Development and Evaluation of a Digital Health Intervention to Prevent Type 2 Diabetes in Primary Care: The PREDIABETEXT Study Protocol for a Randomised Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Aims
- To develop a multifaceted, digital health intervention to prevent T2DM.
- To pilot-test and optimize the components of a digital health intervention.
- To explore the effects of the digital health intervention on glycated haemoglobin (primary outcome) and on additional clinical, physiological, behavioural and psychological outcomes through a phase II, 3-arm, 6-month clinical trial.
- To test the feasibility of a future full-scale phase III clinical trial, quantifying the number of eligible patients, recruitment rate, and follow-up rate.
2.2. Hypothesis
- It is feasible to develop a multifaceted, digital health intervention to prevent T2DM based on: (1) the use of a system comprising mobile health technology integrated with electronic health records to send automated, tailored brief text messages supporting lifestyle changes in people at risk of T2DM and (2) the provision of online education to primary healthcare professionals about T2DM prevention.
- The proposed interventions are feasible to deliver and acceptable to patients and primary healthcare professionals.
- Compared to the control group, the proposed interventions reduce HbA1c (primary outcome) at least 0.3% and improve additional clinical, physiological, behavioural and psychological outcomes.
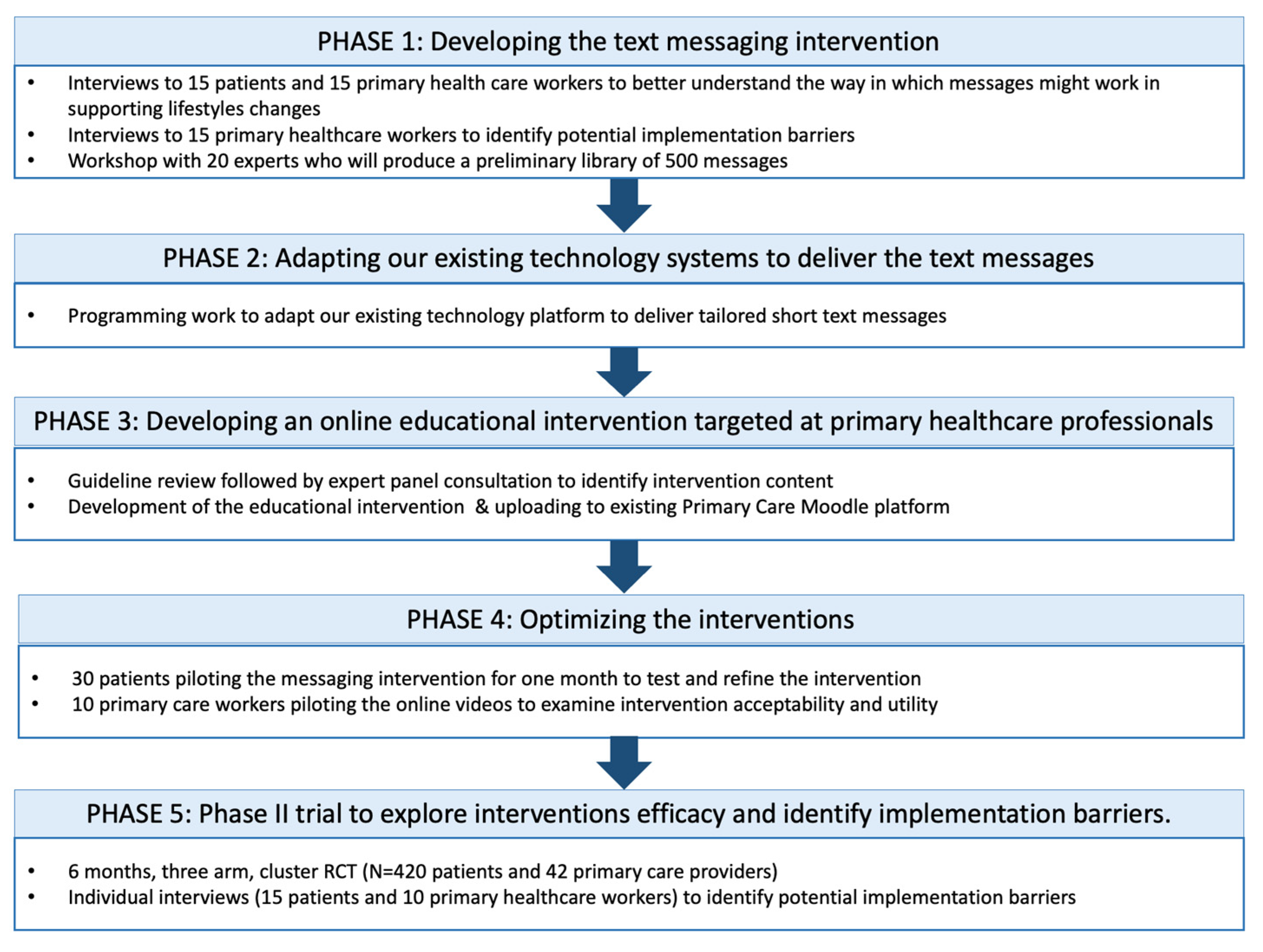
2.3. Design
2.3.1. STAGE 1: Developing the Text Messaging Intervention
2.3.2. STAGE 2: Adapting Our Existing Technology Systems to Deliver Text Messages
2.3.3. STAGE 3: Developing an Online Educational Intervention Targeted at Primary Healthcare Professionals
2.3.4. STAGE 4: Optimizing the Interventions
2.3.5. STAGE 5: Phase II Trial to Explore Interventions Efficacy and Identify Implementation Barriers
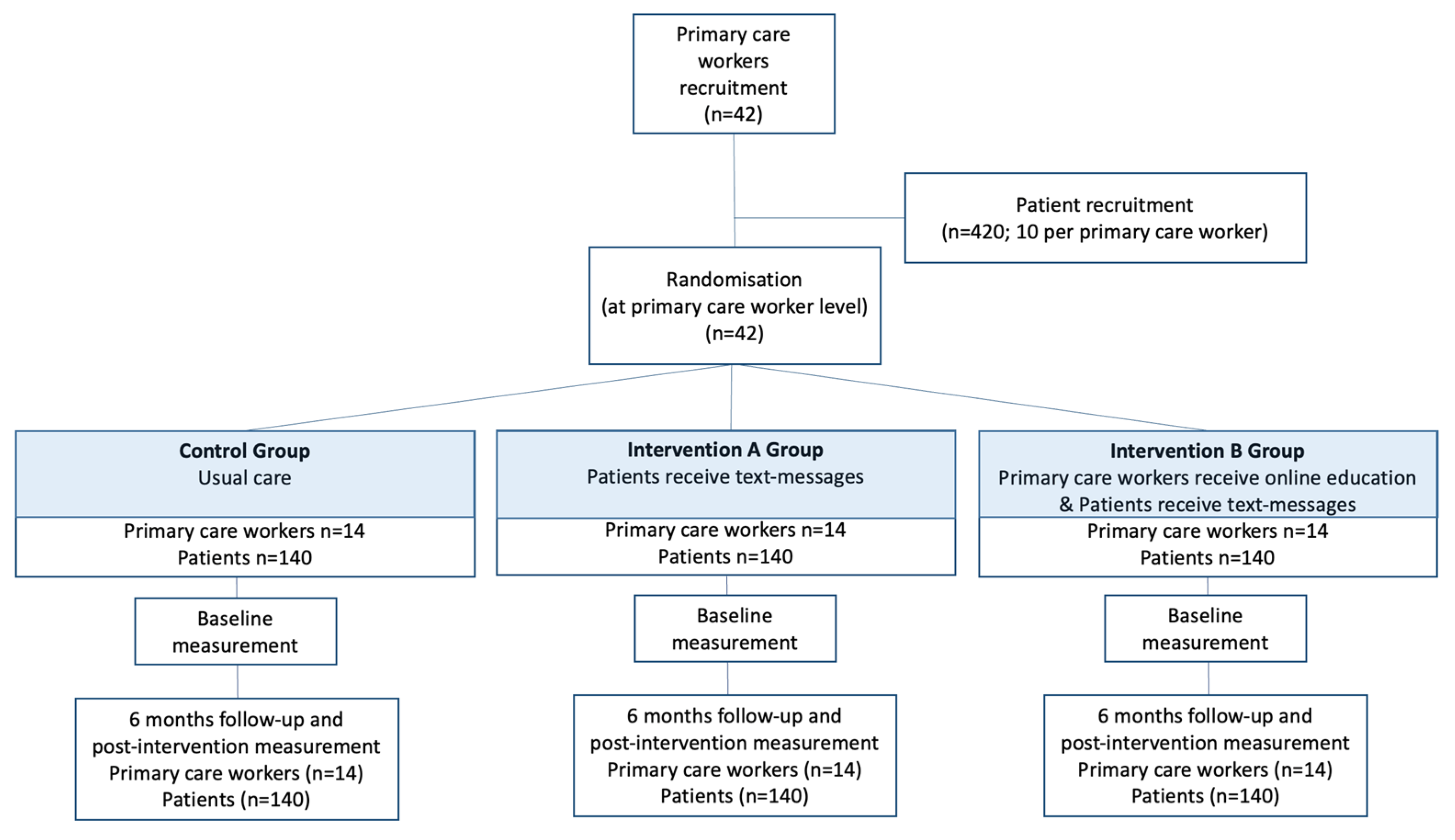
2.4. Participants
2.4.1. Primary Healthcare Professionals
2.4.2. Patients
2.5. Sample Size Determination
2.6. Randomization and Masking
2.7. Interventions
Description of the Intervention Group (SMSs)
2.8. Participant Timeline
2.9. Data Collection
2.10. Outcome Measures
2.11. Data Analysis
2.12. Ethical Considerations
2.13. Validity and Reliability
3. Discussion
Limitations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALT | alanine aminotransferase |
| AST | aspartate aminotransferase enzyme |
| GGT | gamma glutamil transpeptidase |
| GP | general practitioners |
| HbA1c | glycated haemoglobin |
| HDL | high density lipoprotein cholesterol |
| ITT | intention to treat |
| LDL | low density lipoprotein cholesterol |
| NICE | National Institute for Health and Care Excellence |
| SMS | short, automated messages |
| SNS | Spanish National Health System |
| T2DM | type 2 diabetes mellitus |
References
- World Health Organization. Preparing a Health Care Workforce for the 21st Century: The Challenge of Chronic Conditions. Available online: https://apps.who.int/iris/handle/10665/43044 (accessed on 20 February 2022).
- Bauer, M.S.; Damschroder, L.; Hagedorn, H.; Smith, J.; Kilbourne, A.M. An introduction to implementation science for the non-specialist. BMC Psychol. 2015, 3, 32. [Google Scholar] [CrossRef] [PubMed]
- Bury, G.; Twomey, L.; Egan, M. General practice nursing: The views and experience of practice nurses and GPs in one county. Ir. J. Med. Sci. 2021, 190, 193–196. [Google Scholar] [CrossRef] [PubMed]
- James, S.; McInnes, S.; Halcomb, E.; Desborough, J. General practice nurses’ communication strategies for lifestyle risk reduction: A content analysis. J. Adv. Nurs. 2020, 76, 3082–3091. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Guidance on the Use of Patient Education Models for Diabetes. NICE Technology Appraisal Guidance; National Institute for Health and Care Excellence: London, UK, 2015. [Google Scholar]
- Skoglund, G.; Nilsson, B.B.; Olsen, C.F.; Bergland, A.; Hilde, G. Facilitators and barriers for lifestyle change in people with prediabetes: A meta-synthesis of qualitative studies. BMC Public Health 2022, 22, 553. [Google Scholar] [CrossRef]
- Patterson, E.; Munn, Z.; Jennings, C. Experiences of providers in delivering nutrition-focused lifestyle interventions for adults with obesity or metabolic syndrome in primary healthcare settings: A qualitative systematic review protocol. JBI Evid. Synth. 2020, 18, 1573–1579. [Google Scholar] [CrossRef]
- Abbate, M.; Fresneda, S.; Yanez, A.; Ricci-Cabello, I.; Galmes-Panades, A.M.; Aguilo, A.; Bennasar-Veny, M.; PREDIPHONE trial group. Nurse-led telephone intervention for lifestyle changes on glycaemic control in people with prediabetes: Study protocol for a randomized controlled trial. J. Adv. Nurs. 2021, 77, 3204–3217. [Google Scholar] [CrossRef] [PubMed]
- Free, C.; Phillips, G.; Galli, L.; Watson, L.; Felix, L.; Edwards, P.; Patel, V.; Haines, A. The effectiveness of mobile-health technology-based health behaviour change or disease management interventions for health care consumers: A systematic review. PLoS Med. 2013, 10, e1001362. [Google Scholar] [CrossRef] [PubMed]
- Bian, R.R.; Piatt, G.A.; Sen, A.; Plegue, M.A.; De Michele, M.L.; Hafez, D.; Czuhajewski, C.M.; Buis, L.R.; Kaufman, N.; Richardson, C.R. The Effect of Technology-Mediated Diabetes Prevention Interventions on Weight: A Meta-Analysis. J. Med. Internet Res. 2017, 19, e76. [Google Scholar] [CrossRef]
- Fischer, H.H.; Fischer, I.P.; Pereira, R.I.; Furniss, A.L.; Rozwadowski, J.M.; Moore, S.L.; Durfee, M.J.; Raghunath, S.G.; Tsai, A.G.; Havranek, E.P. Text Message Support for Weight Loss in Patients With Prediabetes: A Randomized Clinical Trial. Diabetes Care 2016, 39, 1364–1370. [Google Scholar] [CrossRef]
- Bobrow, K.; Farmer, A.J.; Springer, D.; Shanyinde, M.; Yu, L.M.; Brennan, T.; Rayner, B.; Namane, M.; Steyn, K.; Tarassenko, L.; et al. Mobile Phone Text Messages to Support Treatment Adherence in Adults With High Blood Pressure (SMS-Text Adherence Support [StAR]): A Single-Blind, Randomized Trial. Circulation 2016, 133, 592–600. [Google Scholar] [CrossRef]
- Chow, C.K.; Redfern, J.; Hillis, G.S.; Thakkar, J.; Santo, K.; Hackett, M.L.; Jan, S.; Graves, N.; de Keizer, L.; Barry, T.; et al. Effect of Lifestyle-Focused Text Messaging on Risk Factor Modification in Patients With Coronary Heart Disease: A Randomized Clinical Trial. JAMA 2015, 314, 1255–1263. [Google Scholar] [CrossRef] [PubMed]
- Arambepola, C.; Ricci-Cabello, I.; Manikavasagam, P.; Roberts, N.; French, D.P.; Farmer, A. The Impact of Automated Brief Messages Promoting Lifestyle Changes Delivered Via Mobile Devices to People with Type 2 Diabetes: A Systematic Literature Review and Meta-Analysis of Controlled Trials. J. Med. Internet Res. 2016, 18, e86. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, A.; Snehalatha, C.; Ram, J.; Selvam, S.; Simon, M.; Nanditha, A.; Shetty, A.S.; Godsland, I.F.; Chaturvedi, N.; Majeed, A.; et al. Effectiveness of mobile phone messaging in prevention of type 2 diabetes by lifestyle modification in men in India: A prospective, parallel-group, randomised controlled trial. Lancet Diabetes Endocrinol. 2013, 1, 191–198. [Google Scholar] [CrossRef]
- Wong, C.K.; Fung, C.S.; Siu, S.C.; Lo, Y.Y.; Wong, K.W.; Fong, D.Y.; Lam, C.L. A short message service (SMS) intervention to prevent diabetes in Chinese professional drivers with pre-diabetes: A pilot single-blinded randomized controlled trial. Diabetes Res. Clin. Pract. 2013, 102, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Nanditha, A.; Thomson, H.; Susairaj, P.; Srivanichakorn, W.; Oliver, N.; Godsland, I.F.; Majeed, A.; Darzi, A.; Satheesh, K.; Simon, M.; et al. A pragmatic and scalable strategy using mobile technology to promote sustained lifestyle changes to prevent type 2 diabetes in India and the UK: A randomised controlled trial. Diabetologia 2020, 63, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Soriguer, F.; Goday, A.; Bosch-Comas, A.; Bordiu, E.; Calle-Pascual, A.; Carmena, R.; Casamitjana, R.; Castano, L.; Castell, C.; Catala, M.; et al. Prevalence of diabetes mellitus and impaired glucose regulation in Spain: The Di@bet.es Study. Diabetologia 2012, 55, 88–93. [Google Scholar] [CrossRef]
- International Diabetes Federation. IDF Diabetes Atlas 2021. Available online: https://diabetesatlas.org/atlas/tenth-edition/ (accessed on 25 May 2022).
- Lopez-Bastida, J.; Boronat, M.; Moreno, J.O.; Schurer, W. Costs, outcomes and challenges for diabetes care in Spain. Glob. Health 2013, 9, 17. [Google Scholar] [CrossRef]
- Spanish Ministry of Health. Spanish National Health Survey 2017. Available online: https://www.ine.es/en/metodologia/t15/t153041917_en.pdf (accessed on 28 February 2022).
- National Institute for Health and Care Excellence. Type 2 Diabetes: Prevention in People at High Risk; NIHCE: London, UK, 2017. [Google Scholar]
- Mata-Cases, M.; Artola, S.; Escalada, J.; Ezkurra-Loyola, P.; Ferrer-García, J.; Fornos, J.; Girbés, J.; Rica, I. Consensus on the detection and management of prediabetes. Consensus and Clinical Guidelines Working Group of the Spanish Diabetes Society. Rev. Clin. Esp. 2015, 215, 117–129. [Google Scholar] [CrossRef]
- Bennasar-Veny, M.; Fresneda, S.; Lopez-Gonzalez, A.; Busquets-Cortes, C.; Aguilo, A.; Yanez, A.M. Lifestyle and Progression to Type 2 Diabetes in a Cohort of Workers with Prediabetes. Nutrients 2020, 12, 1538. [Google Scholar] [CrossRef]
- Tabak, A.G.; Herder, C.; Rathmann, W.; Brunner, E.J.; Kivimaki, M. Prediabetes: A high-risk state for diabetes development. Lancet 2012, 379, 2279–2290. [Google Scholar] [CrossRef]
- Selvin, E.; Steffes, M.W.; Zhu, H.; Matsushita, K.; Wagenknecht, L.; Pankow, J.; Coresh, J.; Brancati, F.L. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N. Engl. J. Med. 2010, 362, 800–811. [Google Scholar] [CrossRef] [PubMed]
- González Marantea, C.A.B.C.S.; Valle Alonsob, J.; Fernández Quesadaa, J. Knowledge of type 2 diabetics about their disease: A study in a health centre. Med. Gen. Y Fam. 2015, 4, 10–15. [Google Scholar]
- Spanish Agency for Food Safety and Nutrition. Evaluation and Monitoring of the NAOS Strategy: Minimum Set of Indicators 2020; Spanish Ministry of Health: Madrid, Spain, 2021. [Google Scholar]
- National Institute of Statistics. Survey on Equipment and Use of Information and Communication Technologies in Households; Ministry of Economic Affairs and Digital Transformation: Madrid, Spain, 2018. [Google Scholar]
- Skivington, K.; Matthews, L.; Simpson, S.A.; Craig, P.; Baird, J.; Blazeby, J.M.; Boyd, K.A.; Craig, N.; French, D.P.; McIntosh, E.; et al. A new framework for developing and evaluating complex interventions: Update of Medical Research Council guidance. BMJ 2021, 374, n2061. [Google Scholar] [CrossRef] [PubMed]
- Kitzinger, J. Qualitative research. Introducing focus groups. BMJ 1995, 311, 299–302. [Google Scholar] [CrossRef]
- Nowell, L.S.; Norris, J.M.; White, D.E.; Moules, N.J. Thematic Analysis: Striving to Meet the Trustworthiness Criteria. Int. J. Qual. Methods 2017, 16, 1–13. [Google Scholar] [CrossRef]
- Zamanillo-Campos, R.; Serrano-Ripoll, M.J.; Taltavull-Aparicio, J.M.; Gervilla-Garcia, E.; Ripoll, J.; Fiol-deRoque, M.A.; Boylan, A.M.; Ricci-Cabello, I. Patients’ Views on the Design of DiabeText, a New mHealth Intervention to Improve Adherence to Oral Antidiabetes Medication in Spain: A Qualitative Study. Int. J. Environ. Res. Public Health 2022, 19, 1902. [Google Scholar] [CrossRef]
- Michie, S.; Richardson, M.; Johnston, M.; Abraham, C.; Francis, J.; Hardeman, W.; Eccles, M.P.; Cane, J.; Wood, C.E. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: Building an international consensus for the reporting of behavior change interventions. Ann. Behav. Med. 2013, 46, 81–95. [Google Scholar] [CrossRef]
- Domingo-Salvany, A.; Bacigalupe, A.; Carrasco, J.M.; Espelt, A.; Ferrando, J.; Borrell, C. Proposals for social class classification based on the Spanish National Classification of Occupations 2011 using neo-Weberian and neo-Marxist approaches. Gac. Sanit. 2013, 27, 263–272. [Google Scholar] [CrossRef]
- World Health Organization. International Standards for Clinical Trial Registries—Version 3.0; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Adams, G.; Gulliford, M.C.; Ukoumunne, O.C.; Eldridge, S.; Chinn, S.; Campbell, M.J. Patterns of intra-cluster correlation from primary care research to inform study design and analysis. J. Clin. Epidemiol. 2004, 57, 785–794. [Google Scholar] [CrossRef]
- Dunkley, A.J.; Bodicoat, D.H.; Greaves, C.J.; Russell, C.; Yates, T.; Davies, M.J.; Khunti, K. Diabetes prevention in the real world: Effectiveness of pragmatic lifestyle interventions for the prevention of type 2 diabetes and of the impact of adherence to guideline recommendations: A systematic review and meta-analysis. Diabetes Care 2014, 37, 922–933. [Google Scholar] [CrossRef]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef] [PubMed]
- Dyson, P.A.; Twenefour, D.; Breen, C.; Duncan, A.; Elvin, E.; Goff, L.; Hill, A.; Kalsi, P.; Marsland, N.; McArdle, P.; et al. Diabetes UK evidence-based nutrition guidelines for the prevention and management of diabetes. Diabet Med. 2018, 35, 541–547. [Google Scholar] [CrossRef]
- Martinez-Gonzalez, M.A.; Buil-Cosiales, P.; Corella, D.; Bullo, M.; Fito, M.; Vioque, J.; Romaguera, D.; Martinez, J.A.; Warnberg, J.; Lopez-Miranda, J.; et al. Cohort Profile: Design and methods of the PREDIMED-Plus randomized trial. Int. J. Epidemiol. 2019, 48, 387–388. [Google Scholar] [CrossRef] [PubMed]
- Galmes-Panades, A.M.; Konieczna, J.; Varela-Mato, V.; Abete, I.; Babio, N.; Fiol, M.; Antonio de Paz, J.; Casas, R.; Olbeyra, R.; Ruiz-Canela, M.; et al. Targeting body composition in an older population: Do changes in movement behaviours matter? Longitudinal analyses in the PREDIMED-Plus trial. BMC Med. 2021, 19, 3. [Google Scholar] [CrossRef] [PubMed]
- Galmes-Panades, A.M.; Varela-Mato, V.; Konieczna, J.; Warnberg, J.; Martinez-Gonzalez, M.A.; Salas-Salvado, J.; Corella, D.; Schroder, H.; Vioque, J.; Alonso-Gomez, A.M.; et al. Isotemporal substitution of inactive time with physical activity and time in bed: Cross-sectional associations with cardiometabolic health in the PREDIMED-Plus study. Int. J. Behav. Nutr. Phys. Act. 2019, 16, 137. [Google Scholar] [CrossRef]
- Ramos, R.; Solanas, P.; Cordon, F.; Rohlfs, I.; Elosua, R.; Sala, J.; Masia, R.; Faixedas, M.T.; Marrugat, J. Comparison of population coronary heart disease risk estimated by the Framingham original and REGICOR calibrated functions. Med. Clin. 2003, 121, 521–526. [Google Scholar] [CrossRef]
- Schroder, H.; Fito, M.; Estruch, R.; Martinez-Gonzalez, M.A.; Corella, D.; Salas-Salvado, J.; Lamuela-Raventos, R.; Ros, E.; Salaverria, I.; Fiol, M.; et al. A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. J. Nutr. 2011, 141, 1140–1145. [Google Scholar] [CrossRef]
- Bassett, D.R., Jr. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1396. [Google Scholar] [CrossRef]
- Martinez-Gonzalez, M.A.; Lopez-Fontana, C.; Varo, J.J.; Sanchez-Villegas, A.; Martinez, J.A. Validation of the Spanish version of the physical activity questionnaire used in the Nurses’ Health Study and the Health Professionals’ Follow-up Study. Public Health Nutr. 2005, 8, 920–927. [Google Scholar] [CrossRef]
- Royston, P.; White, I.R. Multiple imputation by chained equations (MICE): Implementation in Stata. J. Stat. Softw. 2011, 45, 1–20. [Google Scholar] [CrossRef]
- Farmer, A.J.; McSharry, J.; Rowbotham, S.; McGowan, L.; Ricci-Cabello, I.; French, D.P. Effects of interventions promoting monitoring of medication use and brief messaging on medication adherence for people with Type 2 diabetes: A systematic review of randomized trials. Diabet. Med. 2016, 33, 565–579. [Google Scholar] [CrossRef] [PubMed]
- Omboni, S.; McManus, R.J.; Bosworth, H.B.; Chappell, L.C.; Green, B.B.; Kario, K.; Logan, A.G.; Magid, D.J.; McKinstry, B.; Margolis, K.L.; et al. Evidence and Recommendations on the Use of Telemedicine for the Management of Arterial Hypertension: An International Expert Position Paper. Hypertension 2020, 76, 1368–1383. [Google Scholar] [CrossRef] [PubMed]


| Data Category | Information |
|---|---|
| Trial identification number | ClinicalTrials.gov NCT05110625 |
| Date of registration | 8 November 2021 |
| Sponsor | Balearic Islands Research Institute (IdISBa), Spain |
| Contact for public and scientific queries | Dr Ignacio Ricci Cabello. Primary Care Research Unit of Mallorca (IB-Salut), Balearic Health Service, Palma de Mallorca, Spain, Palma de Mallorca, ignacio.ricci@ibsalut.es |
| Scientific title | Effects of a low intensity, multifaceted, mHealth intervention to prevent type 2 diabetes mellitus in adults with prediabetes in the primary care setting (the PREDIABETEXT trial) |
| Country of recruitment | Spain |
| Health condition studies | Prediabetes |
| Interventions | Intervention A: Participants will receive text messages (three per week) in their mobile phones during six months Intervention B: Participants will receive messaging intervention plus online education to their primary healthcare workers |
| Control: Participants will receive usual care only, and their healthcare workers will not receive online education | |
| Inclusion/exclusion criteria | Eligible age: 15–75 years; eligible sex: males and females. |
| Inclusion criteria: Registered in the Public Health Service of the Balearic Islands. HbA1c from 6% to 6.4% or fasting plasma glucose 110–125 mg/dL, or both. With access to a mobile device able to receive text messages. | |
| Exclusion criteria: documented history of T2D and/or use of oral antidiabetic medication. Younger than 18 years old or older than 75 years old. People not able to read messages in Spanish. Patients with severe mental conditions. | |
| Study type | Interventional |
| Allocation: randomized; Intervention model: parallel assignment; masking: none (open label) | |
| Primary purpose: Change from Baseline HbA1C at 6 months | |
| Date of first enrolment | 1 September 2021 |
| Target sample size | 420 (140 participants in each group) |
| Recruitment status | Actively recruiting |
| Primary outcomes | Reduction of HbA1C |
| Secondary outcomes | Fasting blood glucose, body weight, waist circumference, blood pressure, lipids, cardiovascular disease risk, proportion of patients developing T2DM, adherence to Mediterranean diet, physical activity, sedentary behaviour, smoking habit, alcohol consumption |
| Main Visits and Assessment Schedules | |||||
|---|---|---|---|---|---|
| Visit | V −3 | V −2 | V −1 | V 0 | V 1 |
| Time point 1 | −60 d | −45 d | −7 d | 0 d | 6 m |
| Participants—patients | |||||
| Invitation by SMS | X | ||||
| Informed consent | X | ||||
| Inclusion/exclusion criteria | X | ||||
| SB assessment 2 | X | X | |||
| Dietary assessment 3 | X | X | |||
| Motivation Questionnaire (ad hoc) 4 | X | X | |||
| PA assessment 5 | X | X | |||
| Randomization 6 | X | ||||
| Blood laboratory examinations 7 | X | X | |||
| Anthropometric measurements 8 | X | X | |||
| Blood pressure measurement 9 | X | X | |||
| Initiation of the intervention 10 | X | ||||
| Trial feasibility: follow-up rate | X | ||||
| Participants—health care workers | |||||
| Invitation by email | X | ||||
| Informed consent | X | ||||
| Inclusion/exclusion criteria | X | ||||
| Randomization | X | ||||
| Online education (intervention B group) | X | ||||
| Individual interviews (intervention B group) | X | ||||
| Interview: Knowledge and attitudes about prediabetes, and communication skills | X | ||||
| Trial feasibility: follow-up rate | X | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galmes-Panades, A.M.; Angullo, E.; Mira-Martínez, S.; Bennasar-Veny, M.; Zamanillo-Campos, R.; Gómez-Juanes, R.; Konieczna, J.; Jiménez, R.; Serrano-Ripoll, M.J.; Fiol-deRoque, M.A.; et al. Development and Evaluation of a Digital Health Intervention to Prevent Type 2 Diabetes in Primary Care: The PREDIABETEXT Study Protocol for a Randomised Clinical Trial. Int. J. Environ. Res. Public Health 2022, 19, 14706. https://doi.org/10.3390/ijerph192214706
Galmes-Panades AM, Angullo E, Mira-Martínez S, Bennasar-Veny M, Zamanillo-Campos R, Gómez-Juanes R, Konieczna J, Jiménez R, Serrano-Ripoll MJ, Fiol-deRoque MA, et al. Development and Evaluation of a Digital Health Intervention to Prevent Type 2 Diabetes in Primary Care: The PREDIABETEXT Study Protocol for a Randomised Clinical Trial. International Journal of Environmental Research and Public Health. 2022; 19(22):14706. https://doi.org/10.3390/ijerph192214706
Chicago/Turabian StyleGalmes-Panades, Aina M., Escarlata Angullo, Sofía Mira-Martínez, Miquel Bennasar-Veny, Rocío Zamanillo-Campos, Rocío Gómez-Juanes, Jadwiga Konieczna, Rafael Jiménez, Maria Jesús Serrano-Ripoll, Maria Antonia Fiol-deRoque, and et al. 2022. "Development and Evaluation of a Digital Health Intervention to Prevent Type 2 Diabetes in Primary Care: The PREDIABETEXT Study Protocol for a Randomised Clinical Trial" International Journal of Environmental Research and Public Health 19, no. 22: 14706. https://doi.org/10.3390/ijerph192214706
APA StyleGalmes-Panades, A. M., Angullo, E., Mira-Martínez, S., Bennasar-Veny, M., Zamanillo-Campos, R., Gómez-Juanes, R., Konieczna, J., Jiménez, R., Serrano-Ripoll, M. J., Fiol-deRoque, M. A., Miralles, J., Yañez, A. M., Romaguera, D., Vidal-Thomas, M. C., Llobera-Canaves, J., García-Toro, M., Vicens, C., Gervilla-García, E., Oña, J. I., ... Ricci-Cabello, I. (2022). Development and Evaluation of a Digital Health Intervention to Prevent Type 2 Diabetes in Primary Care: The PREDIABETEXT Study Protocol for a Randomised Clinical Trial. International Journal of Environmental Research and Public Health, 19(22), 14706. https://doi.org/10.3390/ijerph192214706









