 (100.0) b,c | 181 (85.0) a,c | 291 (50.9) a,b | 0.018 |
| Hearing problems | 198 (24.7) | 6 (35.2) b,c | 91 (42.7) a,c | 101 (17.6) a,b | 0.024 |
| Musculoarticular problems | 46 (5.7) | 9 (53.0) c | 12 (5.6) c | 25 (4.4) a,b | 0.044 |
| Anthropometry | |||||
| Height (kg) | 159.05 ± 8.69 | 159.14 ± 7.34 | 159.60 ± 8.16 | 158.84 ± 8.91 | 0.553 * |
| Weight (cm) | 74.77 ± 13.06 | 50.85 ± 5.68 b,c | 63.88 ± 7.75 a,c | 79.54 ± 11.50 a,b | <0.001 * |
| BMI (kg/m2) | 29.51 ± 4.34 | 20.01 ± 1.02 b,c | 25.00 ± 1.48 a,c | 31.47 ± 3.39 a,b | <0.001 * |
| WC (cm) | 97.19 ± 11.27 | 76.30 ± 5.38 b,c | 87.67 ± 7.26 a,c | 101.35 ± 9.68 a,b | <0.001 * |
| Men ≥ 102 cm (f) | 197 (49.1) | — | 6 (3.0) | 191 (97.0) b | <0.001 * |
| Women ≥ 88 cm (f) | 358 (89.3) | — | 22 (7.5) | 272 (92.5) b | <0.001 * |
| HC (cm) | 101.47 ± 9.04 | 85.52 ± 3.92 b,c | 93.97 ± 4.61 a,c | 104.47 ± 8.20 a,b | <0.001 * |
| WHR (cm) | 0.95 ± 0.08 | 0.89 ± 0.69 b,c | 0.93 ± 07 a,c | 0.97 ± 0.95 a | <0.001 * |
| Men ≥ 90 cm (f) | 392 (97.8) | 10 (2.6) | 116 (29.6) a,c | 266 (67.9) a,b | 0.036 * |
| Women ≥ 85 cm (f) | — | — | — | — | — |
| PA (n) | 7.30 ± 1.23 | 7.33 ± 1.12 | 7.42 ± 1.28 | 7.26 ± 1.21 | 0.122 |
| PF (n) | 581.58 ± 144.21 | 589.80 ± 181.41 | 602.44 ± 149.72 c | 573.55 ± 140.20 b | 0.031 * |
| HRQoL (n) | 68.57 ± 17.96 | 71.96 ± 10.09 | 72.91 ± 18.17 | 67.60 ± 17.96 | 0.036 * |
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| 1. BMI | 1.00 | ||||||
| 2. WC | 0.760 ** | 1.00 | |||||
| 3. WHR | 0.930 ** | 0.850 ** | 1 | ||||
| 4. Sex | 0.170 ** | −0.274 ** | −0.105 * | 1.00 | |||
| 5. Age | −0.033 ns | −0.066 ns | −0.085 * | −0.010 ns | 1 | ||
| 6. PA | −0.380 ** | −0.288 ** | −0.098 ** | 0.250 ** | −0.148 ** | 1.00 | |
| 7. PF | −0.320 ** | −0.254 ** | −0.096 ** | −0.284 ** | −0.406 ** | 0.511 ** | 1 |
| 8. HRQoL | −0.462 ** | −0.321 ** | −0.082 ** | −0.301 ** | −0.191 ** | 0.536 ** | 0.591 ** |
| Variable | Model 1 | Model 2 | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | Value | OR | 95% CI | p-Value | |
| BMI | −0.091 | −0.630–0.114 | <0.001 | −0.069 | −0.534–0.025 | 0.032 |
| PA | 0.055 | 0.864–1.762 | 0.038 | 0.082 | 0.231–2.129 | 0.015 |
| PF | 0.453 | 0.047–0.063 | <0.001 | 0.400 | 0.039–0.057 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nascimento, M.d.M.; Gouveia, É.R.; Gouveia, B.R.; Marques, A.; Campos, P.; García-Mayor, J.; Przednowek, K.; Ihle, A. The Mediating Role of Physical Activity and Physical Function in the Association between Body Mass Index and Health-Related Quality of Life: A Population-Based Study with Older Adults. Int. J. Environ. Res. Public Health 2022, 19, 13718. https://doi.org/10.3390/ijerph192113718
Nascimento MdM, Gouveia ÉR, Gouveia BR, Marques A, Campos P, García-Mayor J, Przednowek K, Ihle A. The Mediating Role of Physical Activity and Physical Function in the Association between Body Mass Index and Health-Related Quality of Life: A Population-Based Study with Older Adults. International Journal of Environmental Research and Public Health. 2022; 19(21):13718. https://doi.org/10.3390/ijerph192113718
Chicago/Turabian StyleNascimento, Marcelo de Maio, Élvio Rúbio Gouveia, Bruna R. Gouveia, Adilson Marques, Pedro Campos, Jesús García-Mayor, Krzysztof Przednowek, and Andreas Ihle. 2022. "The Mediating Role of Physical Activity and Physical Function in the Association between Body Mass Index and Health-Related Quality of Life: A Population-Based Study with Older Adults" International Journal of Environmental Research and Public Health 19, no. 21: 13718. https://doi.org/10.3390/ijerph192113718
APA StyleNascimento, M. d. M., Gouveia, É. R., Gouveia, B. R., Marques, A., Campos, P., García-Mayor, J., Przednowek, K., & Ihle, A. (2022). The Mediating Role of Physical Activity and Physical Function in the Association between Body Mass Index and Health-Related Quality of Life: A Population-Based Study with Older Adults. International Journal of Environmental Research and Public Health, 19(21), 13718. https://doi.org/10.3390/ijerph192113718











