Palliative Effect of Resveratrol against Nanosized Iron Oxide-Induced Oxidative Stress and Steroidogenesis-Related Genes Dysregulation in Testicular Tissue of Adult Male Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals and Experimental Design
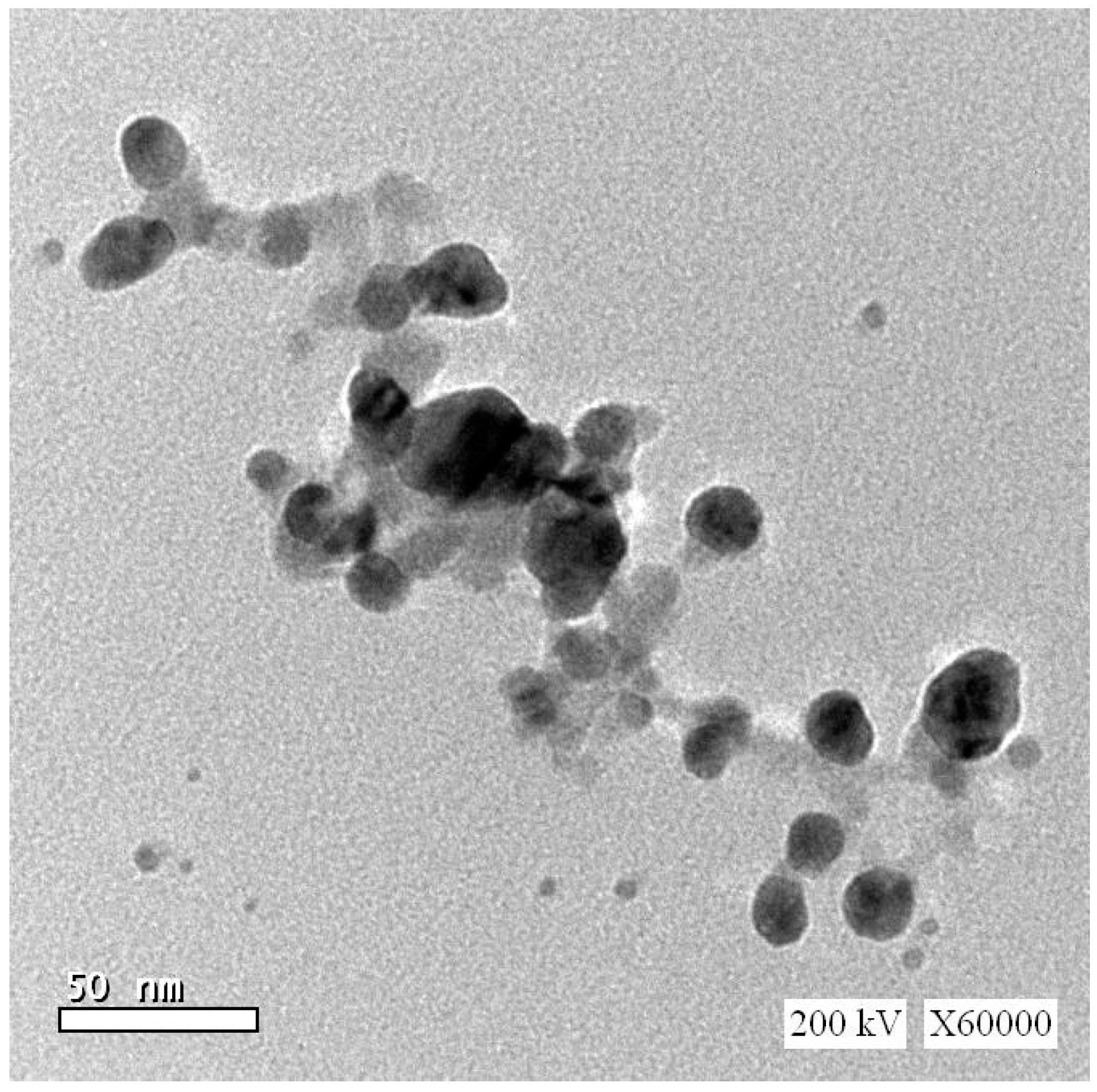
2.3. The Dose and Route Selection of RSV and Fe2O3-NPs
2.4. Blood and Tissue Sampling
2.5. Semen Analysis
2.6. Hormonal Assay
2.7. Testicular Oxidative/Antioxidant Status
2.8. Extraction of Total RNA and Real Time-PCR
2.9. Statistical Analysis
3. Results
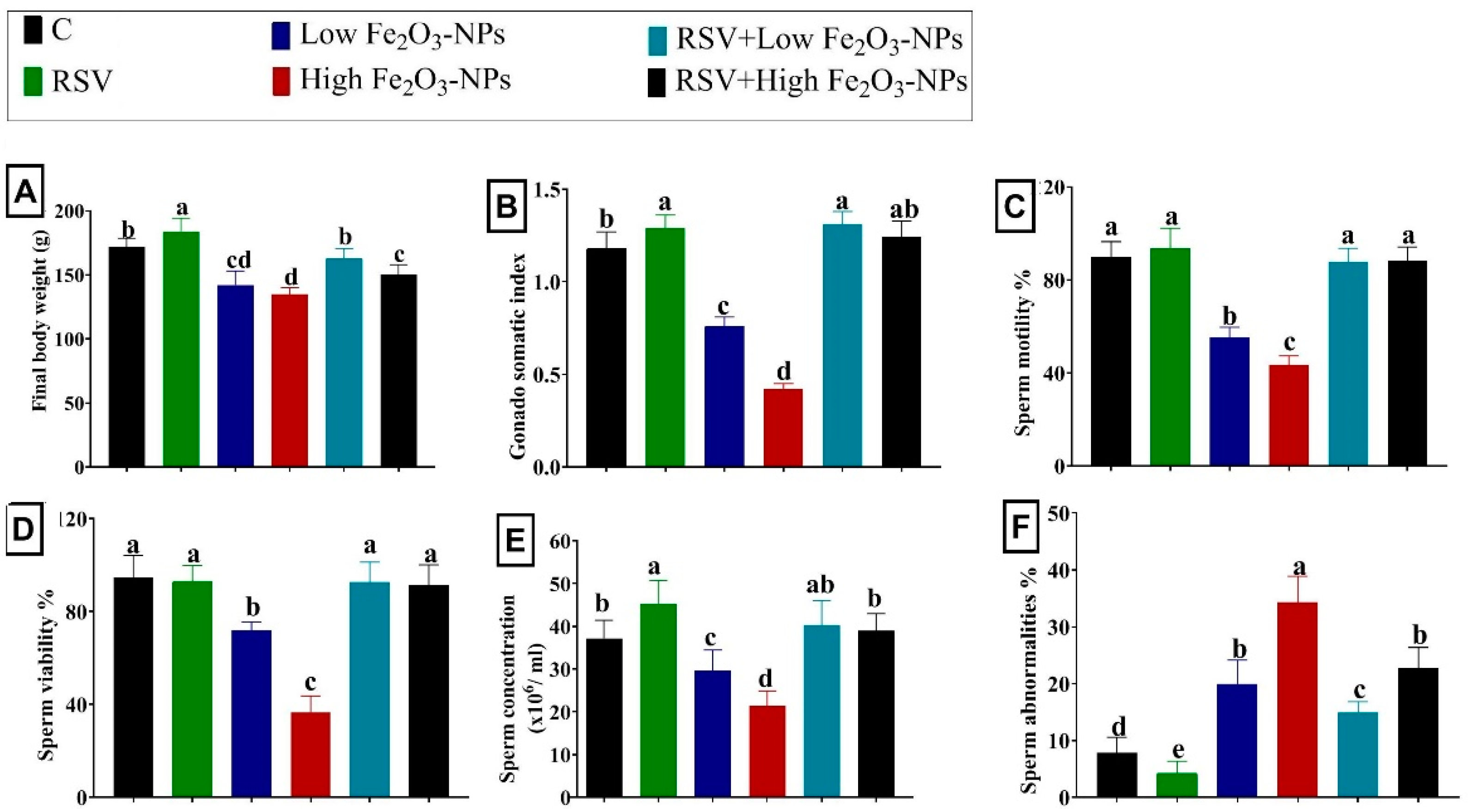
3.1. Effect of RSV and/or Fe2O3-NPs on the Bodyweight Change and Gonadosomatic Index
3.2. Effect of RSV and/or Fe2O3-NPs on Spermiogram
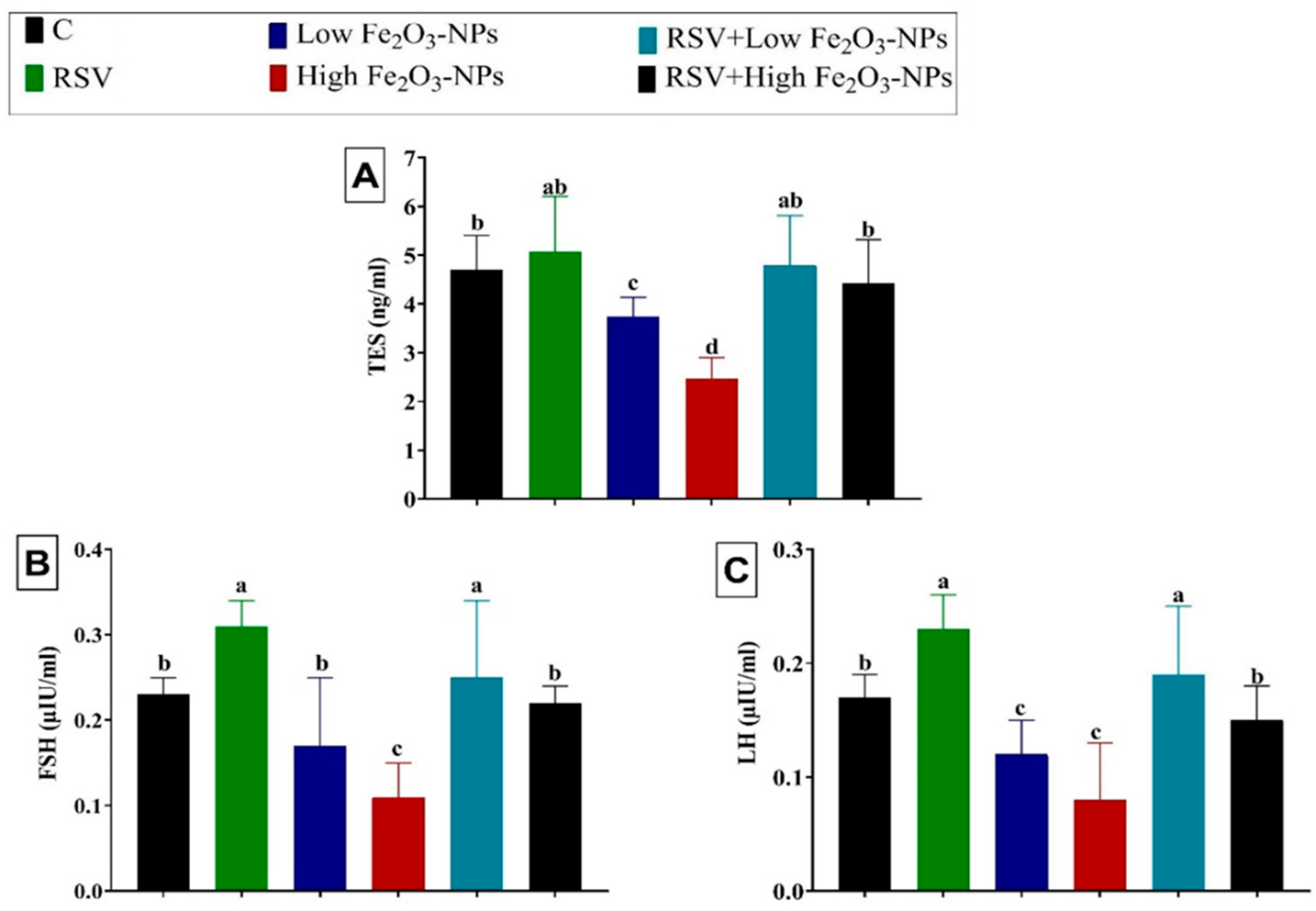
3.3. Effect of RSV and/or Fe2O3-NPs on Male Sex Hormones
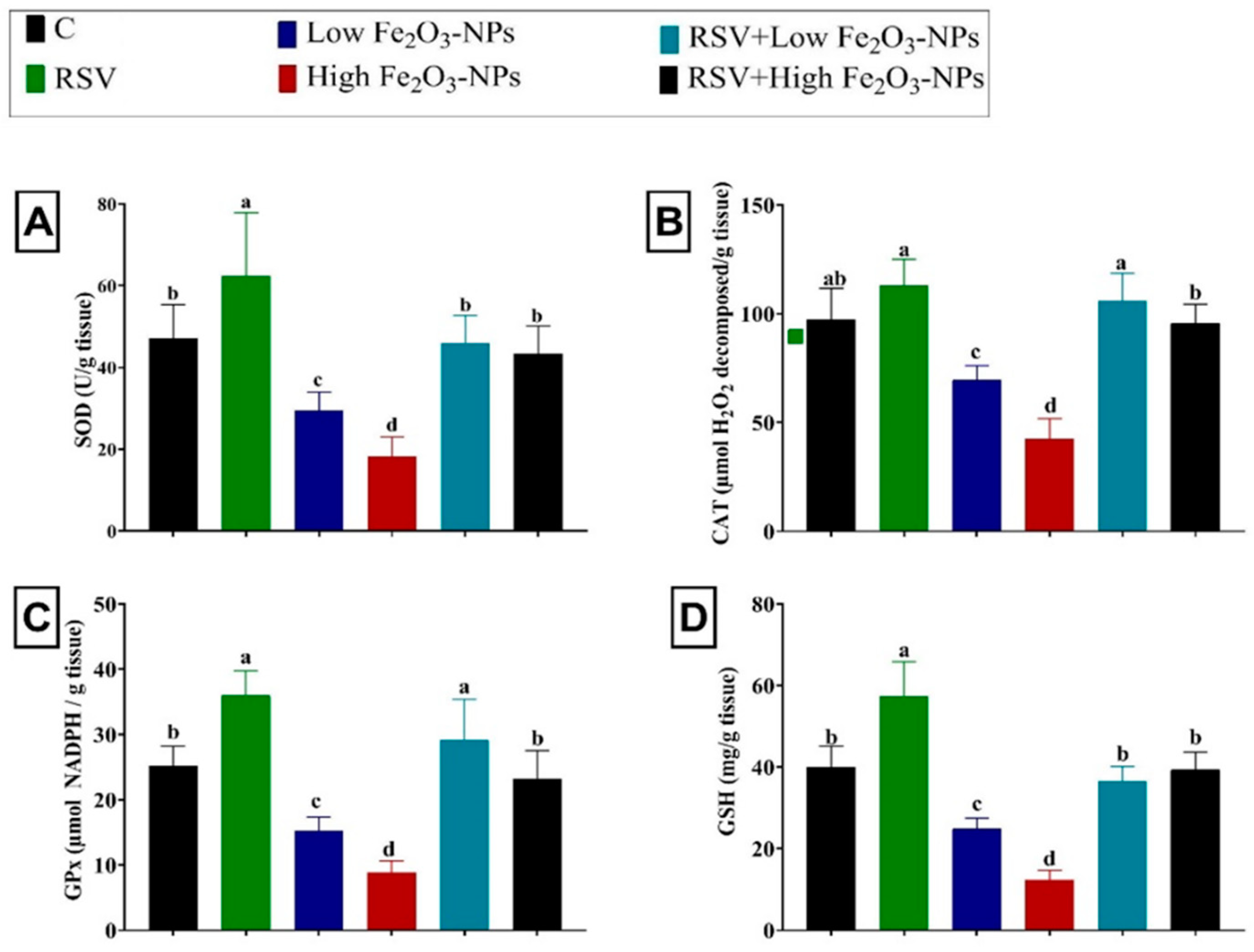
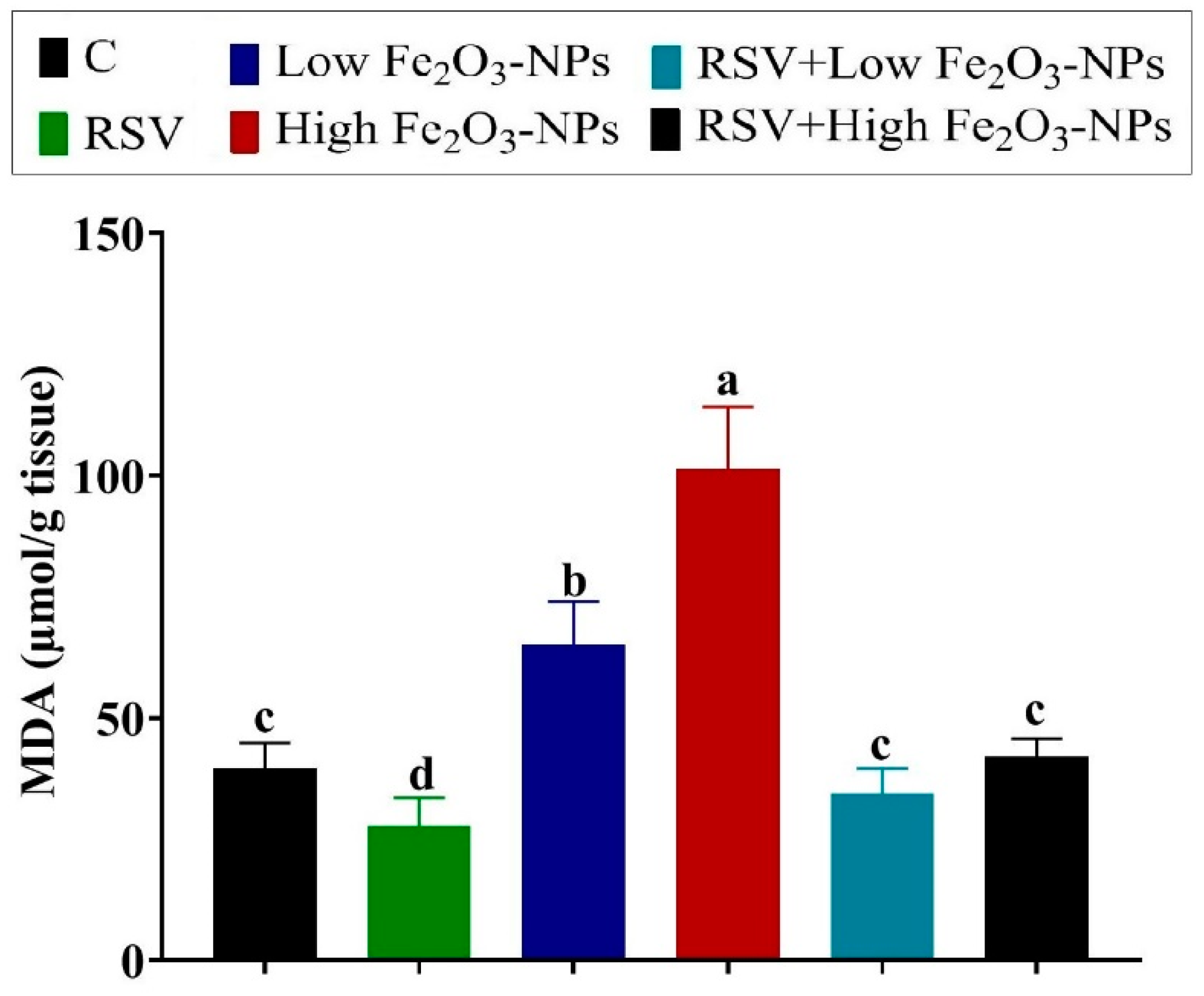
3.4. Effect of RSV and/or Fe2O3-NPs on Testicular Antioxidants and Lipid Peroxidation Level
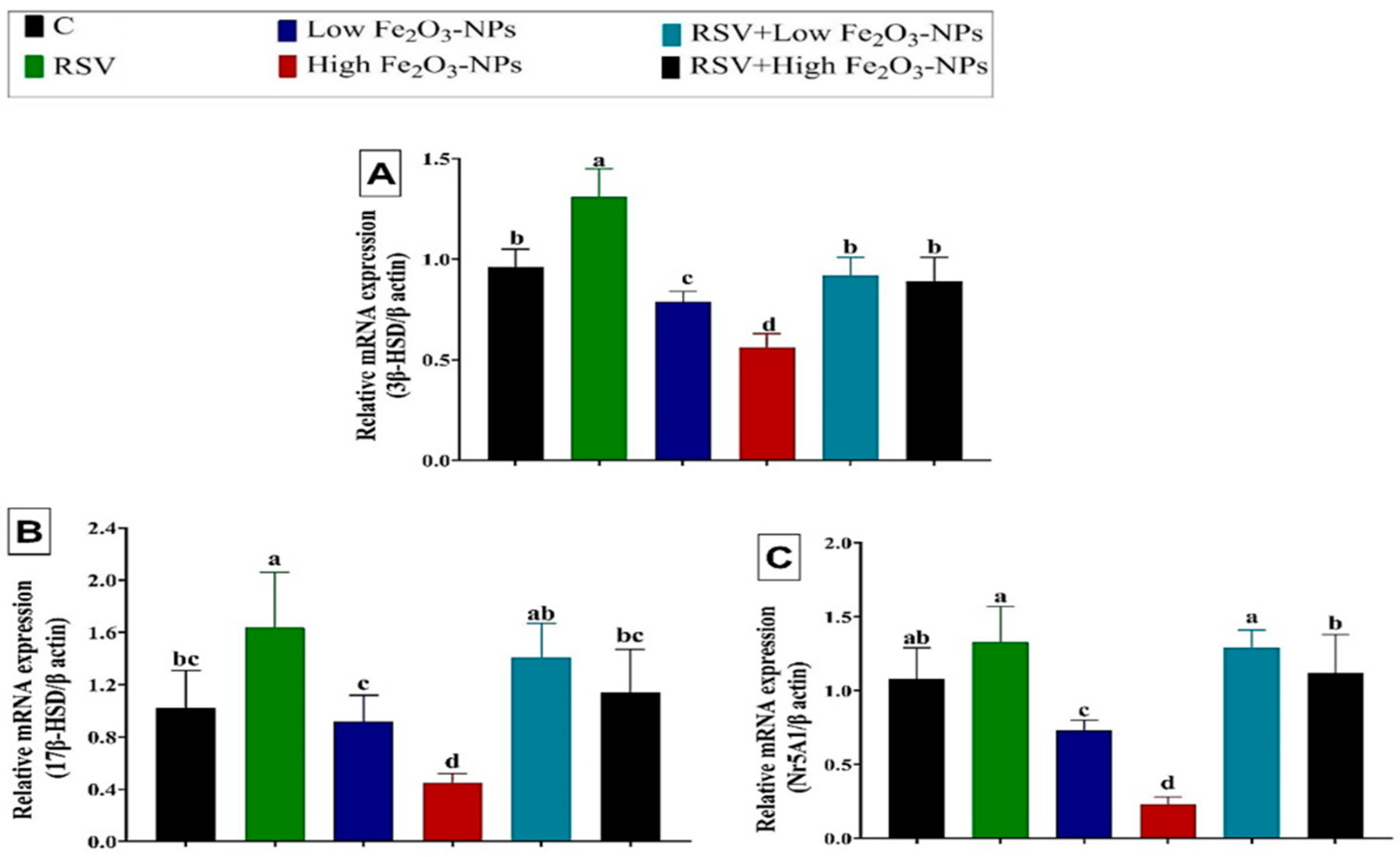
3.5. Effect of RSV and/or Fe2O3-NPs on Gene Expression in Testicular Tissue
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pyrgiotakis, G.; Vedantam, P.; Cirenza, C.; McDevitt, J.; Eleftheriadou, M.; Leonard, S.S.; Demokritou, P. Optimization of a nanotechnology based antimicrobial platform for food safety applications using Engineered Water Nanostructures (EWNS). Sci. Rep. 2016, 6, 21073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abd-Elhakim, Y.M.; Hashem, M.M.; Abo-EL-Sooud, K.; Hassan, B.A.; Elbohi, K.M.; Al-Sagheer, A.A. Effects of Co-Exposure of Nanoparticles and Metals on Different Organisms: A Review. Toxics 2021, 9, 284. [Google Scholar] [CrossRef]
- ECHA. European Union Observatory for Nanomaterial: Catalogue of Cosmetic Ingredients. 2019. Available online: https://euon.echa.europa.eu/catalogue-ofcosmetic-ingredients (accessed on 12 April 2022).
- Häfeli, U.O.; Riffle, J.S.; Harris-Shekhawat, L.; Carmichael-Baranauskas, A.; Mark, F.; Dailey, J.P.; Bardenstein, D. Cell uptake and in vitro toxicity of magnetic nanoparticles suitable for drug delivery. Mol. Pharm. 2009, 6, 1417–1428. [Google Scholar] [CrossRef] [PubMed]
- Gonzales-Weimuller, M.; Zeisberger, M.; Krishnan, K.M. Size-dependant heating rates of iron oxide nanoparticles for magnetic fluid hyperthermia. J. Magn. Magn. Mater. 2009, 321, 1947–1950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, J.; Huang, J.; Li, X.; Sun, S.; Chen, X. Iron oxide nanoparticle platform for biomedical applications. Curr. Med. Chem. 2009, 16, 1278–1294. [Google Scholar] [CrossRef] [PubMed]
- Lobel, B.; Eyal, O.; Kariv, N.; Katzir, A. Temperature controlled CO2 laser welding of soft tissues: Urinary bladder welding in different animal models (rats, rabbits, and cats). Lasers Surg. Med. Off. J. Am. Soc. Laser Med. Surg. 2000, 26, 4–12. [Google Scholar] [CrossRef]
- Naqvi, S.; Samim, M.; Abdin, M.; Ahmed, F.J.; Maitra, A.; Prashant, C.; Dinda, A.K. Concentration-dependent toxicity of iron oxide nanoparticles mediated by increased oxidative stress. Int. J. Nanomed. 2010, 5, 983. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.P.; Rahman, M.F.; Murty, U.S.N.; Mahboob, M.; Grover, P. Comparative study of genotoxicity and tissue distribution of nano and micron sized iron oxide in rats after acute oral treatment. Toxicol. Appl. Pharmacol. 2013, 266, 56–66. [Google Scholar] [CrossRef]
- Sadeghi, L.; Espanani, H. Toxic effects of the Fe2O3 nanoparticles on the liver and lung tissue. Bratisl. Lek. Listy 2015, 116, 373–378. [Google Scholar] [CrossRef]
- Askri, D.; Ouni, S.; Galai, S.; Chovelon, B.; Arnaud, J.; Lehmann, S.G.; Sakly, M.; Seve, M.; Amara, S. Sub-acute intravenous exposure to Fe2O3 nanoparticles does not alter cognitive performances and catecholamine levels, but slightly disrupts plasma iron level and brain iron content in rats. J. Trace Elem. Med. Biol. 2018, 50, 73–79. [Google Scholar] [CrossRef]
- Hadrup, N.; Saber, A.T.; Kyjovska, Z.O.; Jacobsen, N.R.; Vippola, M.; Sarlin, E.; Ding, Y.; Schmid, O.; Wallin, H.; Jensen, K.A. Pulmonary toxicity of Fe2O3, ZnFe2O4, NiFe2O4 and NiZnFe4O8 nanomaterials: Inflammation and DNA strand breaks. Environ. Toxicol. Pharmacol. 2020, 74, 103303. [Google Scholar] [CrossRef]
- Gaharwar, U.S.; Meena, R.; Rajamani, P. Iron oxide nanoparticles induced cytotoxicity, oxidative stress and DNA damage in lymphocytes. J. Appl. Toxicol. 2017, 37, 1232–1244. [Google Scholar] [CrossRef] [PubMed]
- Yarjanli, Z.; Ghaedi, K.; Esmaeili, A.; Rahgozar, S.; Zarrabi, A. Iron oxide nanoparticles may damage to the neural tissue through iron accumulation, oxidative stress, and protein aggregation. BMC Neurosci. 2017, 18, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundarraj, K.; Manickam, V.; Raghunath, A.; Periyasamy, M.; Viswanathan, M.P.; Perumal, E. Repeated exposure to iron oxide nanoparticles causes testicular toxicity in mice. Environ. Toxicol. 2017, 32, 594–608. [Google Scholar] [CrossRef] [PubMed]
- Dora, M.F.; Taha, N.M.; Lebda, M.A.; Hashem, A.E.; Elfeky, M.S.; El-Sayed, Y.S.; Jaouni, S.A.; El-Far, A.H. Quercetin Attenuates Brain Oxidative Alterations Induced by Iron Oxide Nanoparticles in Rats. Int. J. Mol. Sci. 2021, 22, 3829. [Google Scholar] [CrossRef]
- Sundarraj, K.; Raghunath, A.; Panneerselvam, L.; Perumal, E. Iron oxide nanoparticles modulate heat shock proteins and organ specific markers expression in mice male accessory organs. Toxicol. Appl. Pharmacol. 2017, 317, 12–24. [Google Scholar] [CrossRef]
- Mirzaei Varzeghani, S.; Parivar, K.; Abdollahifar, M.-A.; Karamian, A. Effects of Iron Oxide Nanoparticles on Mouse Sperm Parameters and Testicular Tissue. Iran. J. Toxicol. 2018, 12, 39–44. [Google Scholar] [CrossRef]
- Abd-Elhakim, Y.M.; Ghoneim, M.H.; Ebraheim, L.L.; Imam, T.S. Taurine and hesperidin rescues carbon tetrachloride-triggered testicular and kidney damage in rat via modulating oxidative stress and inflammation. Life Sci. 2020, 254, 117782. [Google Scholar] [CrossRef]
- Abd El-Hakim, Y.M.; Mohamed, W.A.; El-Metwally, A.E. Spirulina platensis attenuates furan reprotoxicity by regulating oxidative stress, inflammation, and apoptosis in testis of rats. Ecotoxicol. Environ. Saf. 2018, 161, 25–33. [Google Scholar] [CrossRef]
- Behairy, A.; El-Sharkawy, N.I.; Saber, T.M.; Soliman, M.M.; Metwally, M.M.M.; Abd El-Rahman, G.I.; Abd-Elhakim, Y.M.; El Deib, M.M. The Modulatory Role of Vitamin C in Boldenone Undecylenate Induced Testicular Oxidative Damage and Androgen Receptor Dysregulation in Adult Male Rats. Antioxidants 2020, 9, 1053. [Google Scholar] [CrossRef]
- Sahoo, D.K.; Roy, A.; Chainy, G.B. Protective effects of vitamin E and curcumin on L-thyroxine-induced rat testicular oxidative stress. Chem. Biol. Interact. 2008, 176, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Elewa, Y.H.A.; Mohamed, A.A.-R.; Galal, A.A.A.; El-naseery, N.I.; Ichii, O.; Kon, Y. Food Yellow4 reprotoxicity in relation to localization of DMC1 and apoptosis in rat testes: Roles of royal jelly and cod liver oil. Ecotoxicol. Environ. Saf. 2019, 169, 696–706. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef] [Green Version]
- Oh, W.Y.; Shahidi, F. Antioxidant activity of resveratrol ester derivatives in food and biological model systems. Food Chem. 2018, 261, 267–273. [Google Scholar] [CrossRef]
- Nie, Z.; Zhang, L.; Chen, W.; Zhang, Y.; Hua, R.; Wang, W.; Zhang, T.; Wu, H. The protective effects of pretreatment with resveratrol in cyclophosphamide-induced rat ovarian granulosa cell injury: In vitro study. Reprod. Toxicol. 2020, 95, 66–74. [Google Scholar] [CrossRef]
- Lee, I.T.; Lin, C.-F.; Huang, Y.-L.; Chong, K.-Y.; Hsieh, M.-F.; Huang, T.-H.; Cheng, C.-Y. Protective mechanisms of resveratrol derivatives against TNF-α-induced inflammatory responses in rat mesangial cells. Cytokine 2019, 113, 380–392. [Google Scholar] [CrossRef]
- Pannu, N.; Bhatnagar, A. Resveratrol: From enhanced biosynthesis and bioavailability to multitargeting chronic diseases. Biomed. Pharmacother. 2019, 109, 2237–2251. [Google Scholar] [CrossRef] [PubMed]
- Carrizzo, A.; Forte, M.; Damato, A.; Trimarco, V.; Salzano, F.; Bartolo, M.; Maciag, A.; Puca, A.A.; Vecchione, C. Antioxidant effects of resveratrol in cardiovascular, cerebral and metabolic diseases. Food Chem. Toxicol. 2013, 61, 215–226. [Google Scholar] [CrossRef]
- AlBasher, G.; Abdel-Daim, M.M.; Almeer, R.; Ibrahim, K.A.; Hamza, R.Z.; Bungau, S.; Aleya, L. Synergistic antioxidant effects of resveratrol and curcumin against fipronil-triggered oxidative damage in male albino rats. Environ. Sci. Pollut. Res. 2020, 27, 6505–6514. [Google Scholar] [CrossRef]
- Xia, N.; Daiber, A.; Förstermann, U.; Li, H. Antioxidant effects of resveratrol in the cardiovascular system. Br. J. Pharmacol. 2017, 174, 1633–1646. [Google Scholar] [CrossRef] [Green Version]
- Filardo, S.; Di Pietro, M.; Mastromarino, P.; Sessa, R. Therapeutic potential of resveratrol against emerging respiratory viral infections. Pharmacol. Ther. 2020, 214, 107613. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.H.; Akter, R.; Bhattacharya, T.; Abdel-Daim, M.M.; Alkahtani, S.; Arafah, M.W.; Al-Johani, N.S.; Alhoshani, N.M.; Alkeraishan, N.; Alhenaky, A.; et al. Resveratrol and Neuroprotection: Impact and Its Therapeutic Potential in Alzheimer’s Disease. Front. Pharmacol. 2020, 11, 2272. [Google Scholar] [CrossRef]
- Rai, R.C.; Bagul, P.K.; Banerjee, S.K. NLRP3 inflammasome drives inflammation in high fructose fed diabetic rat liver: Effect of resveratrol and metformin. Life Sci. 2020, 253, 117727. [Google Scholar] [CrossRef]
- Buys-Gonçalves, G.F.; Sampaio, F.J.B.; Silva, M.E.M.; Pereira-Sampaio, M.A.; De Souza, D.B. Histomorphometric evaluation of the rat kidney submitted to warm ischemia and the protective effect of resveratrol. Am. J. Surg. 2020, 220, 1119–1123. [Google Scholar] [CrossRef] [PubMed]
- Mojica-Villegas, M.A.; Izquierdo-Vega, J.A.; Chamorro-Cevallos, G.; Sánchez-Gutiérrez, M. Protective effect of resveratrol on biomarkers of oxidative stress induced by iron/ascorbate in mouse spermatozoa. Nutrients 2014, 6, 489–503. [Google Scholar] [CrossRef]
- Bucak, M.; Ataman, M.; Başpınar, N.; Uysal, O.; Taşpınar, M.; Bilgili, A.; Öztürk, C.; Güngör, Ş.; Inanc, M.; Akal, E. Lycopene and resveratrol improve post-thaw bull sperm parameters: Sperm motility, mitochondrial activity and DNA integrity. Andrologia 2015, 47, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Collodel, G.; Federico, M.; Geminiani, M.; Martini, S.; Bonechi, C.; Rossi, C.; Figura, N.; Moretti, E. Effect of trans-resveratrol on induced oxidative stress in human sperm and in rat germinal cells. Reprod. Toxicol. 2011, 31, 239–246. [Google Scholar] [CrossRef]
- Adler, I.-D. Comparison of the duration of spermatogenesis between male rodents and humans. Mutat. Res. /Fundam. Mol. Mech. Mutagenesis 1996, 352, 169–172. [Google Scholar] [CrossRef]
- Eleawa, S.M.; Alkhateeb, M.A.; Alhashem, F.H.; Bin-Jaliah, I.; Sakr, H.F.; Elrefaey, H.M.; Elkarib, A.O.; Alessa, R.M.; Haidara, M.A.; Shatoor, A.S.; et al. Resveratrol reverses cadmium chloride-induced testicular damage and subfertility by downregulating p53 and Bax and upregulating gonadotropins and Bcl-2 gene expression. J. Reprod. Dev. 2014, 60, 115–127. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Huq, A.U.; Singh, R. Cypermethrin-induced reproductive toxicity in the rat is prevented by resveratrol. J. Hum. Reprod. Sci. 2014, 7, 99. [Google Scholar]
- Jiang, Y.-g.; Tao, P.; Yong, L.; Li, M.-c.; Lin, Y.-h. Resveratrol reestablishes spermatogenesis after testicular injury in rats caused by 2, 5-hexanedione. Chin. Med. J. 2008, 121, 1204–1209. [Google Scholar] [CrossRef] [PubMed]
- Juan, M.E.; Vinardell, M.P.; Planas, J.M. The daily oral administration of high doses of trans-resveratrol to rats for 28 days is not harmful. J. Nutr. 2002, 132, 257–260. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.S.; Yoon, T.-J.; Yu, K.N.; Kim, B.G.; Park, S.J.; Kim, H.W.; Lee, K.H.; Park, S.B.; Lee, J.-K.; Cho, M.H. Toxicity and tissue distribution of magnetic nanoparticles in mice. Toxicol. Sci. 2006, 89, 338–347. [Google Scholar] [CrossRef]
- Kumari, M.; Rajak, S.; Singh, S.P.; Kumari, S.I.; Kumar, P.U.; Murty, U.S.N.; Mahboob, M.; Grover, P.; Rahman, M.F. Repeated Oral Dose Toxicity of Iron Oxide Nanoparticles: Biochemical and Histopathological Alterations in Different Tissues of Rats. J. Nanosci. Nanotechnol. 2012, 12, 2149–2159. [Google Scholar] [CrossRef] [PubMed]
- Askri, D.; Cunin, V.; Ouni, S.; Béal, D.; Rachidi, W.; Sakly, M.; Amara, S.; Lehmann, S.G.; Sève, M. Effects of Iron Oxide Nanoparticles (γ-Fe2O3) on Liver, Lung and Brain Proteomes following Sub-Acute Intranasal Exposure: A New Toxicological Assessment in Rat Model Using iTRAQ-Based Quantitative Proteomics. Int. J. Mol. Sci. 2019, 20, 5186. [Google Scholar] [CrossRef] [Green Version]
- Dhakshinamoorthy, V.; Manickam, V.; Perumal, E. Neurobehavioural Toxicity of Iron Oxide Nanoparticles in Mice. Neurotox. Res. 2017, 32, 187–203. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Song, J.; Zhang, D.-S. Nanosized As2O3/Fe2O3 complexes combined with magnetic fluid hyperthermia selectively target liver cancer cells. World J. Gastroenterol. 2009, 15, 2995. [Google Scholar] [CrossRef]
- Sadeghiani, N.; Barbosa, L.; Silva, L.; Azevedo, R.; Morais, P.; Lacava, Z. Genotoxicity and inflammatory investigation in mice treated with magnetite nanoparticles surface coated with polyaspartic acid. J. Magn. Magn. Mater. 2005, 289, 466–468. [Google Scholar] [CrossRef]
- Pham, B.T.; Colvin, E.K.; Pham, N.T.; Kim, B.J.; Fuller, E.S.; Moon, E.A.; Barbey, R.; Yuen, S.; Rickman, B.H.; Bryce, N.S. Biodistribution and clearance of stable superparamagnetic maghemite iron oxide nanoparticles in mice following intraperitoneal administration. Int. J. Mol. Sci. 2018, 19, 205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prodan, A.M.; Iconaru, S.L.; Ciobanu, C.S.; Chifiriuc, M.C.; Stoicea, M.; Predoi, D. Iron Oxide Magnetic Nanoparticles: Characterization and Toxicity Evaluation by In Vitro and In Vivo Assays. J. Nanomater. 2013, 2013, 587021. [Google Scholar] [CrossRef] [Green Version]
- Adebayo, A.; Oke, B.; Akinloye, A. Characterizing the gonadosomatic index and its relationship with age in greater cane rat (Thryonomys swinderianus, Temminck). J. Vet. Anat. 2009, 2, 53–59. [Google Scholar] [CrossRef] [Green Version]
- Slott, V.L.; Suarez, J.D.; Perreault, S.D. Rat sperm motility analysis: Methodologic considerations. Reprod. Toxicol. 1991, 5, 449–458. [Google Scholar] [CrossRef]
- Mailankot, M.; Kunnath, A.P.; Jayalekshmi, H.; Koduru, B.; Valsalan, R. Radio frequency electromagnetic radiation (RF-EMR) from GSM (0.9/1.8 GHz) mobile phones induces oxidative stress and reduces sperm motility in rats. Clinics 2009, 64, 561–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filler, R. Methods for evaluation of rat epididymal sperm morphology. In Methods in Toxicology: Male Reproductive Toxicology; Chapin, R.E., Heildel, J.J., Eds.; Elsevier: Amsterdam, The Netherlands, 1993; pp. 334–343. [Google Scholar]
- Nishikimi, M.; Rao, N.A.; Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972, 46, 849–854. [Google Scholar] [CrossRef]
- Aebi, H. [13] Catalase in vitro. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar]
- Beutler, E. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Hussein, M.M. Neurotoxic effects of silver nanoparticles and the protective role of rutin. Biomed. Pharmacother. 2017, 90, 731–739. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Shahare, B.; Yashpal, M.; Gajendra. Toxic effects of repeated oral exposure of silver nanoparticles on small intestine mucosa of mice. Toxicol. Mech. Methods 2013, 23, 161–167. [Google Scholar] [CrossRef]
- Xu, J.; Shi, H.; Ruth, M.; Yu, H.; Lazar, L.; Zou, B.; Yang, C.; Wu, A.; Zhao, J. Acute toxicity of intravenously administered titanium dioxide nanoparticles in mice. PLoS ONE 2013, 8, e70618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birkebaek, N.H.; Lange, A.; Holland-Fischer, P.; Kristensen, K.; Rittig, S.; Vilstrup, H.; Handberg, A.; Gronbaek, H. Effect of weight reduction on insulin sensitivity, sex hormone-binding globulin, sex hormones and gonadotrophins in obese children. Eur. J. Endocrinol. 2010, 163, 895–900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kilarkaje, N.; Al-hussaini, H.; Al-Bader, M. Diabetes-induced DNA damage and apoptosis are associated with poly (ADP ribose) polymerase 1 inhibition in the rat testis. Eur. J. Pharmacol. 2014, 737, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Faid, I.; Al-Hussaini, H.; Kilarkaje, N. Resveratrol alleviates diabetes-induced testicular dysfunction by inhibiting oxidative stress and c-Jun N-terminal kinase signaling in rats. Toxicol. Appl. Pharmacol. 2015, 289, 482–494. [Google Scholar] [CrossRef]
- Kumar, N.; Singh, A.K. Impact of environmental factors on human semen quality and male fertility: A narrative review. Environ. Sci. Eur. 2022, 34, 6. [Google Scholar] [CrossRef]
- Sarkar, M.; Gangopadhyay, P.; Basak, B.; Chakrabarty, K.; Banerji, J.; Adhikary, P.; Chatterjee, A. The reversible antifertility effect of Piper betle Linn. on Swiss albino male mice. Contraception 2000, 62, 271–274. [Google Scholar] [CrossRef]
- Gonzales, G.; Kortebani, G.; Mazzolli, A. Hyperviscosity and hypofunction of the seminal vesicles. Arch. Androl. 1993, 30, 63–68. [Google Scholar] [CrossRef]
- Chirumbolo, S. Resveratrol in spermatogenesis. Cell Biol. Int. 2015, 39, 775–776. [Google Scholar] [CrossRef]
- Li, E.; Guo, Y.; Wang, G.; Chen, F.; Li, Q. Effect of resveratrol on restoring spermatogenesis in experimental cryptorchid mice and analysis of related differentially expressed proteins. Cell Biol. Int. 2015, 39, 733–740. [Google Scholar] [CrossRef]
- Banerjee, B.; Nandi, P.; Chakraborty, S.; Raha, S.; Sen, P.C.; Jana, K. Resveratrol ameliorates benzo (a) pyrene-induced testicular dysfunction and apoptosis: Involvement of p38 MAPK/ATF2/iNOS signaling. J. Nutr. Biochem. 2016, 34, 17–29. [Google Scholar] [CrossRef]
- Türedi, S.; Yuluğ, E.; Alver, A.; Kutlu, Ö.; Kahraman, C. Effects of resveratrol on doxorubicin induced testicular damage in rats. Exp. Toxicol. Pathol. 2015, 67, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.A.; El Bohy, K.M.; El Sharkawy, N.I.; Imam, T.S.; El-Metwally, A.E.; Hamed Arisha, A.; Mohammed, H.A.; Abd-Elhakim, Y.M. Iprodione and chlorpyrifos induce testicular damage, oxidative stress, apoptosis and suppression of steroidogenic-and spermatogenic-related genes in immature male albino rats. Andrologia 2021, 53, e13978. [Google Scholar] [CrossRef] [PubMed]
- Chainy, G.B.N.; Sahoo, D.K. Hormones and oxidative stress: An overview. Free Radic. Res. 2020, 54, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, D.K.; Jena, S.; Chainy, G.B.N. Chapter 2.7—Thyroid Dysfunction and Testicular Redox Status: An Intriguing Association. In Oxidants, Antioxidants and Impact of the Oxidative Status in Male Reproduction; Henkel, R., Samanta, L., Agarwal, A., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 149–170. [Google Scholar]
- Negahdary, M.; Arefian, Z.; Dastjerdi, H.A.; Ajdary, M. Toxic effects of Mn2O3 nanoparticles on rat testis and sex hormone. J. Nat. Sci. Biol. Med. 2015, 6, 335. [Google Scholar]
- Juan, M.E.; Gonzalez-Pons, E.; Munuera, T.; Ballester, J.; Rodríguez-Gil, J.E.; Planas, J.M. trans-Resveratrol, a natural antioxidant from grapes, increases sperm output in healthy rats. J. Nutr. 2005, 135, 757–760. [Google Scholar] [CrossRef]
- Abou-Zeid, L.A.; El-Mowafy, A.M. Differential recognition of resveratrol isomers by the human estrogen receptor-α: Molecular dynamics evidence for stereoselective ligand binding. Chirality 2004, 16, 190–195. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Hofmann, H.; Rothen-Rutishauser, B.; Petri-Fink, A. Assessing the in vitro and in vivo toxicity of superparamagnetic iron oxide nanoparticles. Chem. Rev. 2012, 112, 2323–2338. [Google Scholar] [CrossRef] [Green Version]
- Jurczuk, M.; Brzóska, M.M.; Moniuszko-Jakoniuk, J.; Gałażyn-Sidorczuk, M.; Kulikowska-Karpińska, E. Antioxidant enzymes activity and lipid peroxidation in liver and kidney of rats exposed to cadmium and ethanol. Food Chem. Toxicol. 2004, 42, 429–438. [Google Scholar] [CrossRef]
- Abarikwu, S.O.; Adesiyan, A.C.; Oyeloja, T.O.; Oyeyemi, M.O.; Farombi, E.O. Changes in sperm characteristics and induction of oxidative stress in the testis and epididymis of experimental rats by a herbicide, atrazine. Arch. Environ. Contam. Toxicol. 2010, 58, 874–882. [Google Scholar] [CrossRef]
- Mittra, E.S.; Goris, M.L.; Iagaru, A.H.; Kardan, A.; Burton, L.; Berganos, R.; Chang, E.; Liu, S.; Shen, B.; Chin, F.T. Pilot pharmacokinetic and dosimetric studies of 18F-FPPRGD2: A PET radiopharmaceutical agent for imaging αvβ3 integrin levels. Radiology 2011, 260, 182–191. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.J.; Choi, J.S.; Lee, H.J.; Kim, W.-H.; Park, S.I.; Song, J. Effect of excess iron on oxidative stress and gluconeogenesis through hepcidin during mitochondrial dysfunction. J. Nutr. Biochem. 2015, 26, 1414–1423. [Google Scholar] [CrossRef]
- Galaris, D.; Barbouti, A.; Pantopoulos, K. Iron homeostasis and oxidative stress: An intimate relationship. Biochim. Biophys. Acta BBA Mol. Cell Res. 2019, 1866, 118535. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Roman, S.D. Antioxidant systems and oxidative stress in the testes. In Molecular Mechanisms in Spermatogenesis; Springer: Berlin/Heidelberg, Germany, 2009; pp. 154–171. [Google Scholar]
- Li, H.; Xia, N.; Förstermann, U. Cardiovascular effects and molecular targets of resveratrol. Nitric Oxide 2012, 26, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Stivala, L.A.; Savio, M.; Carafoli, F.; Perucca, P.; Bianchi, L.; Maga, G.; Forti, L.; Pagnoni, U.M.; Albini, A.; Prosperi, E. Specific structural determinants are responsible for the antioxidant activity and the cell cycle effects of resveratrol. J. Biol. Chem. 2001, 276, 22586–22594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szekeres, T.; Fritzer-Szekeres, M.; Saiko, P.; Jäger, W. Resveratrol and resveratrol analogues—Structure—Activity relationship. Pharm. Res. 2010, 27, 1042–1048. [Google Scholar] [CrossRef]
- Iuga, C.; Alvarez-Idaboy, J.R.l.; Russo, N. Antioxidant activity of trans-resveratrol toward hydroxyl and hydroperoxyl radicals: A quantum chemical and computation al kinetics study. J. Org. Chem. 2012, 77, 3868–3877. [Google Scholar] [CrossRef]
- Xia, N.; Forstermann, U.; Li, H. Resveratrol as a gene regulator in the vasculature. Curr. Pharm. Biotechnol. 2014, 15, 401–408. [Google Scholar] [CrossRef]
- Jia, Z.; Zhu, H.; Misra, B.R.; Mahaney, J.E.; Li, Y.; Misra, H.P. EPR studies on the superoxide-scavenging capacity of the nutraceutical resveratrol. Mol. Cell. Biochem. 2008, 313, 187–194. [Google Scholar] [CrossRef]
- Ungvari, Z.; Labinskyy, N.; Mukhopadhyay, P.; Pinto, J.T.; Bagi, Z.; Ballabh, P.; Zhang, C.; Pacher, P.; Csiszar, A. Resveratrol attenuates mitochondrial oxidative stress in coronary arterial endothelial cells. Am. J. Physiol.—Heart Circ. Physiol. 2009, 297, H1876–H1881. [Google Scholar] [CrossRef] [Green Version]
- Bremer, A.; Miller, W. Chapter 13-Regulation of Steroidogenesis. In Cellular Endocrinology in Health and Disease; Elsevier: Amsterdam, The Netherlands, 2014; pp. 207–227. [Google Scholar]
- Abd-Elhakim, Y.M.; El Sharkawy, N.I.; El Bohy, K.M.; Hassan, M.A.; Gharib, H.S.; El-Metwally, A.E.; Arisha, A.H.; Imam, T.S. Iprodione and/or chlorpyrifos exposure induced testicular toxicity in adult rats by suppression of steroidogenic genes and SIRT1/TERT/PGC-1α pathway. Environ. Sci. Pollut. Res. 2021, 28, 56491–56506. [Google Scholar] [CrossRef]
- Abd El-Hakim, Y.M.; Abdel-Rahman Mohamed, A.; Khater, S.I.; Hamed Arisha, A.; Metwally, M.M.; Nassan, M.A.; Hassan, M.E. Chitosan-stabilized selenium nanoparticles and metformin synergistically rescue testicular oxidative damage and steroidogenesis-related genes dysregulation in high-fat diet/streptozotocin-induced diabetic rats. Antioxidants 2020, 10, 17. [Google Scholar] [CrossRef]
- Qin, F.; Shen, T.; Li, J.; Qian, J.; Zhang, J.; Zhou, G.; Tong, J. SF-1 mediates reproductive toxicity induced by Cerium oxide nanoparticles in male mice. J. Nanobiotechnol. 2019, 17, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Vassal, M.; Rebelo, S.; Pereira, M.d.L. Metal Oxide Nanoparticles: Evidence of Adverse Effects on the Male Reproductive System. Int. J. Mol. Sci. 2021, 22, 8061. [Google Scholar] [CrossRef]
- Hussein, M.M.; Ali, H.A.; Saadeldin, I.M.; Ahmed, M.M. Querectin alleviates zinc oxide nanoreprotoxicity in male albino rats. J. Biochem. Mol. Toxicol. 2016, 30, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.Y.; Mokhtar, I.Y.; Maher, A.-N.; Rakhad, A.; Jubran, M.A. Changes in semen characteristics and sex hormones of rats treated with iron oxide nanoparticles, silver nanoparticles and their mixture. GSC Biol. Pharm. Sci. 2020, 12, 229–237. [Google Scholar] [CrossRef]
- Premalatha, R.; Jubendradass, R.; Rani, S.J.A.; Srikumar, K.; Mathur, P.P. A phytooxysterol, 28-homobrassinolide modulates rat testicular steroidogenesis in normal and diabetic rats. Reprod. Sci. 2013, 20, 589–596. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.-Y.; Gong, E.-Y.; Hong, C.Y.; Kim, K.-H.; Han, J.-S.; Ryu, J.C.; Chae, H.Z.; Yun, C.-H.; Lee, K. ROS inhibit the expression of testicular steroidogenic enzyme genes via the suppression of Nur77 transactivation. Free Radic. Biol. Med. 2009, 47, 1591–1600. [Google Scholar] [CrossRef]
- Tsai, S.C.; Lu, C.C.; Lin, C.S.; Wang, P.S. Antisteroidogenic actions of hydrogen peroxide on rat Leydig cells. J. Cell. Biochem. 2003, 90, 1276–1286. [Google Scholar] [CrossRef]
- Stocco, D.M.; Wells, J.; Clark, B.J. The effects of hydrogen peroxide on steroidogenesis in mouse Leydig tumor cells. Endocrinology 1993, 133, 2827–2832. [Google Scholar] [CrossRef]
- Turner, T.T.; Lysiak, J.J. Oxidative stress: A common factor in testicular dysfunction. J. Androl. 2008, 29, 488–498. [Google Scholar] [CrossRef] [Green Version]
- Abidi, P.; Zhang, H.; Zaidi, S.M.; Shen, W.-J.; Leers-Sucheta, S.; Cortez, Y.; Han, J.; Azhar, S. Oxidative stress-induced inhibition of adrenal steroidogenesis requires participation of p38 mitogen-activated protein kinase signaling pathway. J. Endocrinol. 2008, 198, 193–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peltola, V.; Huhtaniemi, I.; Metsa-Ketela, T.; Ahotupa, M. Induction of lipid peroxidation during steroidogenesis in the rat testis. Endocrinology 1996, 137, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Abarikwu, S.; Duru, Q.; Chinonso, O.; Njoku, R.C. Antioxidant enzymes activity, lipid peroxidation, oxidative damage in the testis and epididymis, and steroidogenesis in rats after co-exposure to atrazine and ethanol. Andrologia 2016, 48, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, B.; Chakraborty, S.; Chakraborty, P.; Ghosh, D.; Jana, K. Protective Effect of Resveratrol on Benzo(a)Pyrene Induced Dysfunctions of Steroidogenesis and Steroidogenic Acute Regulatory Gene Expression in Leydig Cells. Front. Endocrinol. 2019, 10, 272. [Google Scholar] [CrossRef] [PubMed]
| Gene | Primer Sequences | Reaction Conditions | Accession No. | PCR Product Size | |
|---|---|---|---|---|---|
| 3β-HSD | F | 5′–GCATTAACCCCACTCCCACT–3′ | 95 °C, 10 min/60 °C, 30 s/72 °C, 5 min (35 cycles) | NM 017265 | 146 bp |
| R | 5′–GGACCCTGACCTCCTTCAGA–3′ | ||||
| 17β-HSD | F | 5′–GTGTGCACATTTTCCAAGGC–3′ | 95 °C, 10 min/60 °C, 30 s/72 °C, 5 min (35 cycles) | NM 054007 | 144 bp |
| R | 5′–TTTAACAAACTCATCGGCGG–3′ | ||||
| Nr5A1 | F | 5′–CGCCAGGAGTTTGTCTGTCT–3′ | 95 °C, 10 min/60 °C, 30 s/72 °C, 5 min (35 cycles) | NM 001191099 | 185 bp |
| R | 5′–ACCTCCACCAGGCACAATAG–3′ | ||||
| β-actin | F | 5′–CCTGCTTGCTGATCCACA–3′ | 95 °C, 10 min/60 °C, 30 s/72 °C, 5 min (35 cycles) | V01217 | 97 bp |
| R | 5′–CTGACCGAGCGTGGCTAC–3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, M.M.; Hussein, M.M.A.; Saber, T.; Abd-Elhakim, Y.M. Palliative Effect of Resveratrol against Nanosized Iron Oxide-Induced Oxidative Stress and Steroidogenesis-Related Genes Dysregulation in Testicular Tissue of Adult Male Rats. Int. J. Environ. Res. Public Health 2022, 19, 8171. https://doi.org/10.3390/ijerph19138171
Ahmed MM, Hussein MMA, Saber T, Abd-Elhakim YM. Palliative Effect of Resveratrol against Nanosized Iron Oxide-Induced Oxidative Stress and Steroidogenesis-Related Genes Dysregulation in Testicular Tissue of Adult Male Rats. International Journal of Environmental Research and Public Health. 2022; 19(13):8171. https://doi.org/10.3390/ijerph19138171
Chicago/Turabian StyleAhmed, Mona M., Mohamed M. A. Hussein, Taisir Saber, and Yasmina M. Abd-Elhakim. 2022. "Palliative Effect of Resveratrol against Nanosized Iron Oxide-Induced Oxidative Stress and Steroidogenesis-Related Genes Dysregulation in Testicular Tissue of Adult Male Rats" International Journal of Environmental Research and Public Health 19, no. 13: 8171. https://doi.org/10.3390/ijerph19138171
APA StyleAhmed, M. M., Hussein, M. M. A., Saber, T., & Abd-Elhakim, Y. M. (2022). Palliative Effect of Resveratrol against Nanosized Iron Oxide-Induced Oxidative Stress and Steroidogenesis-Related Genes Dysregulation in Testicular Tissue of Adult Male Rats. International Journal of Environmental Research and Public Health, 19(13), 8171. https://doi.org/10.3390/ijerph19138171








