Prepregnancy Protein Source and BCAA Intake Are Associated with Gestational Diabetes Mellitus in the CARDIA Study
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Data Collection Instruments
2.3. Outcome Definition and Statistical Analysis
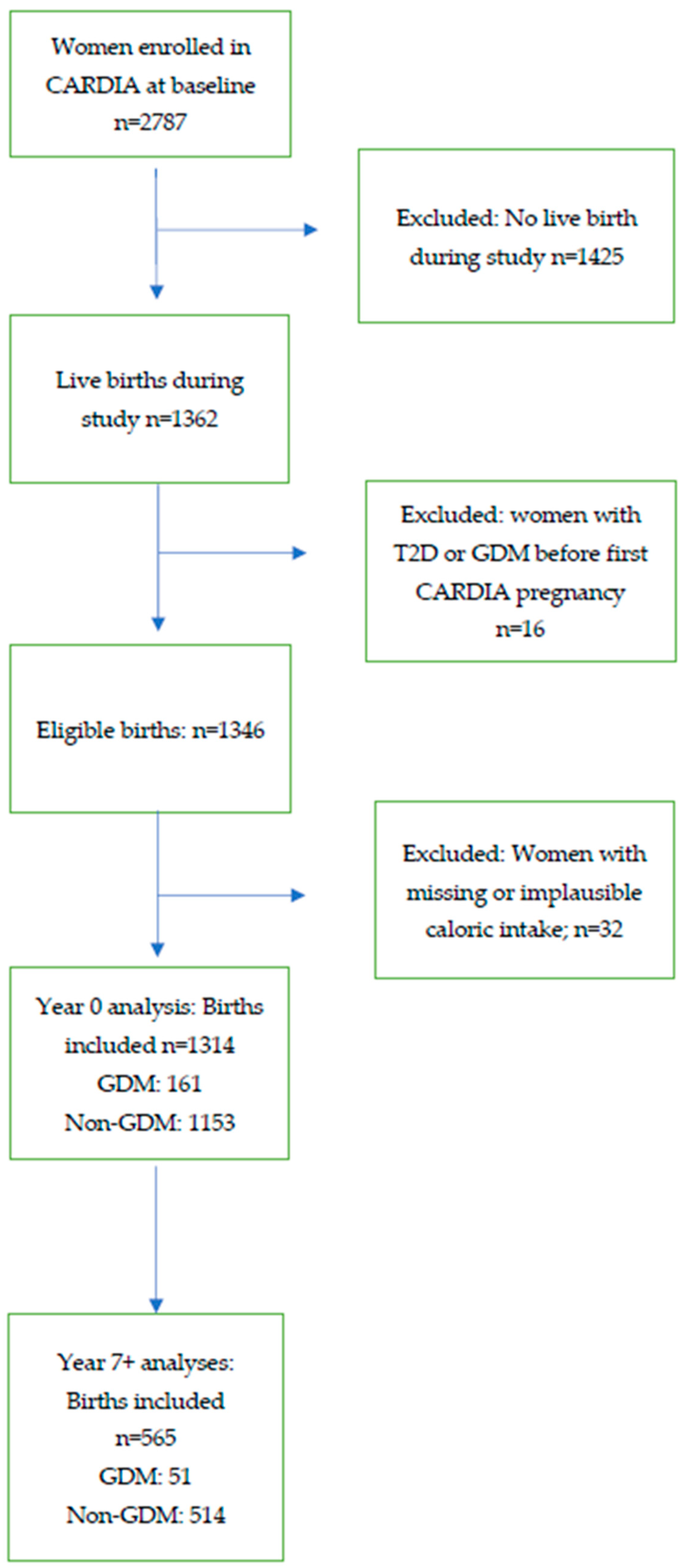
3. Results
4. Discussion
4.1. Animal Proteins and GDM
4.2. Plant-Based Protein
4.3. BCAA and GDM
4.4. Dietary Patterns
4.5. Strengths and Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Construction of the CARDIA A Priori Diet Quality Score (APDQS)
| CARDIA Food Groups | A Priori Diet Quality Score |
| Avocado | + |
| Beans | + |
| Beer | + |
| Coffee | + |
| Fish | + |
| Fruit | + |
| Dark Green Vegetables | + |
| Lean Fish | + |
| Low fat dairy | + |
| Liquor | + |
| Oil | + |
| Other vegetables | + |
| Poultry | + |
| Seeds, nuts | + |
| Soy Products | + |
| Tea | + |
| Tomato | + |
| Whole grains | + |
| Wine | + |
| Yellow vegetables | + |
| Butter | - |
| Frieds foods | - |
| Fried potatoes | - |
| Grain dessert | - |
| Organ meat | - |
| Processed meat | - |
| Regular red meat | - |
| Salty snacks | - |
| Sauces | - |
| Soft drink | - |
| Sweet breads | - |
| Sweet extras | - |
| Whole fat dairy | - |
| Chocolate | 0 |
| Diet soft drink | 0 |
| Eggs | 0 |
| Fruit Juice | 0 |
| Lean red meat | 0 |
| Margarine | 0 |
| Meal replacement | 0 |
| Pickled foods | 0 |
| Potatoes | 0 |
| Refined grains | 0 |
| Shellfish | 0 |
| Soups | 0 |
| Total possible score | 132 |
References
- Ferrara, A. Increasing prevalence of gestational diabetes mellitus: A public health perspective. Diabetes Care 2007, 30, S141–S146. [Google Scholar] [CrossRef]
- Casagrande, S.S.; Linder, B.; Cowie, C.C. Prevalence of gestational diabetes and subsequent Type 2 diabetes among U.S. women. Diabetes Res. Clin. Pract. 2018, 141, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Anna, V.; van der Ploeg, H.P.; Cheung, N.W.; Huxley, R.R.; Bauman, A.E. Sociodemographic Correlates of the Increasing Trend in Prevalence of Gestational Diabetes Mellitus in a Large Population of Women Between 1995 and 2005. Diabetes Care 2008, 31, 2288–2293. [Google Scholar] [CrossRef]
- Hedderson, M.M.; Darbinian, J.A.; Ferrara, A. Disparities in the risk of gestational diabetes by race-ethnicity and country of birth. Paediatr. Périnat. Epidemiol. 2010, 24, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Hedderson, M.; Ehrlich, S.; Sridhar, S.; Darbinian, J.; Moore, S.; Ferrara, A. Racial/Ethnic Disparities in the Prevalence of Gestational Diabetes Mellitus by BMI. Diabetes Care 2012, 35, 1492–1498. [Google Scholar] [CrossRef]
- Jenum, A.K.; Morkrid, K.; Sletner, L.; Vangen, S.; Torper, J.L.; Nakstad, B.; Voldner, N.; Rognerud-Jensen, O.H.; Berntsen, S.; Holme, I.; et al. Impact of ethnicity on gestational diabetes identified with the WHO and the modified International Association of Diabetes and Pregnancy Study Groups criteria: A population-based cohort study. Eur. J. Endocrinol. Eur. Fed. Endocr. Soc. 2012, 166, 317–324. [Google Scholar] [CrossRef]
- Combs, C.A.; Gunderson, E.; Kitzmiller, J.L.; Gavin, L.; Main, E.K. Relationship of Fetal Macrosomia to Maternal Postprandial Glucose Control During Pregnancy. Diabetes Care 1992, 15, 1251–1257. [Google Scholar] [CrossRef] [PubMed]
- Metzger, B.E.; Coustan, D.R.; Trimble, E.R. Hyperglycemia and adverse pregnancy outcomes. N. Engl. J. Med. 2008, 358, 1991–2002. [Google Scholar]
- Fadl, H.; Magnuson, A.; Ostlund, I.; Montgomery, S.; Hanson, U.; Schwarcz, E. Gestational diabetes mellitus and later cardiovascular disease: A Swedish population based case-control study. BJOG Int. J. Obstet. Gynaecol. 2014, 121, 1530–1536. [Google Scholar] [CrossRef]
- Gunderson, E.P.; Chiang, V.; Pletcher, M.J.; Jacobs, D.R.; Quesenberry, C.P.; Sidney, S.; Lewis, C.E. History of Gestational Diabetes Mellitus and Future Risk of Atherosclerosis in Mid-life: The Coronary Artery Risk Development in Young Adults Study. J. Am. Heart Assoc. 2014, 3, e000490. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, L.; Casas, J.-P.; Hingorani, A.D.; Williams, D. Type 2 diabetes mellitus after gestational diabetes: A systematic review and meta-analysis. Lancet 2009, 373, 1773–1779. [Google Scholar] [CrossRef]
- Bao, W.; Yeung, E.; Tobias, D.K.; Hu, F.B.; Vaag, A.A.; Chavarro, J.E.; Mills, J.L.; Grunnet, L.G.; Bowers, K.; Ley, S.H.; et al. Long-term risk of type 2 diabetes mellitus in relation to BMI and weight change among women with a history of gestational diabetes mellitus: A prospective cohort study. Diabetologia 2015, 58, 1212–1219. [Google Scholar] [CrossRef] [PubMed]
- Vounzoulaki, E.; Khunti, K.; Abner, S.C.; Tan, B.K.; Davies, M.J.; Gillies, C.L. Progression to type 2 diabetes in women with a known history of gestational diabetes: Systematic review and meta-analysis. BMJ 2020, 369, m1361. [Google Scholar] [CrossRef]
- Shah, N.S.; Wang, M.C.; Freaney, P.M.; Perak, A.M.; Carnethon, M.R.; Kandula, N.R.; Gunderson, E.P.; Bullard, K.M.; Grobman, W.A.; O’Brien, M.J.; et al. Trends in Gestational Diabetes at First Live Birth by Race and Ethnicity in the US, 2011–2019. JAMA 2021, 326, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Gunderson, E.P.; Quesenberry, C.P.; Jacobs, D.R.; Feng, J.; Lewis, C.E.; Sidney, S. Longitudinal Study of Prepregnancy Cardiometabolic Risk Factors and Subsequent Risk of Gestational Diabetes Mellitus: The CARDIA Study. Am. J. Epidemiol. 2010, 172, 1131–1143. [Google Scholar] [CrossRef] [PubMed]
- Catov, J.M.; Sun, B.; Bertolet, M.; Snyder, G.G.; Lewis, C.E.; Allen, N.; Shikany, J.M.; Ingram, K.H.; Appiah, D.; Gunderson, E.P. Changes in Cardiometabolic Risk Factors Before and After Gestational Diabetes: A Prospective Life-Course Analysis in CARDIA Women. Obesity 2020, 28, 1397–1404. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, K.M.; Ingram, K.H.; Appiah, D.; Nicholson, W.K.; Bennett, W.L.; Lewis, C.E.; Reis, J.P.; Schreiner, P.J.; Gunderson, E.P. Prepregnancy Fitness and Risk of Gestational Diabetes: A Longitudinal Analysis. Med. Sci. Sport. Exerc. 2018, 50, 1613–1619. [Google Scholar] [CrossRef]
- Bao, W.; Bowers, K.; Tobias, D.K.; Hu, F.B.; Zhang, C. Prepregnancy dietary protein intake, major dietary protein sources, and the risk of gestational diabetes mellitus: A prospective cohort study. Diabetes Care 2013, 36, 2001–2008. [Google Scholar] [CrossRef] [PubMed]
- Tobias, D.K.; Zhang, C.; Chavarro, J.; Bowers, K.; Rich-Edwards, J.; Rosner, B.; Mozaffarian, D.; Hu, F.B. Prepregnancy adherence to dietary patterns and lower risk of gestational diabetes mellitus. Am. J. Clin. Nutr. 2012, 96, 289–295. [Google Scholar] [CrossRef]
- Bowers, K.; Tobias, D.K.; Yeung, E.; Hu, F.B.; Zhang, C. A prospective study of prepregnancy dietary fat intake and risk of gestational diabetes. Am. J. Clin. Nutr. 2012, 95, 446–453. [Google Scholar] [CrossRef]
- Costello, E.; Goodrich, J.; Patterson, W.B.; Rock, S.; Li, Y.; Baumert, B.; Gilliland, F.; Goran, M.I.; Chen, Z.; Alderete, T.L.; et al. Diet Quality Is Associated with Glucose Regulation in a Cohort of Young Adults. Nutrients 2022, 14, 3734. [Google Scholar] [CrossRef] [PubMed]
- Schoenaker, D.A.J.M.; Soedamah-Muthu, S.S.; Callaway, L.K.; Mishra, G.D. Pre-pregnancy dietary patterns and risk of gestational diabetes mellitus: Results from an Australian population-based prospective cohort study. Diabetologia 2015, 58, 2726–2735. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Bowers, K.; Tobias, D.K.; Olsen, S.F.; Chavarro, J.; Vaag, A.; Kiely, M.; Zhang, C. Prepregnancy low-carbohydrate dietary pattern and risk of gestational diabetes mellitus: A prospective cohort study. Am. J. Clin. Nutr. 2014, 99, 1378–1384. [Google Scholar] [CrossRef] [PubMed]
- Bowers, K.; Yeung, E.; Williams, M.A.; Qi, L.; Tobias, D.K.; Hu, F.B.; Zhang, C. A Prospective Study of Prepregnancy Dietary Iron Intake and Risk for Gestational Diabetes Mellitus. Diabetes Care 2011, 34, 1557–1563. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Drouin-Chartier, J.-P.; Li, Y.; Baden, M.Y.; Manson, J.E.; Willett, W.C.; Voortman, T.; Hu, F.B.; Bhupathiraju, S.N. Changes in Plant-Based Diet Indices and Subsequent Risk of Type 2 Diabetes in Women and Men: Three U.S. Prospective Cohorts. Diabetes Care 2021, 44, 663–671. [Google Scholar] [CrossRef]
- Wang, H.; Huang, L.; Lin, L.; Chen, X.; Zhong, C.; Li, Q.; Li, N.; Gao, D.; Zhou, X.; Chen, R.; et al. The overall plant-based diet index during pregnancy and risk of gestational diabetes mellitus: A prospective cohort study in China. Br. J. Nutr. 2021, 126, 1519–1528. [Google Scholar] [CrossRef]
- Choi, Y.; Larson, N.; Gallaher, D.D.; Odegaard, A.O.; Rana, J.S.; Shikany, J.M.; Steffen, L.M.; Jacobs, D.R. A Shift Toward a Plant-Centered Diet From Young to Middle Adulthood and Subsequent Risk of Type 2 Diabetes and Weight Gain: The Coronary Artery Risk Development in Young Adults (CARDIA) Study. Diabetes Care 2020, 43, 2796–2803. [Google Scholar] [CrossRef]
- Buse, M.G.; Biggers, J.F.; Friderici, K.H.; Buse, J.F. Oxidation of branched chain amino acids by isolated hearts and diaphragms of the rat. The effect of fatty acids, glucose, and pyruvate respiration. J. Biol. Chem. 1972, 247, 8085–8096. [Google Scholar] [CrossRef]
- Cavallaro, N.L.; Garry, J.; Shi, X.; Gerszten, R.E.; Anderson, E.J.; Walford, G.A. A pilot, short-term dietary manipulation of branched chain amino acids has modest influence on fasting levels of branched chain amino acids. Food Nutr. Res. 2016, 60, 28592. [Google Scholar] [CrossRef]
- Elshorbagy, A.; Jernerén, F.; Basta, M.; Basta, C.; Turner, C.; Khaled, M.; Refsum, H. Amino acid changes during transition to a vegan diet supplemented with fish in healthy humans. Eur. J. Nutr. 2016, 56, 1953–1962. [Google Scholar] [CrossRef]
- McCormack, S.E.; Shaham, O.; McCarthy, M.A.; Deik, A.A.; Wang, T.J.; Gerszten, R.E.; Clish, C.B.; Mootha, V.K.; Grinspoon, S.K.; Fleischman, A. Circulating branched-chain amino acid concentrations are associated with obesity and future insulin resistance in children and adolescents. Pediatr. Obes. 2013, 8, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Watkins, S.M.; Lorenzo, C.; Wagenknecht, L.E.; Il’Yasova, D.; Chen, Y.-D.I.; Haffner, S.M.; Hanley, A.J. Branched-Chain Amino Acids and Insulin Metabolism: The Insulin Resistance Atherosclerosis Study (IRAS). Diabetes Care 2016, 39, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Thalacker-Mercer, A.E.; Ingram, K.H.; Guo, F.; Ilkayeva, O.; Newgard, C.B.; Garvey, W.T. BMI, RQ, Diabetes, and Sex Affect the Relationships Between Amino Acids and Clamp Measures of Insulin Action in Humans. Diabetes 2014, 63, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A Branched-Chain Amino Acid-Related Metabolic Signature that Differentiates Obese and Lean Humans and Contributes to Insulin Resistance. Cell Metab. 2009, 9, 311–326. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Li, J.; Wang, H.; Liu, J.; Li, W.; Yang, K.; Huo, X.; Leng, J.; Yu, Z.; Hu, G.; et al. Branched-Chain Amino Acids and Their Interactions With Lipid Metabolites for Increased Risk of Gestational Diabetes. J. Clin. Endocrinol. Metab. 2022, 107, e3058–e3065. [Google Scholar] [CrossRef]
- Jiang, R.; Wu, S.; Fang, C.; Wang, C.; Yang, Y.; Liu, C.; Hu, J.; Huang, Y. Amino acids levels in early pregnancy predict subsequent gestational diabetes. J. Diabetes 2020, 12, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Chorell, E.; Hall, U.A.; Gustavsson, C.; Berntorp, K.; Puhkala, J.; Luoto, R.; Olsson, T.; Holmäng, A. Pregnancy to postpartum transition of serum metabolites in women with gestational diabetes. Metabolism 2017, 72, 27–36. [Google Scholar] [CrossRef]
- Andersson-Hall, U.; Gustavsson, C.; Pedersen, A.; Malmodin, D.; Joelsson, L.; Holmäng, A. Higher Concentrations of BCAAs and 3-HIB Are Associated with Insulin Resistance in the Transition from Gestational Diabetes to Type 2 Diabetes. J. Diabetes Res. 2018, 2018, 4207067. [Google Scholar] [CrossRef]
- Friedman, G.D.; Cutter, G.R.; Donahue, R.P.; Hughes, G.H.; Hulley, S.B.; Jacobs, D.R.; Liu, K.; Savage, P.J. Cardia: Study design, recruitment, and some characteristics of the examined subjects. J. Clin. Epidemiol. 1988, 41, 1105–1116. [Google Scholar] [CrossRef]
- National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979, 28, 1039–1057. [Google Scholar] [CrossRef]
- Carpenter, M.W.; Coustan, D.R. Criteria for screening tests for gestational diabetes. Am. J. Obstet. Gynecol. 1982, 144, 768–773. [Google Scholar] [CrossRef]
- van Horn, L.; Slattery, M.; Hilner, J.; Bragg, C.; Jacobs, D.; Gernhofer, N.; Havlik, D. The CARDIA dietary history: Development, implementation, and evaluation. J. Am. Diet. Assoc. 1991, 91, 1104. [Google Scholar]
- Steffen, L.M.; Kroenke, C.H.; Yu, X.; Pereira, M.; Slattery, M.L.; Van Horn, L.; Gross, M.D.; Jacobs, D.R. Associations of plant food, dairy product, and meat intakes with 15-y incidence of elevated blood pressure in young black and white adults: The Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am. J. Clin. Nutr. 2005, 82, 1169–1177. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, D.R.; Hahn, L.P.; Haskell, W.L.; Pirie, P.; Sidney, S. Validity and Reliability of Short Physical Activity History: Cardia and the Minnesota Heart Health Program. J. Cardiopulm. Rehabil. 1989, 9, 448–459. [Google Scholar] [CrossRef]
- Tobias, D.K.; Hu, F.B.; Chavarro, J.; Rosner, B.; Mozaffarian, D.; Zhang, C. Healthful Dietary Patterns and Type 2 Diabetes Mellitus Risk Among Women With a History of Gestational Diabetes Mellitus. Arch. Intern. Med. 2012, 172, 1566–1572. [Google Scholar] [CrossRef]
- Xiao, R.S.; Simas, T.A.M.; Person, S.D.; Goldberg, R.J.; Waring, M.E. Diet Quality and History of Gestational Diabetes Mellitus Among Childbearing Women, United States, 2007–2010. Prev. Chronic Dis. 2015, 12, E25. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, S.; Solomon, C.G.; Hu, F.B. Dietary fiber intake, dietary glycemic load, and the risk for gestational diabetes mellitus. Diabetes Care 2006, 29, 2223–2230. [Google Scholar] [CrossRef]
- Tobias, D.K.; Clish, C.; Mora, S.; Li, J.; Liang, L.; Hu, F.B.; Manson, J.E.; Zhang, C. Dietary Intakes and Circulating Concentrations of Branched-Chain Amino Acids in Relation to Incident Type 2 Diabetes Risk Among High-Risk Women with a History of Gestational Diabetes Mellitus. Clin. Chem. 2018, 64, 1203–1210. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Y.; Ong, C.-N.; Subramaniam, T.; Choi, H.W.; Yuan, J.-M.; Koh, W.-P.; Pan, A. Metabolic signatures and risk of type 2 diabetes in a Chinese population: An untargeted metabolomics study using both LC-MS and GC-MS. Diabetologia 2016, 59, 2349–2359. [Google Scholar] [CrossRef]
- Wang, T.J.; Larson, M.G.; Vasan, R.S.; Cheng, S.; Rhee, E.P.; McCabe, E.; Lewis, G.D.; Fox, C.S.; Jacques, P.F.; Fernandez, C.; et al. Metabolite profiles and the risk of developing diabetes. Nat. Med. 2011, 17, 448–453. [Google Scholar] [CrossRef]
- Isanejad, M.; LaCroix, A.Z.; Thomson, C.A.; Tinker, L.; Larson, J.C.; Qi, Q.; Cooper-DeHoff, R.M.; Phillips, L.S.; Beasley, J.M. Branched-chain amino acid, meat intake and risk of type 2 diabetes in the Women’s Health Initiative. Br. J. Nutr. 2017, 117, 1523–1530. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ning, Y. Effect of dietary and lifestyle factors on the risk of gestational diabetes: Review of epidemiologic evidence. Am. J. Clin. Nutr. 2011, 94, 1975S–1979S. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Qian, F.; Liu, G.; Li, M.; Voortman, T.; Tobias, D.K.; Ley, S.H.; Bhupathiraju, S.N.; Li, L.-J.; Chavarro, J.; et al. Prepregnancy plant-based diets and the risk of gestational diabetes mellitus: A prospective cohort study of 14,926 women. Am. J. Clin. Nutr. 2021, 114, 1997–2005. [Google Scholar] [CrossRef] [PubMed]
- Hirahatake, K.M.; Jacobs, D.R.; Shikany, J.M.; Jiang, L.; Wong, N.D.; Odegaard, A.O. Cumulative average dietary pattern scores in young adulthood and risk of incident type 2 diabetes: The CARDIA study. Diabetologia 2019, 62, 2233–2244. [Google Scholar] [CrossRef] [PubMed]
- Rehm, C.D.; Peñalvo, J.L.; Afshin, A.; Mozaffarian, D. Dietary Intake Among US Adults, 1999–2012. JAMA 2016, 315, 2542–2553. [Google Scholar] [CrossRef]
| Characteristics | GDM Pregnancies (n = 161) | Non-GDM Pregnancies (n = 1153) | p Value a |
|---|---|---|---|
| Age, years, mean (SD) | 24.5 (3.9) | 24.1 (3.6) | 0.236 |
| Race, Black, n (%) | 78 (48.4) | 576 (50.0) | 0.737 |
| >High school education, n (%) | 110 (68.3) | 749 (65.0) | 0.427 |
| Current smoker, n (%) | 49 (30.4) | 277 (24.0) | 0.080 |
| Family history of diabetes, n (%) | 33 (20.5) | 146 (12.7) | 0.010 |
| Body mass index, n (%) | 0.003 | ||
| Normal weight | 97 (61.8) | 810 (72.8) | |
| Overweight | 30 (19.1) | 187(16.8) | |
| Obese | 30 (19.1) | 116 (10.4) | |
| Nulliparous, n (%) | 107 (66.5) | 792 (68.7) | 0.586 |
| Physical Activity Score | 292 (166–460) | 295 (154–476) | 0.970 |
| Diet Quality Score | 65.0 (12.4) | 63.8 (13.7) | 0.315 |
| Energy Intake, kcal b | 1993 (1553–2696) | 2089 (1623–2821) | 0.118 |
| Protein, g/100 kcal | 3.8 (0.7) | 3.7 (0.7) | 0.140 |
| Carbohydrate, g/100 kcal | 11.6 (1.8) | 11.7 (1.8) | 0.448 |
| Fat, g/100 kcal | 4.2 (0.7) | 4.2 (0.7) | 0.574 |
| Polyunsaturated Fat, g/100 kcal b | 0.75 (0.65–0.89) | 0.74 (0.62–0.90) | 0.593 |
| Saturated Fat, g/100 kcal | 1.6 (0.4) | 1.6 (0.3) | 0.606 |
| Fiber, g/100 kcal b | 0.20 (0.15–0.27) | 0.20 (0.15–0.27) | 0.660 |
| Cholesterol, mg/100 kcal b | 16.2 (12.8–20.2) | 15.4 (12.0–19.4) | 0.051 |
| Alcohol, g/100 kcal b | 0.15 (0.00–0.44) | 0.13 (0.01–0.41) | 0.861 |
| Fasting glucose, mg/dL | 81.2 (9.4) | 79.1 (7.4) | 0.009 |
| Insulin, uU/ML | 11.0 (9.0–15.0) | 10.0 (8.0–13.0) | 0.004 |
| Triglycerides, mg/dL | 64.0 (51.0–81.0) | 57.0 (43.0–77.0) | <0.001 |
| HDL-Cholesterol, mg/dL | 54.9 (13.6) | 56.3 (12.7) | 0.220 |
| Characteristics | GDM Pregnancies (n = 51) | Non-GDM Pregnancies (n = 514) | p Value a |
|---|---|---|---|
| Age, years, b | 30.0 (26.0–33.0) | 30.0 (27.0–33.0) | 0.369 |
| Race, Black, n (%) | 21 (41.2) | 213 (41.4) | 0.971 |
| >High school education, n (%) | 39 (76.5) | 421 (81.9) | 0.347 |
| Current smoker, n (%) | 13 (25.5) | 103 (20.0) | 0.365 |
| Family history of diabetes, n (%) | 13 (25.5) | 72 (14.2) | 0.041 |
| Body mass index, n (%) | 0.122 | ||
| Normal weight | 25 (52.1) | 292 (62.5) | |
| Overweight | 8 (16.7) | 87 (18.6) | |
| Obese | 15 (31.1) | 88 (18.8) | |
| Nulliparous, n (%) | 27 (52.9) | 284 (55.3) | 0.770 |
| Physical Activity Score | 204 (84- 358) | 219 (115–406) | 0.915 |
| Diet Quality Score | 66.5 (13.4) | 69.8 (11.5) | 0.053 |
| Energy Intake, kcal b | 2201 (1725–2680) | 2134 (1675–2901) | 0.777 |
| BCAA, g/100 kcal b | 15.2 (11.7–17.7) | 14.5 (10.8–18.8) | 0.430 |
| Protein, g/100 kcal | 3.8 (0.7) | 3.7 (0.7) | 0.336 |
| Vegetable Protein, g/100 kcal b | 1.2 (1.0–1.3) | 1.3 (1.1–1.5) | 0.013 |
| Veg. Protein/Total Protein, % b | 30.1 (26.5–35.8) | 34.1 (27.8–40.2) | 0.006 |
| Animal Protein, g/100 kcal | 2.7 (0.7) | 2.4 (0.7) | 0.033 |
| Animal Protein/Total Protein, % b | 69.2 (63.6–72.6) | 64.6 (58.8–71.1) | 0.003 |
| Glycine/Total protein, % | 4.0 (0.4) | 3.9 (0.5) | 0.856 |
| Carbohydrate, g/100 kcal | 12.3 (1.4) | 12.8 (1.9) | 0.032 |
| Fat, g/100 kcal b | 4.1 (3.7–4.3) | 3.9 (3.3–4.3) | 0.024 |
| Polyunsaturated Fat, g/100 kcal b | 0.78 (0.62- 0.91) | 0.74 (0.61–0.90) | 0.490 |
| Saturated Fat, g/100 kcal | 1.4 (0.3) | 1.3 (0.3) | 0.014 |
| Fiber, g/100 kcal b | 0.76 (0.62–0.90) | 0.87 (0.70–1.08) | 0.010 |
| Cholesterol, mg/100 kcal b | 13.0 (10.5–15.2) | 11.1 (8.5–13.9) | 0.003 |
| Alcohol, g/100 kcal b | 0.05 (0.01–0.18) | 0.10 (0.00–0.39) | 0.158 |
| Blood test parameters: | |||
| Insulin, uU/ML b | 11.0 (9.0–15.0) | 11.0 (8.0–14.0) | 0.210 |
| Fasting glucose, mg/dL | 87.5 (9.1) | 84.5 (7.8) | 0.014 |
| Triglycerides, mg/dL b | 66.0 (47.0–91.0) | 59.0 (43.0–86.0) | 0.277 |
| HDL-Cholesterol, mg/dL | 51.9 (11.8) | 58.0 (13.7) | 0.003 |
| Dietary Characteristics | OR (95% CI) | p Value |
|---|---|---|
| Diet quality | 0.107 | |
| First tertile | 1 | |
| Second tertile | 1.66 (1.04–2.65) | |
| Third tertile | 1.45 (0.83–2.54) | |
| Per 1-point increase | 1.01 (0.99–1.02) | 0.461 |
| Per 1 standard deviation (13.5) | 1.09 (0.87–1.37) | 0.461 |
| Dietary Characteristics | OR (95% CI) | p Value |
|---|---|---|
| BCAA | 1.08 (0.96–1.20) | 0.195 |
| BCAA/total protein | 1.57 (1.02–2.40) | 0.040 |
| Animal protein | 1.02 (1.00–1.04) | 0.063 |
| Animal protein/total protein | 1.06 (1.02–1.10) | 0.006 |
| Vegetable protein | 0.95 (0.91–1.00) | 0.063 |
| Vegetable protein/total protein | 0.95 (0.91–0.99) | 0.014 |
| Glycine/total protein | 1.03 (0.50–2.10) | 0.944 |
| Diet quality † | 0.081 | |
| First tertile | 1 | |
| Second tertile | 0.41 (0.18–0.93) | |
| Third tertile | 0.53 (0.23–1.20) | |
| Per 1-point increase | 0.97 (0.94–1.00) | 0.060 |
| Per 1 standard deviation (11.8) | 0.70 (0.48–1.02) | 0.060 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gadgil, M.D.; Ingram, K.H.; Appiah, D.; Rudd, J.; Whitaker, K.M.; Bennett, W.L.; Shikany, J.M.; Jacobs, D.R., Jr; Lewis, C.E.; Gunderson, E.P. Prepregnancy Protein Source and BCAA Intake Are Associated with Gestational Diabetes Mellitus in the CARDIA Study. Int. J. Environ. Res. Public Health 2022, 19, 14142. https://doi.org/10.3390/ijerph192114142
Gadgil MD, Ingram KH, Appiah D, Rudd J, Whitaker KM, Bennett WL, Shikany JM, Jacobs DR Jr, Lewis CE, Gunderson EP. Prepregnancy Protein Source and BCAA Intake Are Associated with Gestational Diabetes Mellitus in the CARDIA Study. International Journal of Environmental Research and Public Health. 2022; 19(21):14142. https://doi.org/10.3390/ijerph192114142
Chicago/Turabian StyleGadgil, Meghana D., Katherine H. Ingram, Duke Appiah, Jessica Rudd, Kara M. Whitaker, Wendy L. Bennett, James M. Shikany, David R. Jacobs, Jr, Cora E. Lewis, and Erica P. Gunderson. 2022. "Prepregnancy Protein Source and BCAA Intake Are Associated with Gestational Diabetes Mellitus in the CARDIA Study" International Journal of Environmental Research and Public Health 19, no. 21: 14142. https://doi.org/10.3390/ijerph192114142
APA StyleGadgil, M. D., Ingram, K. H., Appiah, D., Rudd, J., Whitaker, K. M., Bennett, W. L., Shikany, J. M., Jacobs, D. R., Jr, Lewis, C. E., & Gunderson, E. P. (2022). Prepregnancy Protein Source and BCAA Intake Are Associated with Gestational Diabetes Mellitus in the CARDIA Study. International Journal of Environmental Research and Public Health, 19(21), 14142. https://doi.org/10.3390/ijerph192114142





