Administration of Different Doses of Acrylamide Changed the Chemical Coding of Enteric Neurons in the Jejunum in Gilts
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Tissue Collection
2.3. Immunofluorescence Procedures
2.4. Statistical Analysis
3. Results
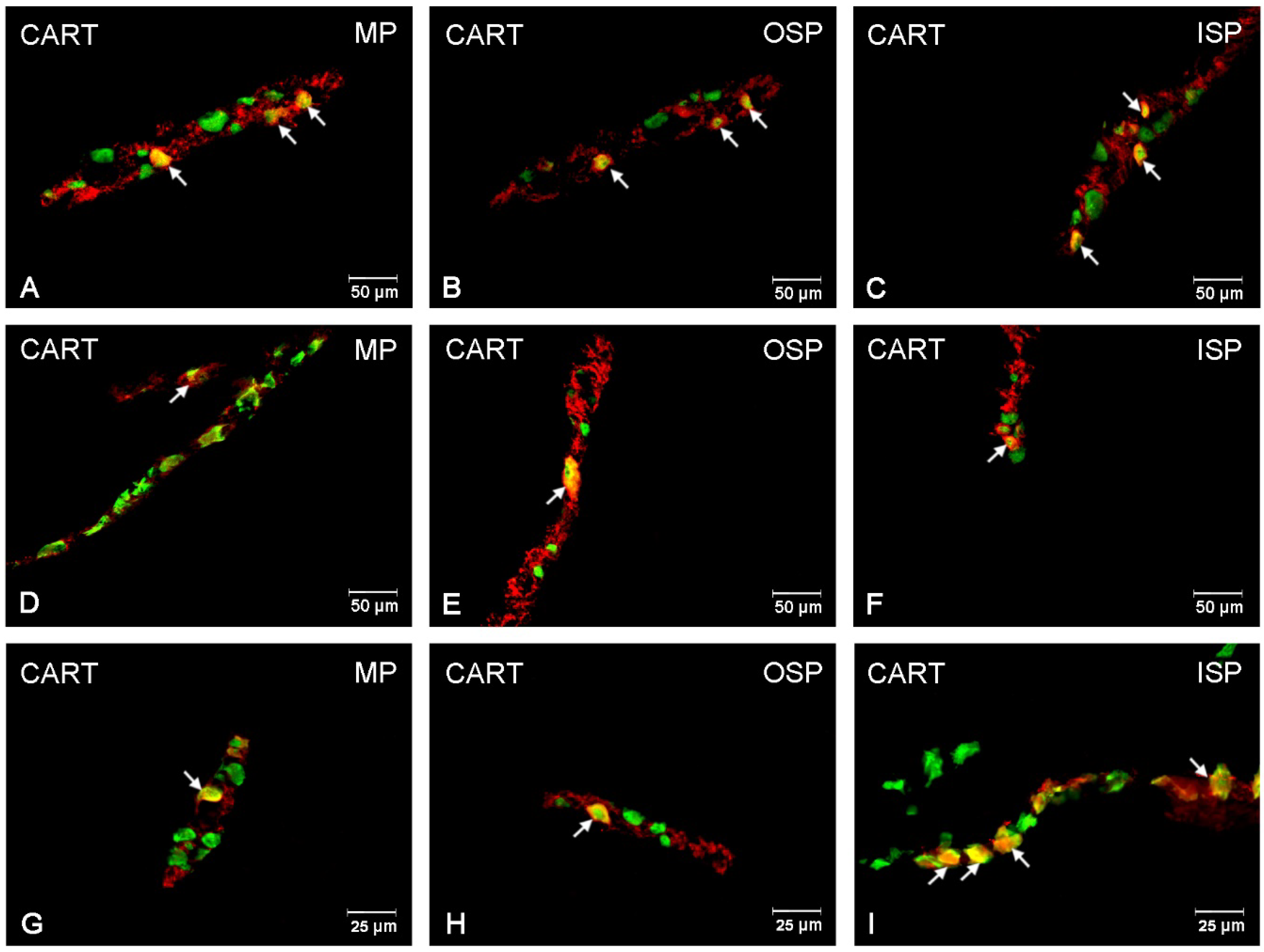
3.1. CART-Immunoreactive Neurons
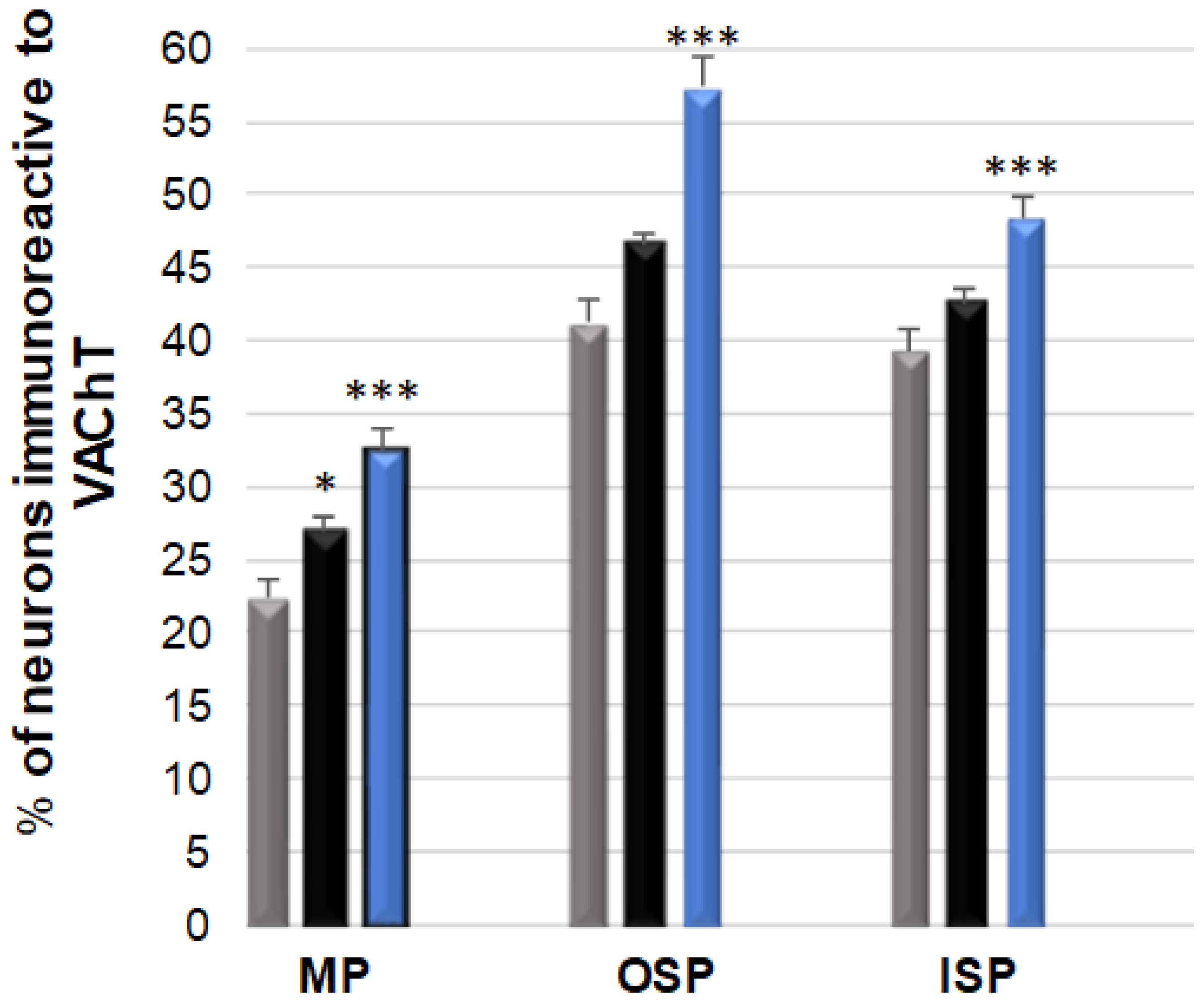
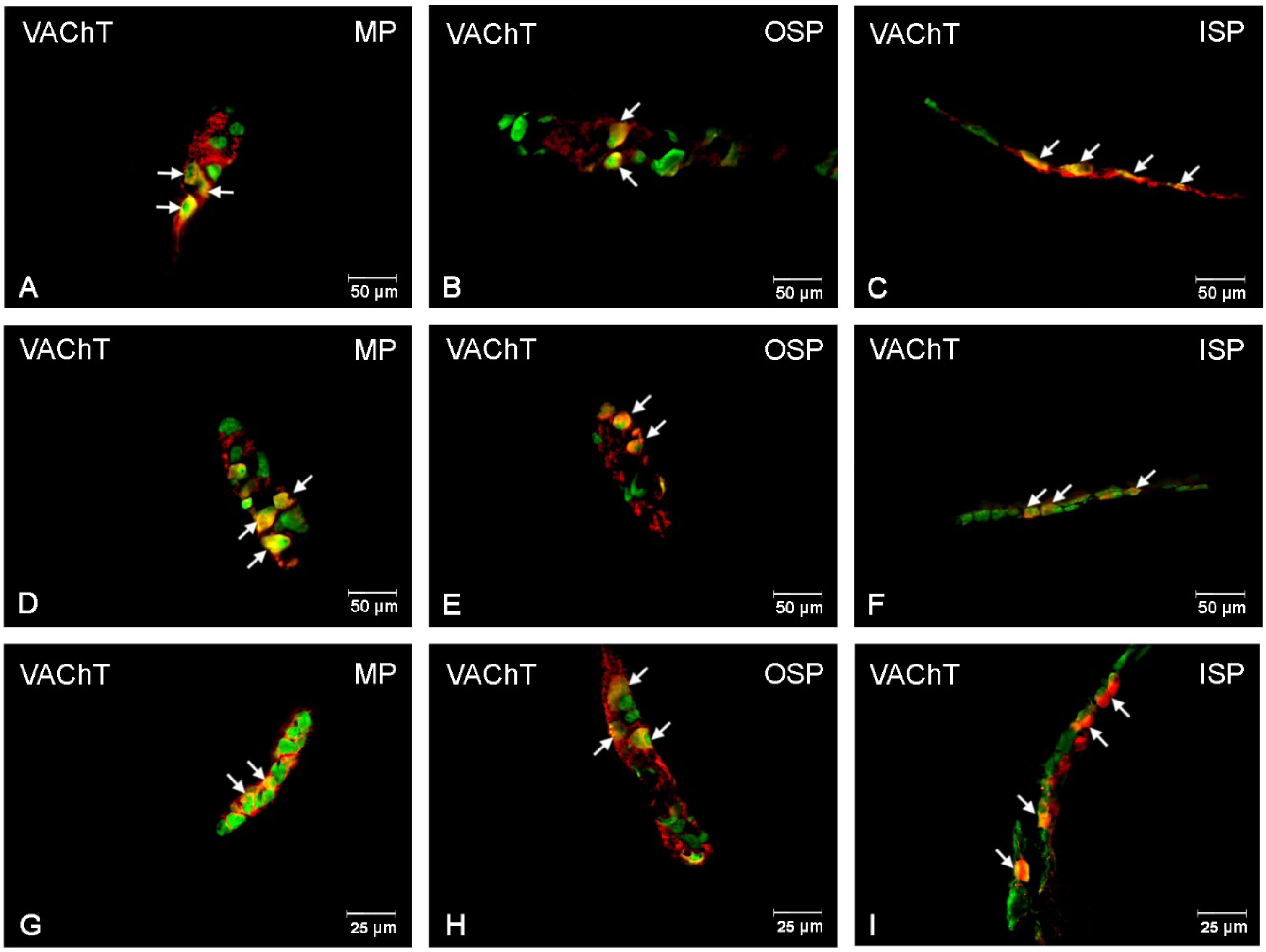
3.2. VAChT-Immunoreactive Neurons
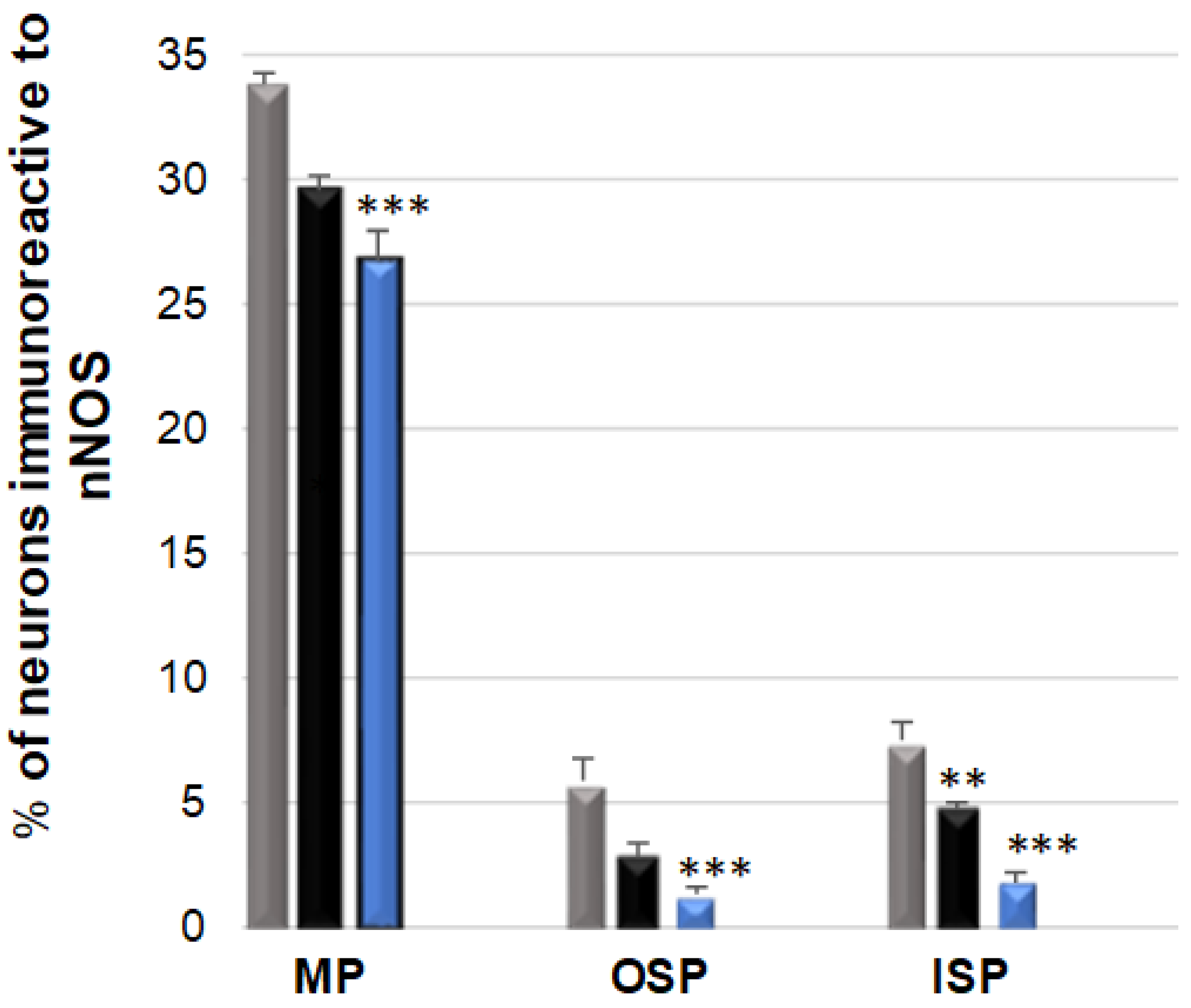
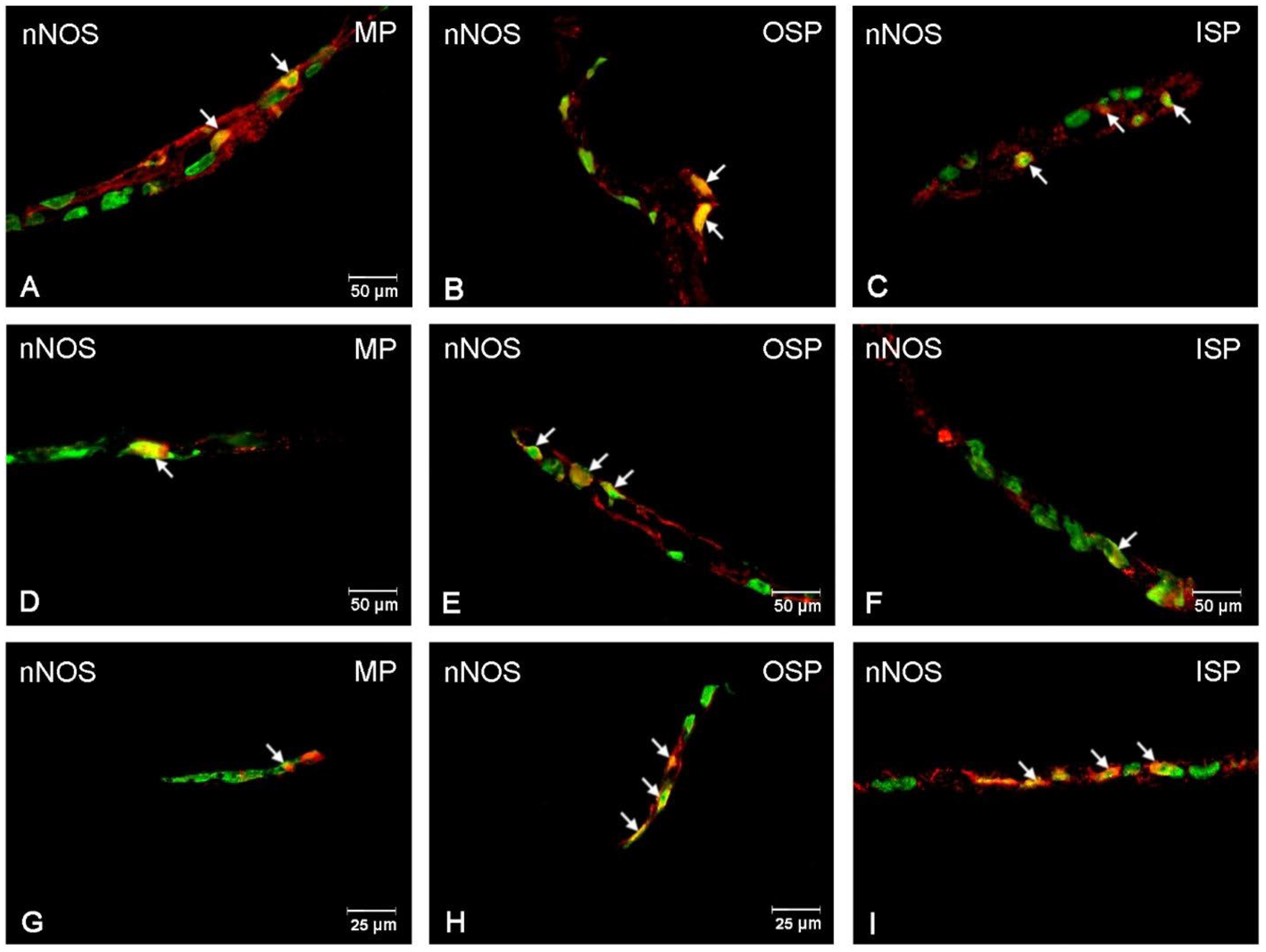
3.3. nNOS-Immunoreactive Neurons
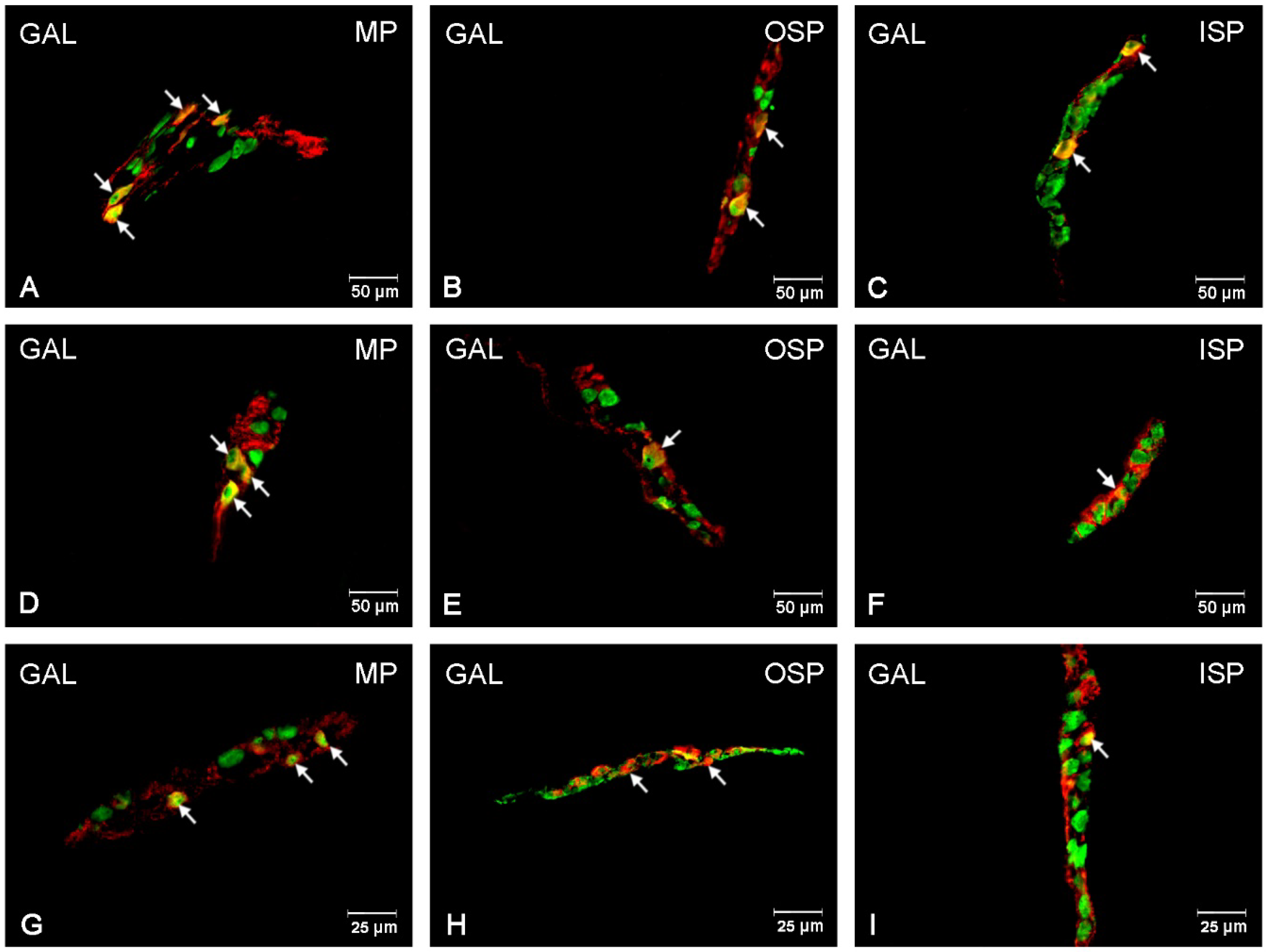
3.4. GAL-Immunoreactive Neurons
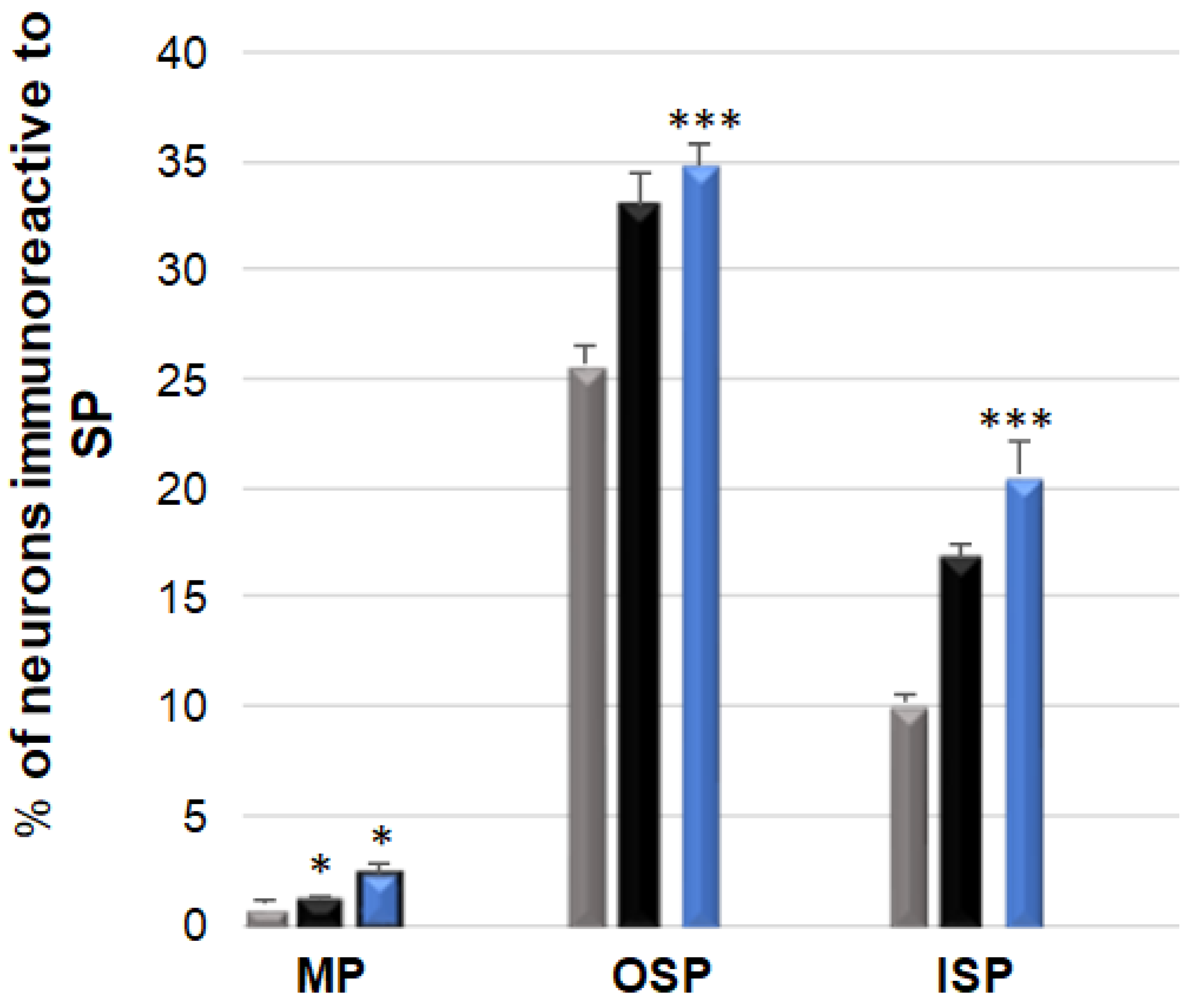
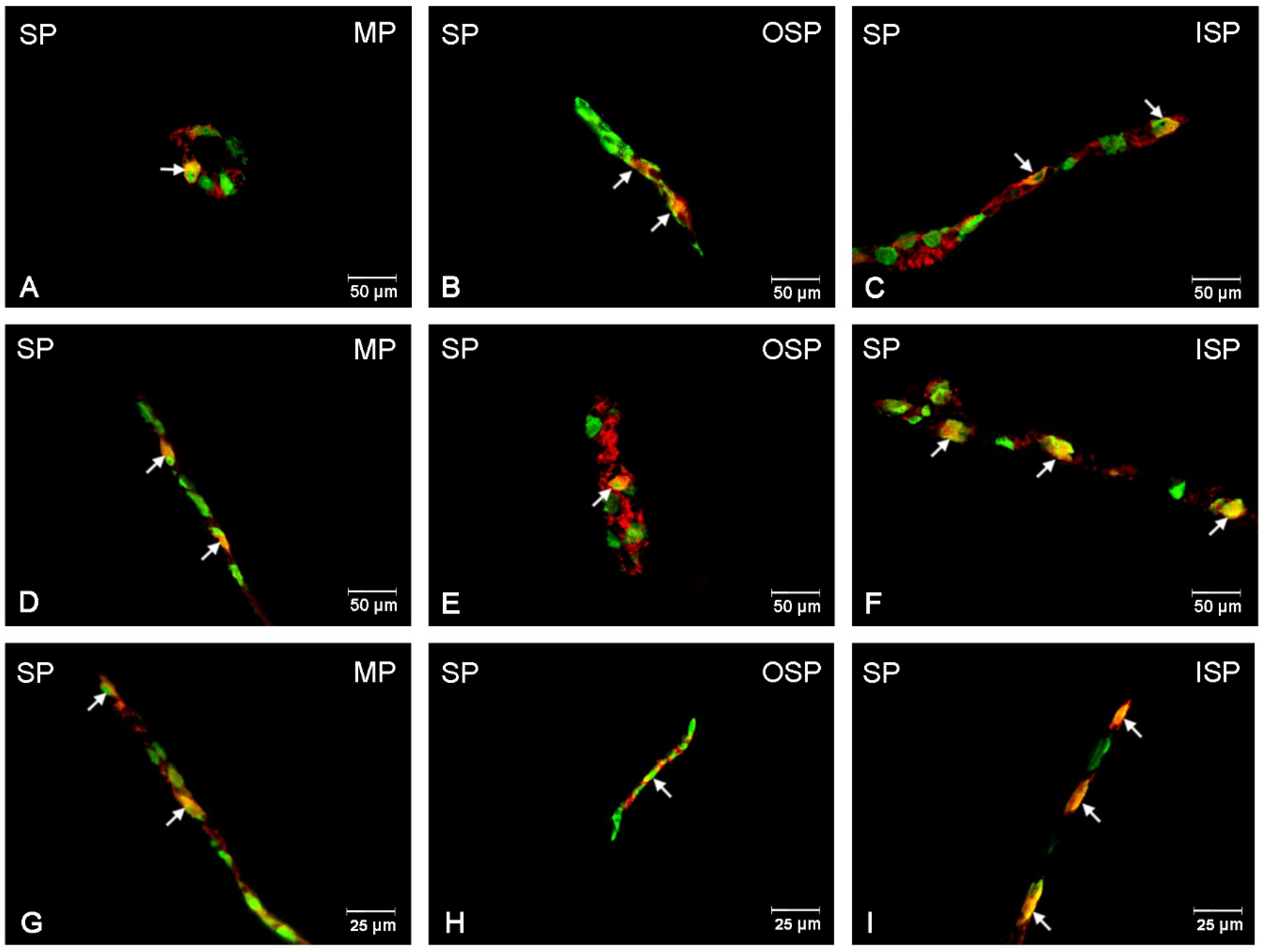
3.5. SP-Immunoreactive Neurons
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smith, E.A.; Oehme, F.W. Acrylamide and polyacrylamide: A review of production, use, environmental fate and neurotoxicity. Rev. Environ. Health 1991, 9, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Girma, K.B.; Lorenz, V.; Blaurock, S.; Edelmann, F. Coordination chemistry of acrylamide. Coord. Chem. Rev. 2005, 249, 1283–1293. [Google Scholar] [CrossRef]
- Friedman, M. Chemistry, Biochemistry, and Safety of Acrylamide. A Review. J. Agric. Food Chem. 2003, 51, 4504–4526. [Google Scholar] [CrossRef] [PubMed]
- Ericsson, S. Acrylamide in Food Products: Identifi Cation, Formation and Analytical Methodology; Institutionen för Miljökemi Stockholms Universitet: Stockholm, Sweden, 2005; pp. 9–53. [Google Scholar]
- Van Lancker, F.; Adams, A.; De Kimpe, N. Chemical modifications of peptides and their impact on food properties. Chem. Rev. 2011, 111, 7876–7903. [Google Scholar] [CrossRef] [PubMed]
- Stadler, R.H.; Scholz, G. Acrylamide: An update on current knowledge in analysis, levels in food, mechanisms of formation, and potential strategies of control. Nutr. Rev. 2004, 62, 449–467. [Google Scholar] [CrossRef] [PubMed]
- Gerrard, J.A. The Maillard reaction in food: Progress made, challenges ahead—Conference report from the eighth international symposium on the maillard reaction. Trends Food Sci. Technol. 2006, 17, 324–330. [Google Scholar] [CrossRef]
- Lee, S.; Park, H.R.; Lee, J.Y.; Cho, J.H.; Song, H.M.; Kim, A.H.; Lee, W.; Lee, Y.; Chang, S.C.; Kim, H.S.; et al. Learning, memory deficits, and impaired neuronal maturation attributed to acrylamide. J. Toxicol. Environ. Health A 2018, 81, 254–265. [Google Scholar] [CrossRef]
- Lo Pachin, R.M. The changing view of acrylamide neurotoxicity. Neurotoxicology 2004, 25, 617–630. [Google Scholar] [CrossRef]
- LoPachin, R.M.; Gavin, T. Molecular mechanism of acrylamide neurotoxicity: Lessons earned from organic chemistry. Environ. Health Perspect. 2012, 120, 1650. [Google Scholar] [CrossRef]
- Zödl, B.; Schmid, D.; Wassler, G.; Gundacker, C.; Leibetseder, V.; Thalhammer, T.; Ekmekcioglu, C. Intestinal transport and metabolism of acrylamide. Toxicology 2007, 232, 99–108. [Google Scholar] [CrossRef]
- Sörgel, F.; Weissenbacher, R.; Kinig-Schippers, M.; Hofmann, A.; Illauer, M.; Skott, A.; Landersdorfer, C. Acrylamide: Increased concentrations in homemade food and first evidence of its variable absorption from food, variable metabolism and placental and breast milk transfer in humans. Chemotherapy 2002, 48, 267–274. [Google Scholar] [CrossRef]
- Furness, J.B. The enteric nervous system and neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 2012, 69, 286–294. [Google Scholar] [CrossRef]
- Furness, J.B.; Callaghan, B.P.; Rivera, L.R.; Cho, H.J. The enteric nervous system and gastrointestinal innervation: Integrated local and central control. Adv. Exp. Med. Biol. 2014, 817, 39–71. [Google Scholar]
- Furness, J.B. Types of neurons in the enteric nervous system. J. Auton. Nerv. Syst. 2000, 81, 87–96. [Google Scholar] [CrossRef]
- Destexhe, A.; Marder, E. Plasticity in single neuron and circuit computations. Nature 2004, 431, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.W.; Qu, J.; Black, D.; Tso, P. Regulation of intestinal lipid metabolism: Current concepts and relevance to disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Gonkowski, S. Substance P as a neuronal factor in the enteric nervous system of the porcine descending colon in physiological conditions and during selected pathogenic processes. Biofactors 2013, 39, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Gonkowski, S.; Burlinski, P.; Skobowiat, C.; Majewski, M.; Całka, J. Inflammation- and axotomy-induced changes in galanin-like immunoreactive (GAL-LI) nerve structures in the porcine descending colon. Acta Vet. Hung. 2010, 58, 91–103. [Google Scholar] [CrossRef]
- Rychlik, A.; Gonkowski, S.; Nowicki, M.; Calka, J. Inflammatory bowel disease affects density of nitrergic nerve fibers in the mucosal layer of the canine gastrointestinal tract. Can. J. Vet. Res. 2017, 81, 129–136. [Google Scholar]
- Arvidsson, U.; Riedl, M.; Elde, R.; Meister, B. Vesicular acetylcholine transporter (VAChT) protein: A novel and unique marker for cholinergic neurons in the central and peripheral nervous systems. J. Comp. Neurol. 1997, 378, 454–467. [Google Scholar] [CrossRef]
- Zalecki, M.; Plywacz, A.; Antushevich, H.; Franke-Radowiecka, A. Cocaine and Amphetamine Regulated Transcript (CART) Expression Changes in the Stomach Wall Affected by Experimentally Induced Gastric Ulcerations. Int. J. Mol. Sci. 2021, 22, 7437. [Google Scholar] [CrossRef]
- Tardiff, R.G.; Gargas, M.L.; Kirman, C.R.; Carson, M.L.; Sweeney, L.M. Estimation of safe dietary intake levels of acrylamide for humans. Food Chem. Toxicol. 2010, 48, 658–667. [Google Scholar] [CrossRef]
- Kararli, T.T. Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals. Biopharm. Drug Dispos. 1995, 16, 351–380. [Google Scholar] [CrossRef]
- Boyaci-Gunduz, C.P. Acrylamide exposure of infants and toddlers through baby foods and current progress on regulations. Curr. Opin. Food Sci. 2022, 46, 100849. [Google Scholar] [CrossRef]
- Mielech, A.; Puścion-Jakubik, A.; Socha, K. Assessment of the Risk of Contamination of Food for Infants and Toddlers. Nutrients 2021, 9, 2358. [Google Scholar] [CrossRef]
- Kobayashi, E.; Hanazono, Y.; Kunita, S. Swine used in the medical university: Overview of 20 years of experience. Exp. Anim. 2018, 67, 7–13. [Google Scholar] [CrossRef]
- Makowska, K.; Obremski, K.; Zielonka, L.; Gonkowski, S. The Influence of Low Doses of Zearalenone and T-2 Toxin on Calcitonin Gene Related Peptide-Like Immunoreactive (CGRP-LI) Neurons in the ENS of the Porcine Descending Colon. Toxins 2017, 9, 98. [Google Scholar] [CrossRef] [PubMed]
- Abelli, L.; Ferri, G.L.; Astolfi, M.; Conte, B.; Geppetti, P.; Parlani, M.; Dahl, D.; Polak, J.M.; Maggi, C.A. Acrylamide-induced visceral neuropathy: Evidence for the involvement of capsaicin-sensitive nerves of the rat urinary bladder. Neuroscience 1991, 41, 311–321. [Google Scholar] [CrossRef]
- Szymanska, K.; Calka, J.; Gonkowski, S. Nitric oxide as an active substance in the enteric neurons of the porcine digestive tract in physiological conditions and under intoxication with bisphenol A (BPA). Nitric Oxide 2018, 80, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Szymanska, K.; Gonkowski, S. Bisphenol A-Induced changes in the enteric nervous system of the porcine duodenum. Neurotoxicology 2018, 66, 78–86. [Google Scholar] [CrossRef]
- Czajkowska, M.; Rychlik, A.; Całka, J. Long-term treatment with naproxen changes the chemical coding of the porcine intramural duodenum neurons. Ann. Anat. 2020, 227, 151425. [Google Scholar] [CrossRef]
- Makowska, K. Chemically induced inflammation and nerve damage affect the distribution of vasoactive intestinal polypeptide-like immunoreactive (VIP-LI) nervous structures in the descending colon of the domestic pig. Neurogastroenterol. Motil. 2018, 30, e13439. [Google Scholar] [CrossRef]
- Bulc, M.; Palus, K.; Zielonka, Ł.; Gajecka, M.; Całka, J. Changes in expression of inhibitory substances in the intramural neurons of the stomach following streptozotocin-induced diabetes in the pig. World J. Gastroenterol. 2017, 23, 6088–6099. [Google Scholar] [CrossRef]
- Takahara, H.; Fujimura, M.; Taniguchi, S.; Hayashi, N.; Nakamura, T.; Fujimiya, M. Changes in serotonin levels and 5-HT receptor activity in duodenum of streptozotocin-diabetic rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 281, 798–808. [Google Scholar] [CrossRef]
- Rzap, D.; Czajkowska, M.; Calka, J. Neurochemical Plasticity of nNOS-, VIP- and CART-Immunoreactive Neurons Following Prolonged Acetylsalicylic Acid Supplementation in the Porcine Jejunum. Int. J. Mol. Sci. 2020, 21, 2157. [Google Scholar] [CrossRef]
- Kolgazi, M.; Uslu, U.; Yuksel, M.; Velioglu-Ogunc, A.; Ercan, F.; Alican, I. The role of cholinergic anti-inflammatory pathway in acetic acid-induced colonic inflammation in the rat. Chem. Biol. Interact. 2013, 205, 72–80. [Google Scholar] [CrossRef]
- Sandgren, K.; Lin, Z.; Fex Svenningsen, A.; Ekblad, E. Vasoactive intestinal peptide and nitric oxide promote survival of adult rat myenteric neurons in culture. J. Neurosci. Res. 2003, 72, 595–602. [Google Scholar] [CrossRef]
- Kaleczyc, J.; Klimczuk, M.; Franke-Radowiecka, A.; Sienkiewicz, W.; Majewski, M.; Łakomy, M. The distribution and chemical coding of intramural neurons supplying the porcine stomach—The study on normal pigs and on animals suffering from swine dysentery. Anat. Histol. Embryol. 2007, 36, 186–193. [Google Scholar] [CrossRef]
- Brancati, S.B.; Zádori, Z.S.; Németh, J.; Gyires, K. Substance P induces gastric mucosal protection at supraspinal level via increasing the level of endomorphin-2 in rats. Brain Res. Bull. 2013, 91, 38–45. [Google Scholar] [CrossRef]
- Ekblad, E. CART in the enteric nervous system. Peptides 2006, 27, 2024–2030. [Google Scholar] [CrossRef]
- Kasacka, I.; Piotrowska, Z. Evaluation of density and distribution of CART-immunoreactive structures in gastrointestinal tract of hypertensive rats. Biofactors 2012, 38, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Gonkowski, S.; Burlinski, P.; Szwajca, P.; Całka, J. Changes in cocaine- and amphetamine-regulated transcript-like immunoreactive (CART-LI) nerve structures of the porcine descending colon during proliferative enteropathy. Bull. Vet. Inst. Pulawy 2012, 56, 199–203. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bulc, M.; Całka, J.; Palus, K. Administration of Different Doses of Acrylamide Changed the Chemical Coding of Enteric Neurons in the Jejunum in Gilts. Int. J. Environ. Res. Public Health 2022, 19, 14514. https://doi.org/10.3390/ijerph192114514
Bulc M, Całka J, Palus K. Administration of Different Doses of Acrylamide Changed the Chemical Coding of Enteric Neurons in the Jejunum in Gilts. International Journal of Environmental Research and Public Health. 2022; 19(21):14514. https://doi.org/10.3390/ijerph192114514
Chicago/Turabian StyleBulc, Michał, Jarosław Całka, and Katarzyna Palus. 2022. "Administration of Different Doses of Acrylamide Changed the Chemical Coding of Enteric Neurons in the Jejunum in Gilts" International Journal of Environmental Research and Public Health 19, no. 21: 14514. https://doi.org/10.3390/ijerph192114514
APA StyleBulc, M., Całka, J., & Palus, K. (2022). Administration of Different Doses of Acrylamide Changed the Chemical Coding of Enteric Neurons in the Jejunum in Gilts. International Journal of Environmental Research and Public Health, 19(21), 14514. https://doi.org/10.3390/ijerph192114514




