Soot Combustion over Cu–Co Spinel Catalysts: The Intrinsic Effects of Precursors on Catalytic Activity
Abstract
1. Introduction
2. Experimental Study
2.1. Catalyst Preparation
2.2. Catalyst Characterizations
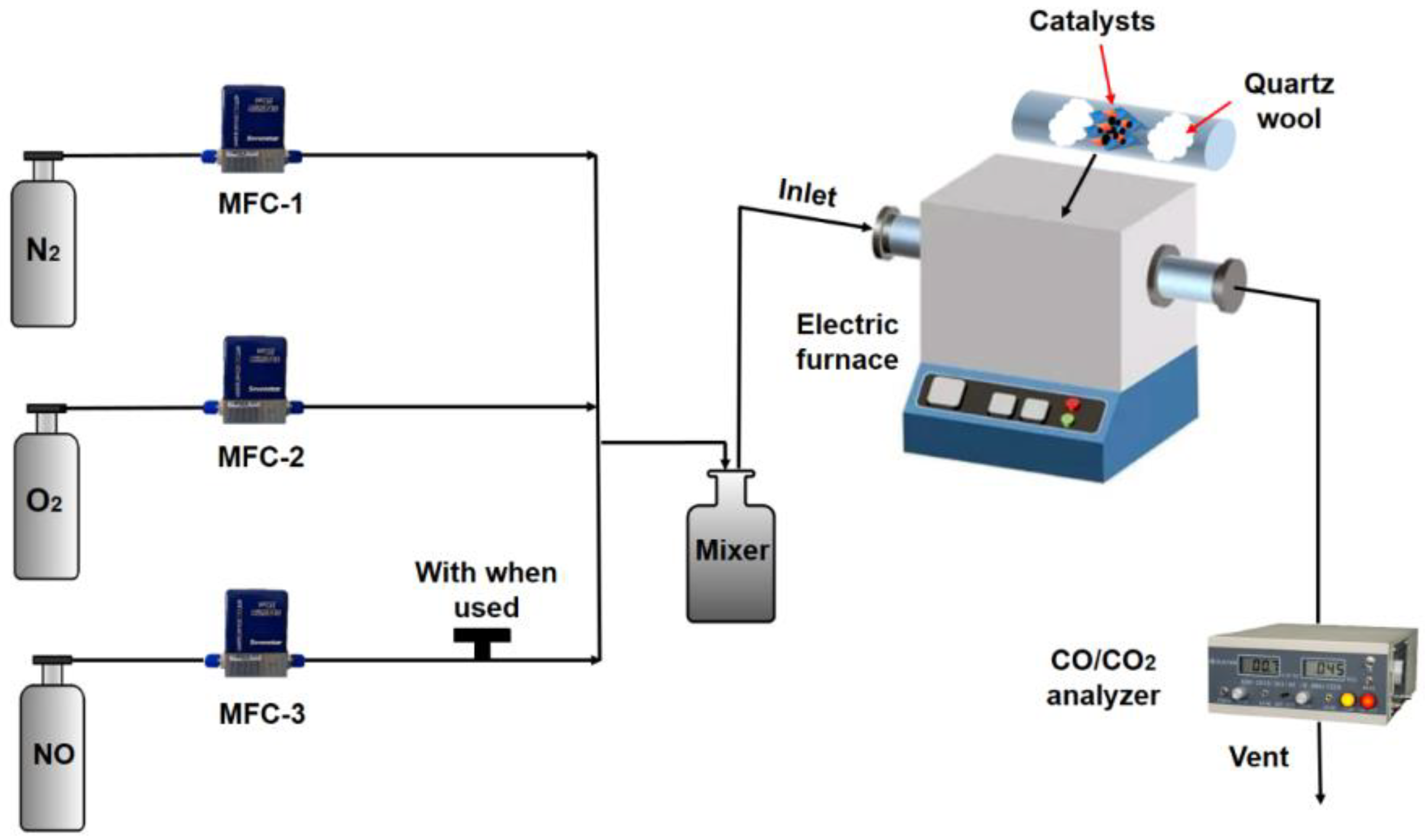
2.3. Experimental System
3. Results and Discussion
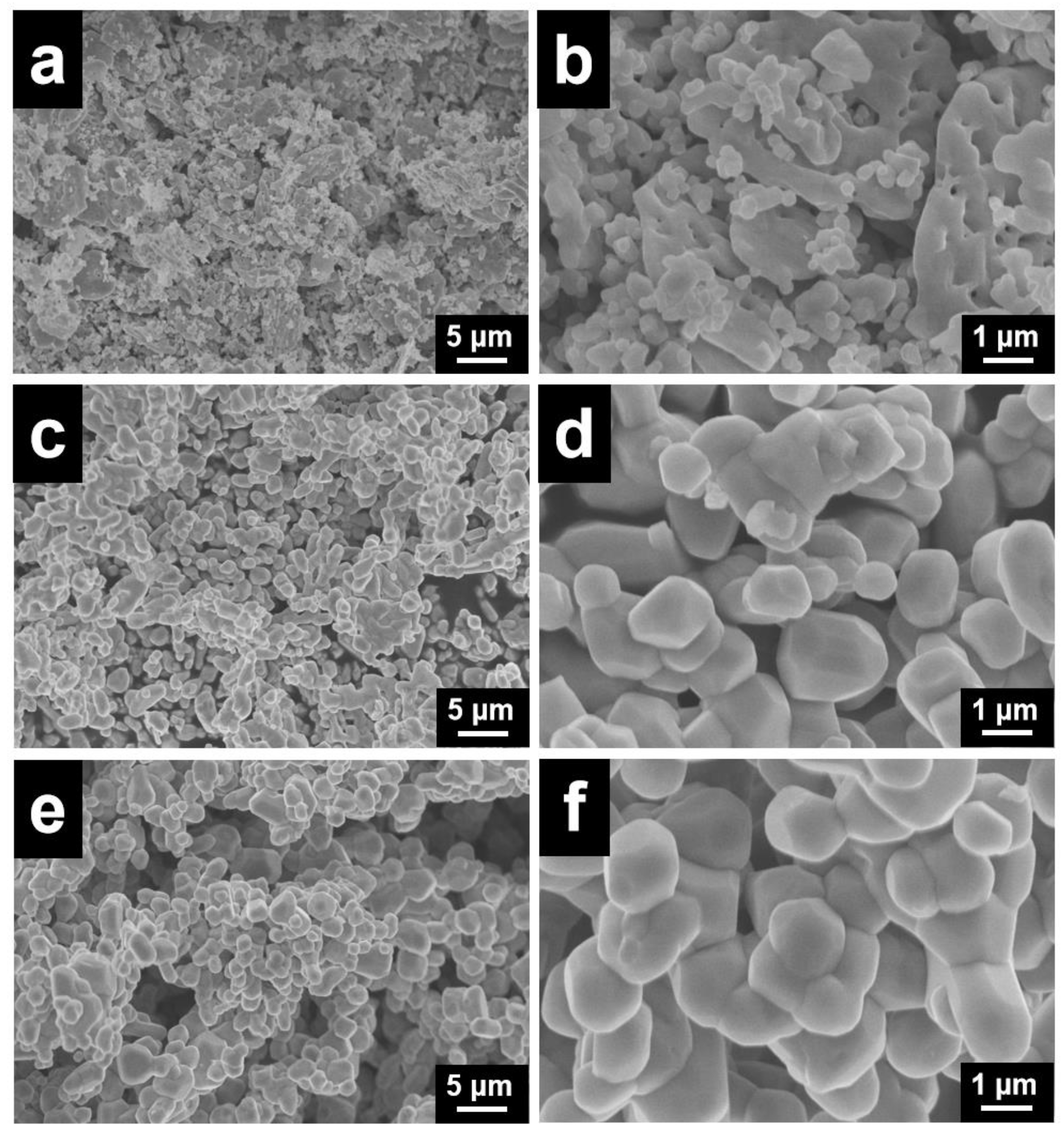
3.1. Textural Properties of the Catalysts
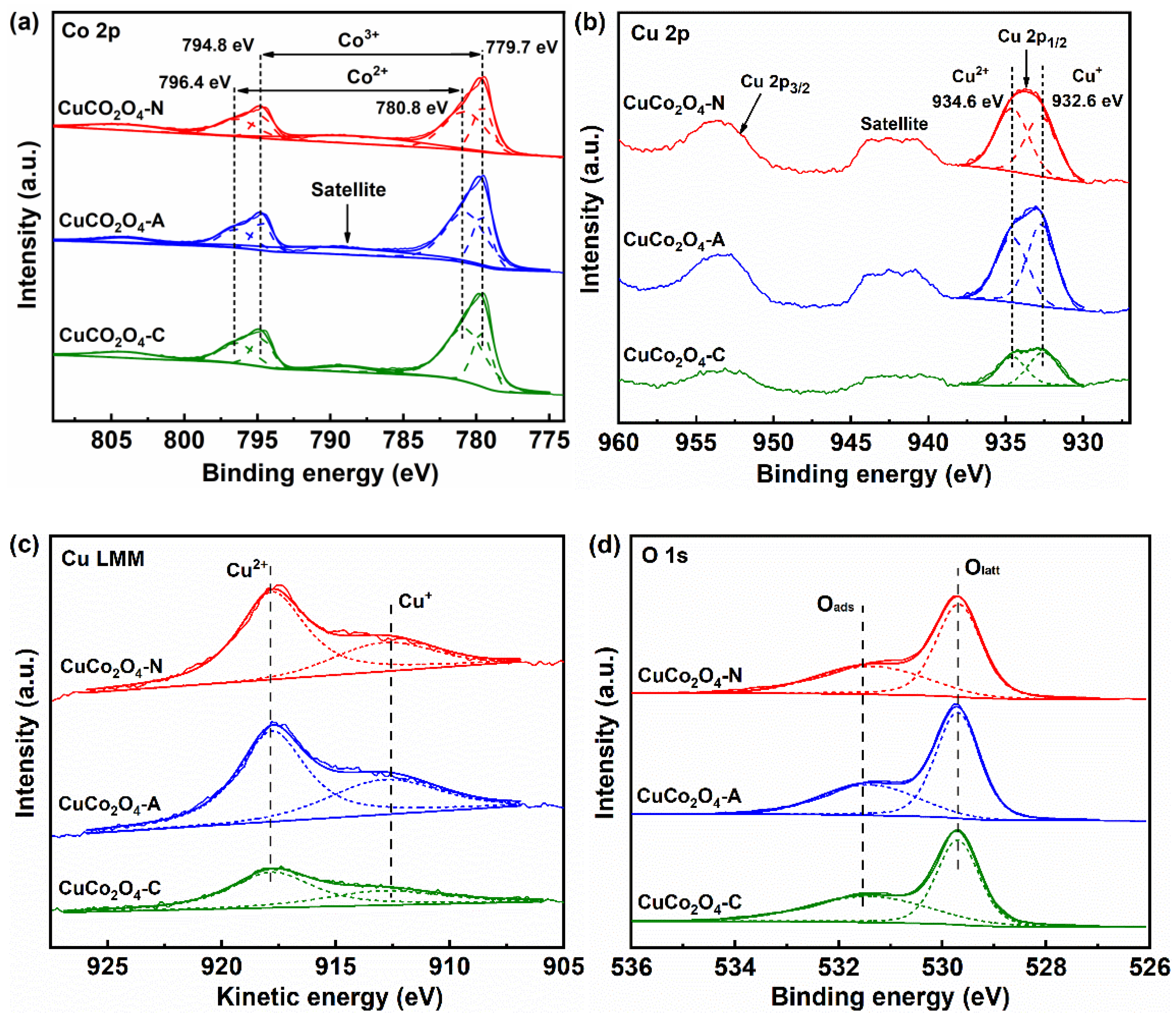
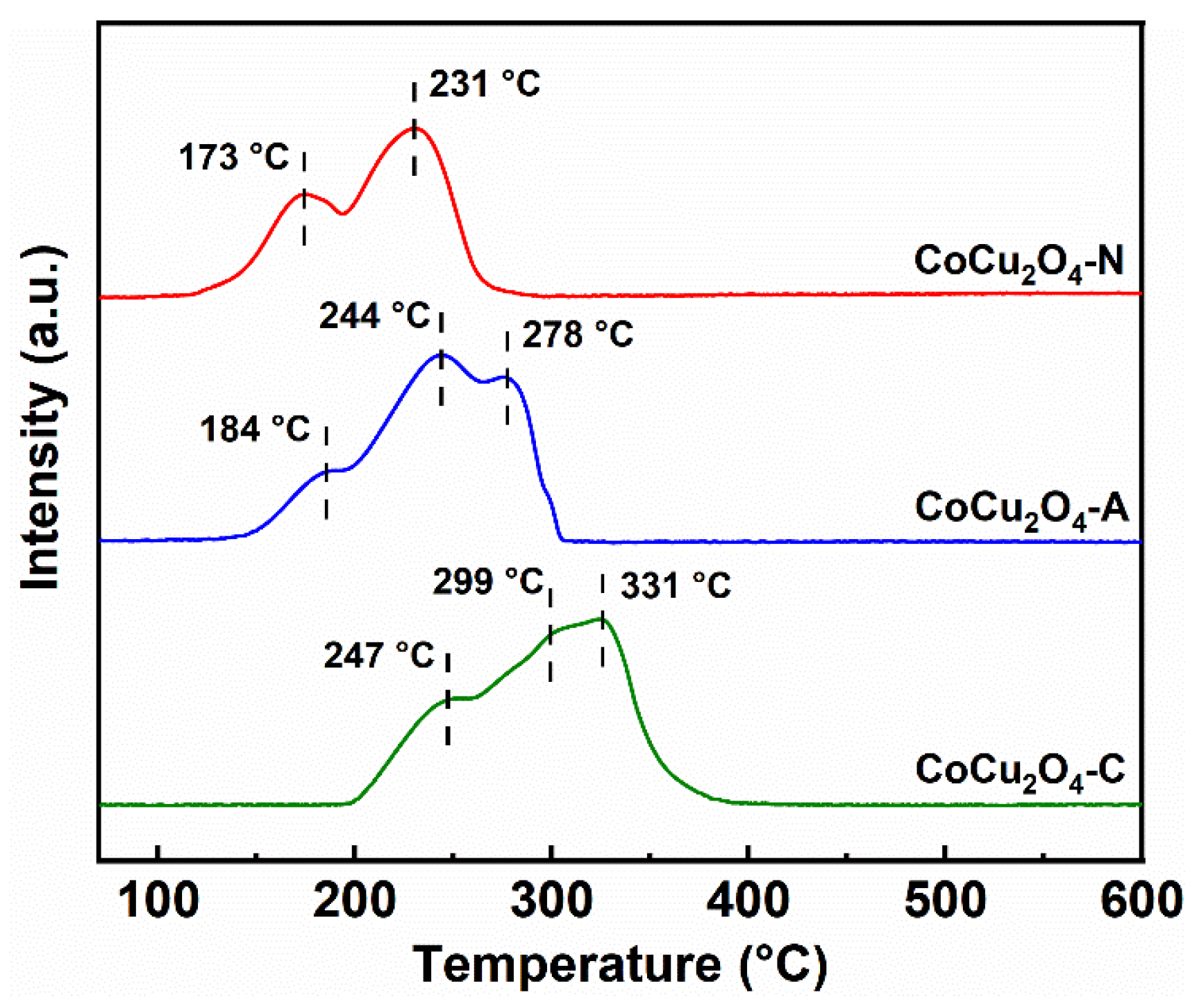
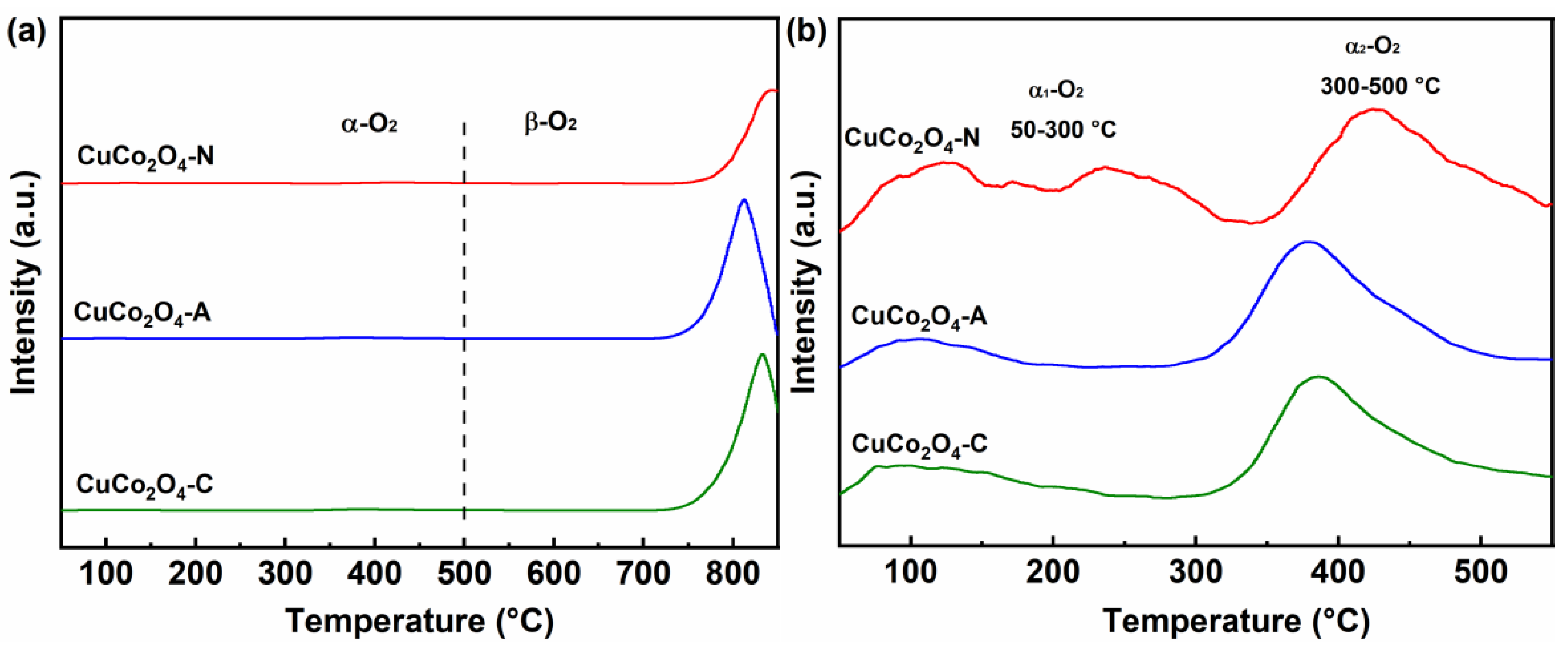
3.2. Redox Properties of the Catalysts
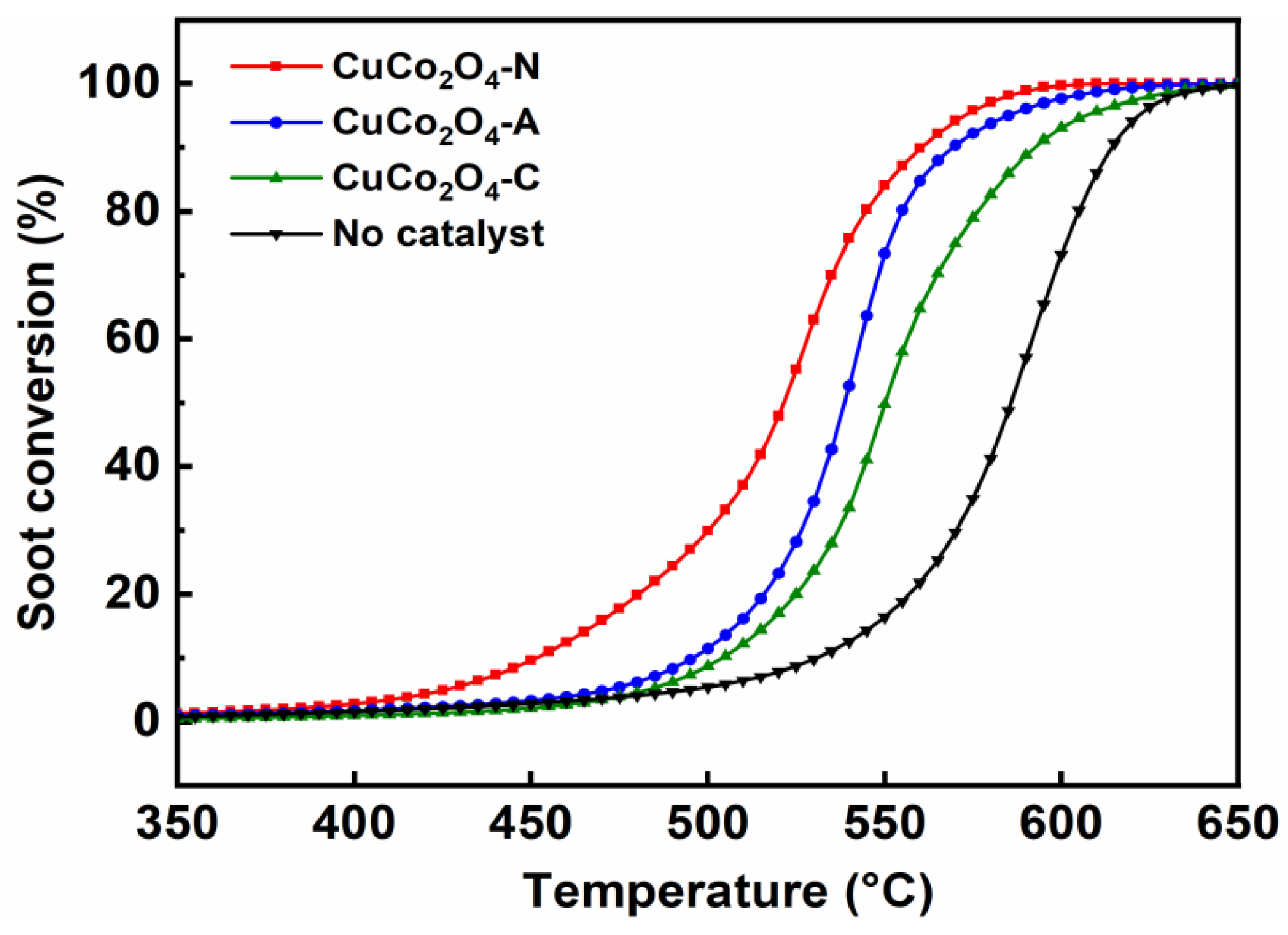
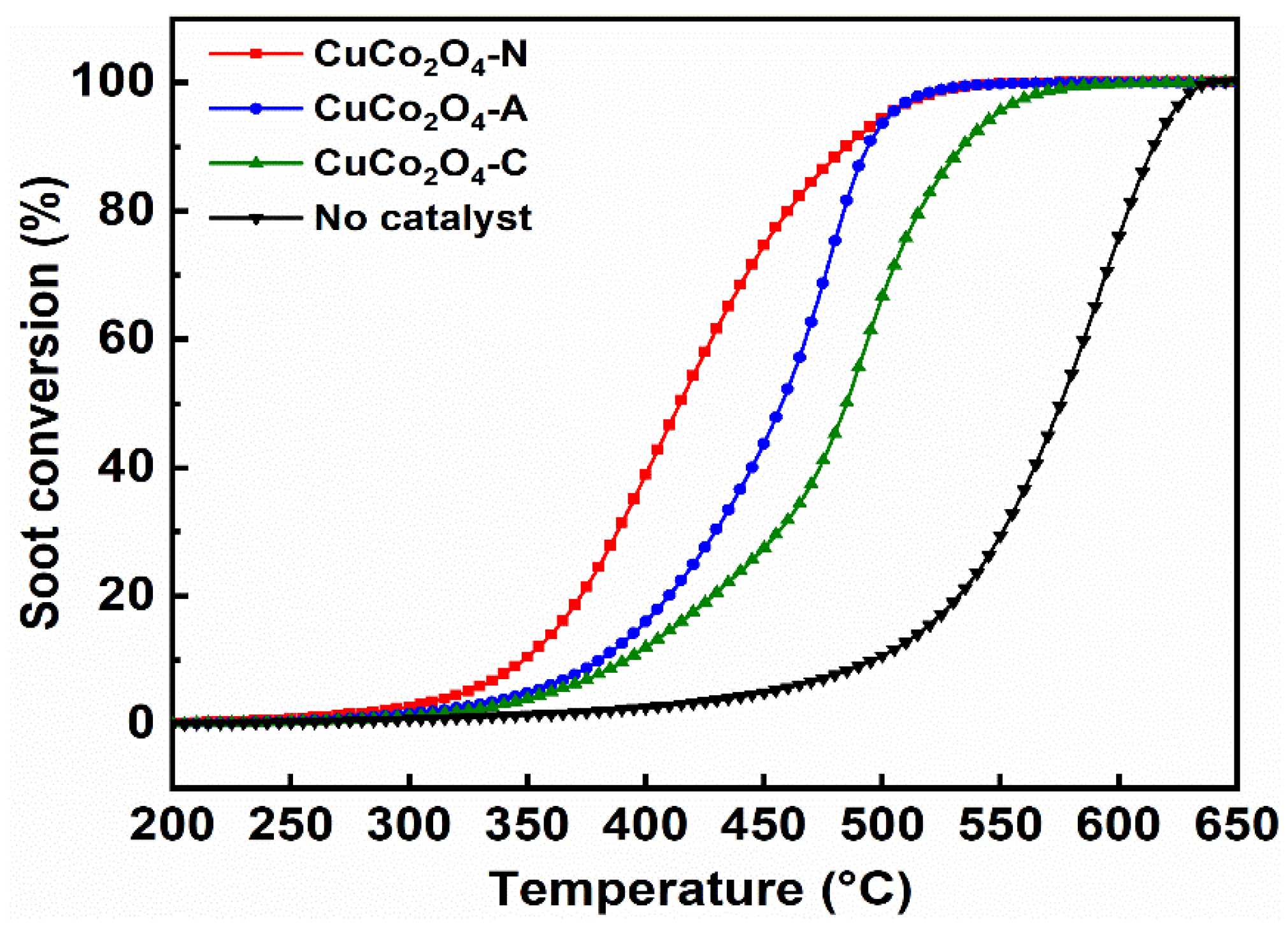
3.3. Activity of CuCo2O4-x for Soot Conversion
3.3.1. Soot Conversion in O2/N2
3.3.2. Soot Conversion in NO/O2/N2
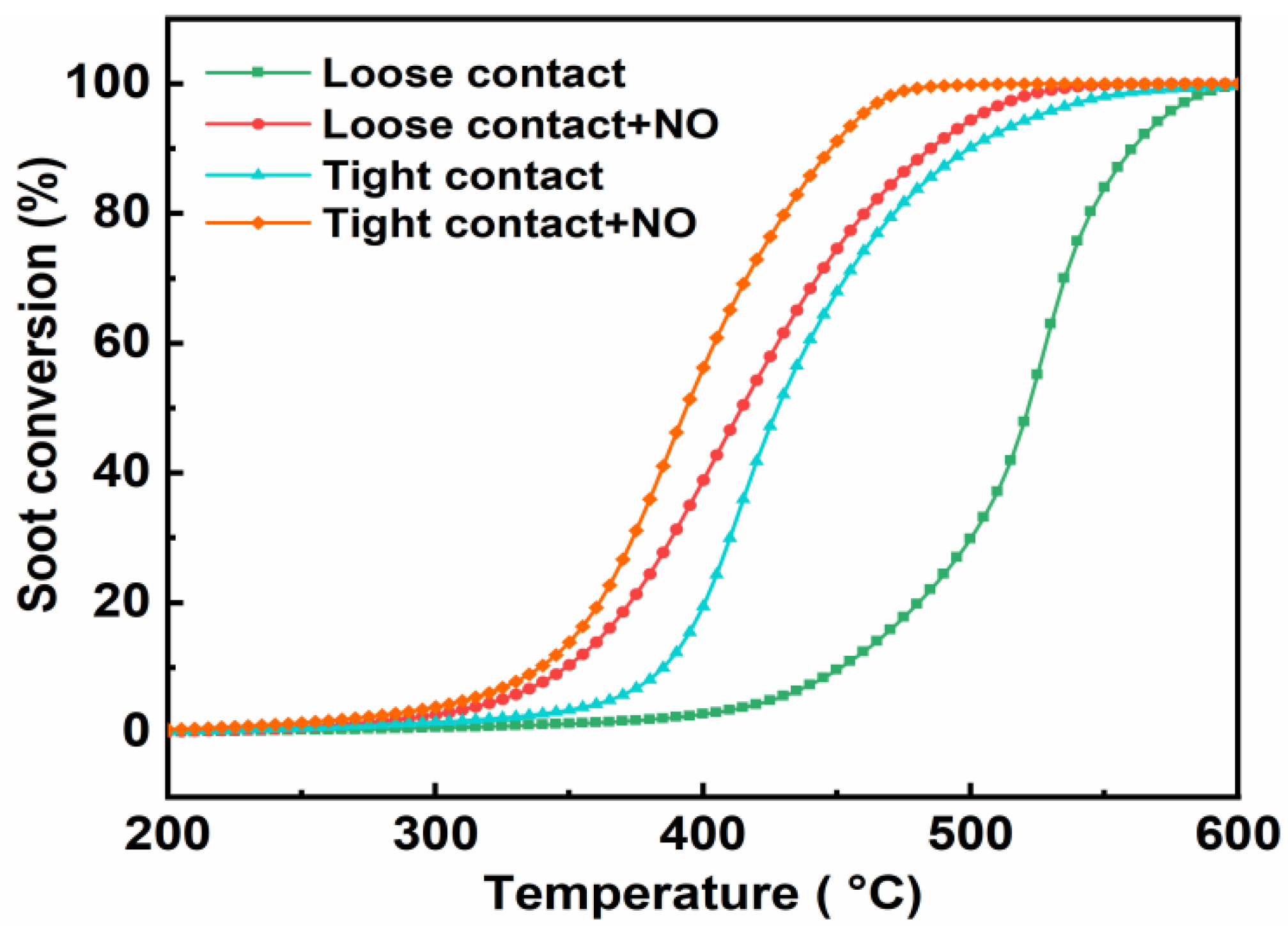
3.3.3. Effect of the Contact Mode
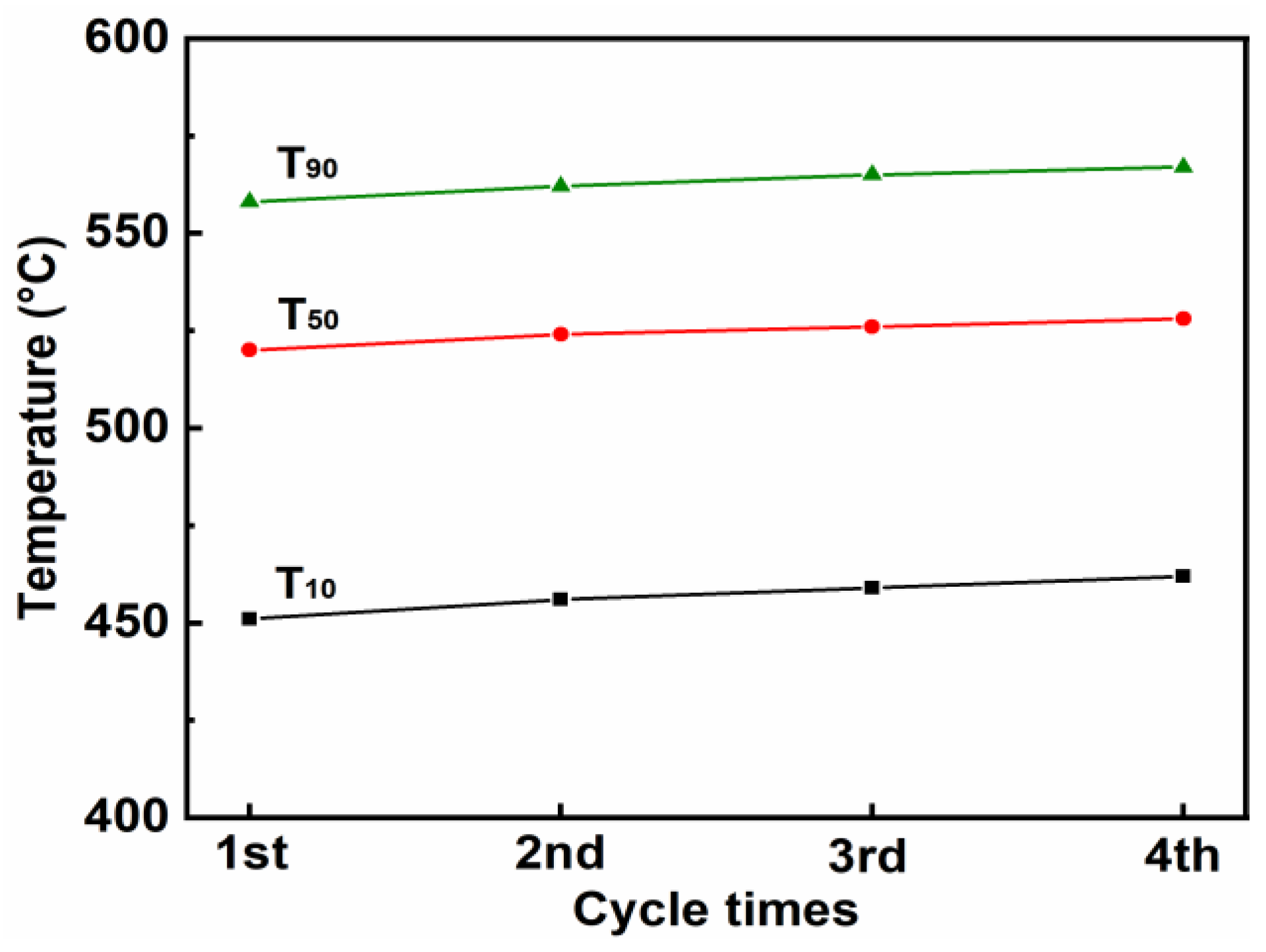
3.3.4. Stability Test
4. Reaction Mechanisms for Catalytic Soot Combustion
5. Conclusions
- (1)
- The tetragonal spinel crystals of CuCo2O4 were formed for all catalysts, while the single-metal oxide CuO species was formed only on the CuCo2O4-N and CuCo2O4-A catalysts. The CuCo2O4-N catalysts exhibited a higher specific surface area and well-developed pore structure.
- (2)
- The type of metal precursors could also profoundly affect the redox properties on CuCo2O4 spinel catalysts. The higher relative content of Co3+ (38.4%), Cu2+ (51.7%) and Oads (46.8%) species were obtained over the CuCo2O4-N catalysts. Meanwhile, CuCo2O4-N catalysts (173 °C) showed a lower reduction temperature over CuCo2O4-A (184 °C) and CuCo2O4-C (247 °C). The highest amount of surface adsorbed oxygen species (41.2 μmol·g−1) was achieved over the CuCo2O4-N catalysts.
- (3)
- The highest soot conversion activity and CO2 selectivity were obtained over the CuCo2O4-N catalysts regardless of the soot combustion conditions. The effects of the contact mode (loose and tight) and NO addition on soot conversion were also investigated. A good correlation between soot conversion and the textural and redox properties of the catalysts were observed. The reaction mechanisms and pathways of the CuCo2O4 for catalytic soot combustion were also established.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choi, M.; Park, S. Optimization of multiple-stage fuel injection and optical analysis of the combustion process in a heavy-duty diesel engine. Fuel Process. Technol. 2022, 228, 107137. [Google Scholar] [CrossRef]
- Jeong, S.; Lee, J.H.; Ha, J.H.; Kim, J.; Kim, I.; Bae, S. An exploratory study of the relationships between diesel engine exhaust particle inhalation, pulmonary inflammation and anxious behavior. Int. J. Environ. Res. Public Health 2021, 18, 1166. [Google Scholar] [CrossRef]
- Jiaqiang, E.; Zheng, P.; Han, D.; Zhao, X.; Deng, Y. Effects analysis on soot combustion performance enhancement in a rotary diesel particulate filter unit during continuous microwave heating. Fuel 2020, 276, 118043. [Google Scholar] [CrossRef]
- Schobing, J.; Tschamber, V.; Brillard, A.; Leyssens, G. Biodiesel soot combustion: Analysis of the soot-catalyst contact in different experimental conditions. Fuel 2022, 312, 122854. [Google Scholar] [CrossRef]
- Wang, X.; Jin, B.; Feng, R.; Liu, W.; Weng, D.; Wu, X.; Liu, S. A robust core-shell silver soot oxidation catalyst driven by Co3O4: Effect of tandem oxygen delivery and Co3O4-CeO2 synergy. Appl. Catal. B Environ. 2019, 250, 132–142. [Google Scholar] [CrossRef]
- Shen, J.; Rao, C.; Fu, Z.; Feng, X.; Liu, J.; Fan, X.; Peng, H.; Xu, X.; Tan, C.; Wang, X. The influence on the structural and redox property of CuO by using different precursors and precipitants for catalytic soot combustion. Appl. Surf. Sci. 2018, 453, 204–213. [Google Scholar] [CrossRef]
- Li, K.; Li, T.; Dai, Y.; Quan, Y.; Zhao, J.; Ren, J. Highly active urchin-like MCo2O4 (M=Co, Cu, Ni or Zn) spinel for toluene catalytic combustion. Fuel 2022, 318, 123648. [Google Scholar] [CrossRef]
- Lin, C.S.; Liu, H.Y.; Guo, M.; Zhao, Y.H.; Su, X.; Zhang, P.Y.; Zhang, Y.F. Plasmon-induced broad spectrum photocatalytic overall water splitting: Through non-noble bimetal nanoparticles hybrid with reduced graphene oxide. Colloids Surf. A Physicochem. Eng. Asp. 2022, 646, 128962. [Google Scholar] [CrossRef]
- Fino, D.; Bensaid, S.; Piumetti, M.; Russo, N. A review on the catalytic combustion of soot in Diesel particulate filters for automotive applications: From powder catalysts to structured reactors. Appl. Catal. A Gen. 2016, 509, 75–96. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, X.; Zheng, C.; Hu, D.; Zhang, J.; Gao, X. Study on catalytic soot oxidation over spinel type ACo2O4 (A=Co, Ni, Cu, Zn) catalysts. Aerosol Air Qual. Res. 2017, 17, 2317–2327. [Google Scholar] [CrossRef]
- Jampaiah, D.; Velisoju, V.K.; Venkataswamy, P.; Coyle, V.E.; Nafady, A.; Reddy, B.M.; Bhargava, S.K. Nanowire morphology of mono- and bidoped α-MnO2 catalysts for remarkable enhancement in soot oxidation. ACS Appl. Mater. Interfaces 2017, 9, 32652–32666. [Google Scholar] [CrossRef]
- Zhang, F.; Zhu, X.; Wu, H.; Wu, X.; Zhou, Z.; Chen, G.; Yang, G. Activity and stability of Cu-based spinel-type complex oxides for diesel soot combustion. ChemistrySelect 2021, 6, 14019–14026. [Google Scholar] [CrossRef]
- Okal, J.; Zawadzki, M.; Kraszkiewicz, P.; Adamska, K. Ru/CeO2 catalysts for combustion of mixture of light hydrocarbons: Effect of preparation method and metal salt precursors. Appl. Catal. A Gen. 2018, 549, 161–169. [Google Scholar] [CrossRef]
- Yang, T.; Zhao, M.; Wang, X.; Ma, R.; Liu, Y.; He, Y.; Li, D. Influence of active metal precursors on the structure and catalytic behavior of Pd/Al2O3 catalysts for selective acetylene hydrogenation. Catal. Lett. 2021, 152, 227–238. [Google Scholar] [CrossRef]
- Wang, Y.; Tao, Z.; Wu, B.; Xu, J.; Huo, C.; Li, K.; Chen, H.; Yang, Y.; Li, Y. Effect of metal precursors on the performance of Pt/ZSM-22 catalysts for n-hexadecane hydroisomerization. J. Catal. 2015, 322, 1–13. [Google Scholar] [CrossRef]
- Yu, Z.; Hu, X.; Jia, P.; Zhang, Z.; Dong, D.; Hu, G.; Hu, S.; Wang, Y.; Xiang, J. Steam reforming of acetic acid over nickel-based catalysts: The intrinsic effects of nickel precursors on behaviors of nickel catalysts. Appl. Catal. B Environ. 2018, 237, 538–553. [Google Scholar] [CrossRef]
- Liu, H.; Zhuang, M.; Zhang, Z.; Dai, X.; Qian, L.; Yan, Z. Catalytic removal of soot particles over MnCo2O4 catalysts prepared by the auto-combustion method. Chem. Pap. 2018, 72, 1973–1979. [Google Scholar] [CrossRef]
- Jin, C.; Cui, Y.; Zhang, G.; Luo, W.; Liu, Y.; Sun, Y.; Tian, Z.; Zheng, W. Synthesis of copper-cobalt hybrid oxide microflowers as electrode material for supercapacitors. Chem. Eng. J. 2018, 343, 331–339. [Google Scholar] [CrossRef]
- Lu, J.; Zhong, J.; Ren, Q.; Li, J.; Song, L.; Mo, S.; Zhang, M.; Chen, P.; Fu, M.; Ye, D. Construction of Cu-Ce interface for boosting toluene oxidation: Study of Cu-Ce interaction and intermediates identified by in situ DRIFTS. Chin. Chem. Lett. 2021, 32, 3435–3439. [Google Scholar] [CrossRef]
- Wen, X.; Xu, L.; Chen, M.; Shi, Y.; Lv, C.; Cui, Y.; Wu, X.; Cheng, G.; Wu, C.-E.; Miao, Z.; et al. Exploring the influence of nickel precursors on constructing efficient Ni-based CO2 methanation catalysts assisted with in-situ technologies. Appl. Catal. B Environ. 2021, 297, 120486. [Google Scholar] [CrossRef]
- Kang, T.; Kim, J. Optimal cobalt-based catalyst containing high-ratio of oxygen vacancy synthesized from metal-organic-framework (MOF) for oxygen evolution reaction (OER) enhancement. Appl. Surf. Sci. 2021, 560, 150035. [Google Scholar] [CrossRef]
- Li, Y.; Han, W.; Wang, R.; Weng, L.-T.; Serrano-Lotina, A.; Bañares, M.A.; Wang, Q.; Yeung, K.L. Performance of an aliovalent-substituted CoCeOx catalyst from bimetallic MOF for VOC oxidation in air. Appl. Catal. B Environ. 2020, 275, 119121. [Google Scholar] [CrossRef]
- Xiao, S.Y.; Liu, Y.; Wu, X.F.; Gan, L.T.; Lin, H.Y.; Zheng, L.R.; Dai, S.; Liu, P.F.; Yang, H.G. A low-valent cobalt oxide co-catalyst to boost photocatalytic water oxidation via enhanced hole-capturing ability. J. Mater. Chem. A 2021, 9, 14786–14792. [Google Scholar] [CrossRef]
- Huang, C.; Yu, Y.; Tang, X.; Liu, Z.; Zhang, J.; Ye, C.; Ye, Y.; Zhang, R. Hydrogen generation by ammonia decomposition over Co/CeO2 catalyst: Influence of support morphologies. Appl. Surf. Sci. 2020, 532, 147335. [Google Scholar] [CrossRef]
- Andana, T.; Piumetti, M.; Bensaid, S.; Veyre, L.; Thieuleux, C.; Russo, N.; Fino, D.; Quadrelli, E.A.; Pirone, R. CuO nanoparticles supported by ceria for NOx-assisted soot oxidation: Insight into catalytic activity and sintering. Appl. Catal. B Environ. 2017, 216, 41–58. [Google Scholar] [CrossRef]
- Du, H.; Ma, X.; Jiang, M.; Yan, P.; Conrad Zhang, Z. Highly efficient Cu/SiO2 catalyst derived from ethanolamine modification for furfural hydrogenation. Appl. Catal. A Gen. 2020, 598, 117598. [Google Scholar] [CrossRef]
- Liang, H.; Jin, B.; Li, M.; Yuan, X.; Wan, J.; Liu, W.; Wu, X.; Liu, S. Highly reactive and thermally stable Ag/YSZ catalysts with macroporous fiber-like morphology for soot combustion. Appl. Catal. B Environ. 2021, 294, 120271. [Google Scholar] [CrossRef]
- Xu, Y.; Qu, Z.P.; Ren, Y.W.; Dong, C. Enhancement of toluene oxidation performance over Cu-Mn composite oxides by regulating oxygen vacancy. Appl. Surf. Sci. 2021, 560, 149983. [Google Scholar] [CrossRef]
- Wang, J.; Chernavskii, P.A.; Khodakov, A.Y.; Wang, Y. Structure and catalytic performance of alumina-supported copper-cobalt catalysts for carbon monoxide hydrogenation. J. Catal. 2012, 286, 51–61. [Google Scholar] [CrossRef]
- Jia, A.-P.; Hu, G.-S.; Meng, L.; Xie, Y.-L.; Lu, J.-Q.; Luo, M.-F. CO oxidation over CuO/Ce1-xCuxO2−δ and Ce1−xCuxO2−δ catalysts: Synergetic effects and kinetic study. J. Catal. 2012, 289, 199–209. [Google Scholar] [CrossRef]
- Song, L.; Xiong, J.; Cheng, H.; Lu, J.; Liu, P.; Fu, M.; Wu, J.; Chen, L.; Huang, H.; Ye, D. In-Situ characterizations to investigate the nature of Co3+ coordination environment to activate surface adsorbed oxygen for methane oxidation. Appl. Surf. Sci. 2021, 556, 149713. [Google Scholar] [CrossRef]
- Lu, S.; Wang, F.; Chen, C.; Huang, F.; Li, K. Catalytic oxidation of formaldehyde over CeO2-Co3O4 catalysts. J. Rare Earths 2017, 35, 867–874. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, D.; Gao, Z.; Tian, Y.; Ding, T.; Zhang, J.; Jiang, Z.; Li, X. Interface interaction induced oxygen activation of cactus-like Co3O4/OMS-2 nanorod catalysts in situ grown on monolithic cordierite for diesel soot combustion. Appl. Catal. B Environ. 2021, 286, 119932. [Google Scholar] [CrossRef]
- Cui, B.; Yan, S.; Xia, Y.; Li, K.; Li, S.; Wang, D.; Ye, Y.; Liu, Y.-Q. Cu Ce1-O2 nanoflakes with improved catalytic activity and thermal stability for diesel soot combustion. Appl. Catal. A Gen. 2019, 578, 20–29. [Google Scholar] [CrossRef]
- He, J.; Yao, P.; Qiu, J.; Zhang, H.; Jiao, Y.; Wang, J.; Chen, Y. Enhancement effect of oxygen mobility over Ce0.5Zr0.5O2 catalysts doped by multivalent metal oxides for soot combustion. Fuel 2021, 286, 119359. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, Z.; Li, Y.; Hou, Y.; Hu, J.; Huang, Z. Effect of the A-site cation over spinel AMn2O4 (A=Cu2+, Ni2+, Zn2+) for toluene combustion: Enhancement of the synergy and the oxygen activation ability. Fuel 2021, 288, 119700. [Google Scholar] [CrossRef]
- Zhao, M.; Deng, J.; Liu, J.; Li, Y.; Liu, J.; Duan, Z.; Xiong, J.; Zhao, Z.; Wei, Y.; Song, W.; et al. Roles of surface-active oxygen species on 3DOM cobalt-based spinel catalysts MxCo3-xO4 (M=Zn and Ni) for NOx-assisted soot oxidation. ACS Catal. 2019, 9, 7548–7567. [Google Scholar] [CrossRef]
- Geng, Q.; Wang, H.; Wang, J.; Hong, J.; Sun, W.; Wu, Y.; Wang, Y. Boosting the capacity of aqueous Li-ion capacitors via pinpoint surgery in nanocoral-like covalent organic frameworks. Small Methods 2022, 6, 2200314. [Google Scholar] [CrossRef]
- Feng, F.; Zheng, Y.; Shen, X.; Zheng, Q.; Dai, S.; Zhang, X.; Huang, Y.; Liu, Z.; Yan, K. Characteristics of back corona discharge in a honeycomb catalyst and its application for treatment of volatile organic compounds. Environ. Sci. Technol. 2015, 49, 6831–6837. [Google Scholar] [CrossRef]
- Roberts, C.A.; Paidi, V.K.; Shepit, M.; Peck, T.C.; Stamm Masias, K.L.; van Lierop, J.; Reddy, G.K. Effect of Cu substitution on the structure and reactivity of CuxCo3-xO4 spinel catalysts for direct NOx decomposition. Catal. Today 2021, 360, 204–212. [Google Scholar] [CrossRef]
- Xiong, J.; Wu, Q.; Mei, X.; Liu, J.; Wei, Y.; Zhao, Z.; Wu, D.; Li, J. Fabrication of spinel-type PdxCo3–xO4 binary active sites on 3D ordered meso-macroporous Ce-Zr-O2 with enhanced activity for catalytic soot oxidation. ACS Catal. 2018, 8, 7915–7930. [Google Scholar] [CrossRef]
- Yu, D.; Peng, C.; Yu, X.; Wang, L.; Li, K.; Zhao, Z.; Li, Z. Facile preparation of amorphous CenMnOx catalysts and their good catalytic performance for soot combustion. Fuel 2022, 307, 121803. [Google Scholar] [CrossRef]
- Zhang, W.; Descorme, C.; Valverde, J.L.; Giroir-Fendler, A. Cu-Co mixed oxide catalysts for the total oxidation of toluene and propane. Catal. Today 2022, 384–386, 238–245. [Google Scholar] [CrossRef]
- Ambrogio, M.; Saracco, G.; Specchia, V. Combining filtration and catalytic combustion in particulate traps for diesel exhaust treatment. Chem. Eng. Sci. 2001, 56, 1613–1621. [Google Scholar] [CrossRef]
- Cao, C.; Xing, L.; Yang, Y.; Tian, Y.; Ding, T.; Zhang, J.; Hu, T.; Zheng, L.; Li, X. The monolithic transition metal oxide crossed nanosheets used for diesel soot combustion under gravitational contact mode. Appl. Surf. Sci. 2017, 406, 245–253. [Google Scholar] [CrossRef]
- Yeste, M.P.; Cauqui, M.Á.; Giménez-Mañogil, J.; Martínez-Munuera, J.C.; Muñoz, M.Á.; García-García, A. Catalytic activity of Cu and Co supported on ceria-yttria-zirconia oxides for the diesel soot combustion reaction in the presence of NOx. Chem. Eng. J. 2020, 380, 122370. [Google Scholar] [CrossRef]
- Chen, Y.; Shen, G.; Lang, Y.; Chen, R.; Jia, L.; Yue, J.; Shen, M.; Du, C.; Shan, B. Promoting soot combustion efficiency by strengthening the adsorption of NOx on the 3DOM mullite catalyst. J. Catal. 2020, 384, 96–105. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, C.; Galvez, M.E.; Da Costa, P.; Chen, Y. MnOx-CeO2 mixed oxides as the catalyst for NO-assisted soot oxidation: The key role of NO adsorption/desorption on catalytic activity. Appl. Surf. Sci. 2018, 462, 678–684. [Google Scholar] [CrossRef]
- Martínez-Munuera, J.C.; Zoccoli, M.; Giménez-Mañogil, J.; García-García, A. Lattice oxygen activity in ceria-praseodymia mixed oxides for soot oxidation in catalysed gasoline particle filters. Appl. Catal. B Environ. 2019, 245, 706–720. [Google Scholar] [CrossRef]
- Chen, L.; Li, T.; Zhang, J.; Wang, J.; Chen, P.; Fu, M.; Wu, J.; Ye, D. Chemisorbed superoxide species enhanced the high catalytic performance of Ag/Co3O4 nanocubes for soot oxidation. ACS Appl. Mater. Interfaces 2021, 13, 21436–21449. [Google Scholar] [CrossRef]
- Guo, X.; Li, J.; Zhou, R. Catalytic performance of manganese doped CuO-CeO2 catalysts for selective oxidation of CO in hydrogen-rich gas. Fuel 2016, 163, 56–64. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, B.J.; Lee, D.-W.; Choung, J.W.; Kim, C.H.; Lee, K.-Y. Synergistic effect of Cu on a Ag-loaded CeO2 catalyst for soot oxidation with improved generation of active oxygen species and reducibility. Fuel 2020, 275, 117930. [Google Scholar] [CrossRef]
- Yang, Q.; Gu, F.; Tang, Y.; Zhang, H.; Liu, Q.; Zhong, Z.; Su, F. A Co3O4-CeO2 functionalized SBA-15 monolith with a three-dimensional framework improves NOx-assisted soot combustion. RSC Adv. 2015, 5, 26815–26822. [Google Scholar] [CrossRef]


| Catalysts | SBET (m2·g−1) | Pore Volume (mm3·g−1) | Average Pore Diameter (nm) | Average Crystal Size (nm) * |
|---|---|---|---|---|
| CuCo2O4-N | 2.1 | 4.5 | 8.3 | 38.0 |
| CuCo2O4-A | 2.0 | 4.2 | 8.6 | 38.3 |
| CuCo2O4-C | 1.2 | 2.5 | 8.5 | 38.8 |
| Catalysts | Co3+/Cototal (%) | Cu2+/Cutotal (%) | Oads/(Oads+ Olatt) (%) | H2 Consumption (mmol·g−1) | O2 Uptake (μmol·g−1) |
|---|---|---|---|---|---|
| CuCo2O4-N | 38.4 | 51.7 | 46.8 | 13.4 | 41.2 |
| CuCo2O4-A | 35.1 | 48.2 | 37.2 | 13.6 | 27.1 |
| CuCo2O4-C | 33.8 | 47.8 | 36.5 | 14.5 | 25.6 |
| Catalysts | 10 vol.% O2/N2 | |||
|---|---|---|---|---|
| T10 (°C) | T50 (°C) | T90 (°C) | SCO2 (%) | |
| CuCo2O4-N | 451 | 520 | 558 | 98.8 |
| CuCo2O4-A | 496 | 539 | 569 | 95.0 |
| CuCo2O4-C | 504 | 550 | 594 | 81.8 |
| No catalysts | 530 | 586 | 615 | 58.7 |
| Catalysts | T10 (°C) | T50 (°C) | T90 (°C) | SCO2 (%) |
|---|---|---|---|---|
| CuCo2O4-N | 349 | 414 | 482 | 96.6 |
| CuCo2O4-A | 381 | 458 | 494 | 94.3 |
| CuCo2O4-C | 392 | 485 | 534 | 79.7 |
| No catalysts | 496 | 575 | 615 | 43.6 |
| Contact Mode | T10 (°C) | T50 (°C) | T90 (°C) | SCO2 (%) |
|---|---|---|---|---|
| Loose contact | 451 | 520 | 558 | 98.8 |
| Loose contact + NO | 349 | 414 | 482 | 96.6 |
| Tight contact | 385 | 428 | 499 | 100 |
| Tight contact + NO | 339 | 394 | 448 | 100 |
| Catalysts | Oads/(Oads + Olatt) (%) |
|---|---|
| CuCo2O4-N (fresh) | 46.8 |
| CuCo2O4-N (used) | 40.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, C.; Zhu, X.; Zhang, F.; Li, X.; Chen, G.; Zhou, Z.; Yang, G. Soot Combustion over Cu–Co Spinel Catalysts: The Intrinsic Effects of Precursors on Catalytic Activity. Int. J. Environ. Res. Public Health 2022, 19, 14737. https://doi.org/10.3390/ijerph192214737
Zhou C, Zhu X, Zhang F, Li X, Chen G, Zhou Z, Yang G. Soot Combustion over Cu–Co Spinel Catalysts: The Intrinsic Effects of Precursors on Catalytic Activity. International Journal of Environmental Research and Public Health. 2022; 19(22):14737. https://doi.org/10.3390/ijerph192214737
Chicago/Turabian StyleZhou, Chunlin, Xinbo Zhu, Fei Zhang, Xinbao Li, Geng Chen, Zijian Zhou, and Guohua Yang. 2022. "Soot Combustion over Cu–Co Spinel Catalysts: The Intrinsic Effects of Precursors on Catalytic Activity" International Journal of Environmental Research and Public Health 19, no. 22: 14737. https://doi.org/10.3390/ijerph192214737
APA StyleZhou, C., Zhu, X., Zhang, F., Li, X., Chen, G., Zhou, Z., & Yang, G. (2022). Soot Combustion over Cu–Co Spinel Catalysts: The Intrinsic Effects of Precursors on Catalytic Activity. International Journal of Environmental Research and Public Health, 19(22), 14737. https://doi.org/10.3390/ijerph192214737




