Do Current Smokers and Ex-Smokers Who Use Nicotine Vaping Products Daily Versus Weekly Differ on Their Reasons for Vaping? Findings from the 2020 ITC Four Country Smoking and Vaping Survey
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Procedure, and Population
2.2. Measures
2.2.1. Independent Variable: Vaping Frequency
2.2.2. Covariates
Smoking Status and Frequency
Sociodemographics
2.2.3. Outcome Measures
| Reasons for Vaping: Survey Question | Outcome: Coding | Asked of Smokers/Ex-Smokers |
| Vaping is less harmful to me than smoking | (0) no vs. (1) yes | Both |
| Vaping is less harmful than smoking to other people around me | Both | |
| I enjoy vaping | Both | |
| I save money by vaping instead of smoking | Both | |
| I like the e-liquid flavours | Both | |
| Vaping is more acceptable than smoking | Both | |
| I can vape in places where I can’t smoke | Both | |
| Vaping helps me cut down on the number of cigarettes I smoke | Smokers only | |
| Vaping might help me stop smoking ordinary cigarettes | Smokers only | |
| Vaping might help me stay quit from smoking cigarettes | Ex-smokers only | |
| Composite quit-reduce measure | (1) selected quitting smoking as a reason for vaping; (2) selected reducing number of cigarettes but did not select quitting smoking; (0) did not select reducing number of cigarettes or quitting smoking (selected other reasons only) | Smokers only |
2.3. Statistical Analysis
3. Results
3.1. Sample Characteristics
3.2. Study Results
3.2.1. Reasons for NVP Use among Current Smokers
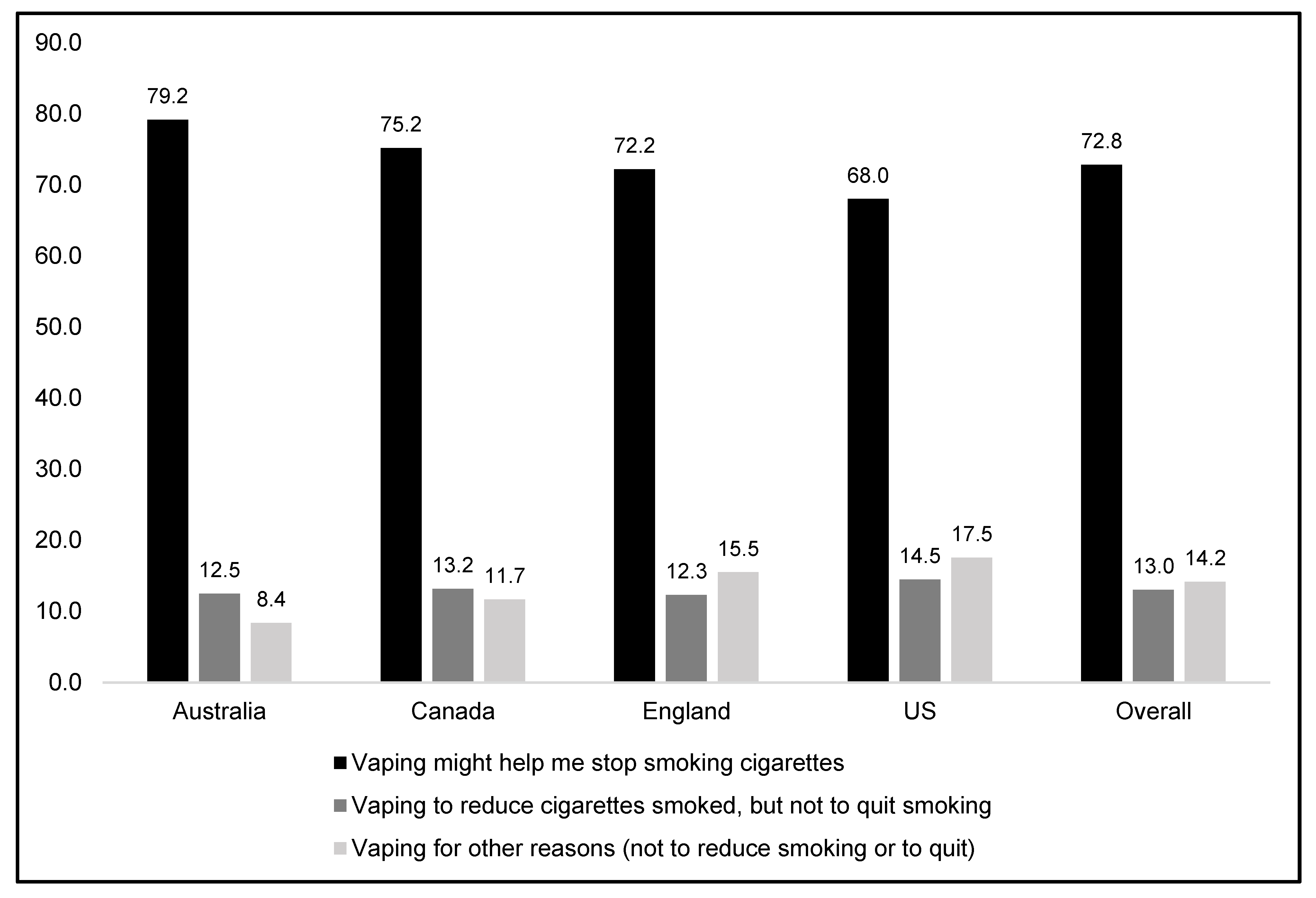
3.2.2. Vaping for Smoking Reduction/Cessation versus Other Reasons among Current Smokers, Overall and by Country
3.2.3. Reasons for NVP Use among Ex-Smokers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- U.S. Department of Health and Human Services. The Health Consequences of Smoking: 50 Years of Progress. A Report of the Surgeon General; U.S. Department of Health and Human Services; Centers for Disease Control and Prevention; National Center for Chronic Disease Prevention and Health Promotion; Office on Smoking and Health: Atlanta, GA, USA, 2014; corrected in 2014. [Google Scholar]
- The NCD Alliance. Tobacco: A Major Risk Factor for Non-Communicable Diseases. NCD Alliance 2017. Available online: https://ncdalliance.org/sites/default/files/rfiles/NCDA_Tobacco_and_Health.pdf (accessed on 19 October 2022).
- Dai, X.; Gakidou, E.; Lopez, A.D. Evolution of the global smoking epidemic over the past half century: Strengthening the evidence base for policy action. Tob. Control 2022, 31, 129–137. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services. Smoking Cessation: A Report of the Surgeon General; U.S. Department of Health and Human Services; Centers for Disease Control and Prevention; National Center for Chronic Disease Prevention and Health Promotion; Office on Smoking and Health: Atlanta, GA, USA, 2020. [Google Scholar]
- Centers for Disease Control and Prevention. Harms of Cigarette Smoking and Health Benefits of Quitting; CDC: Atlanta, GA, USA, 2017. Available online: https://www.cancer.gov/about-cancer/causes-prevention/risk/tobacco/cessation-fact-sheet (accessed on 19 October 2022).
- Centers for Disease Control and Prevention. Smoking Cessation: Fast Facts. Available online: https://www.cdc.gov/tobacco/data_statistics/fact_sheets/cessation/smoking-cessation-fast-facts/index.html#:~:text=Fewer%20than%20one%20in%20ten,succeed%20in%20quitting%20each%20year.&text=Four%20out%20of%20every%20nine,not%20receive%20advice%20to%20quit (accessed on 19 October 2022).
- Chaiton, M.; Diemert, L.; Cohen, J.E.; Bondy, S.J.; Selby, P.; Philipneri, A.; Schwartz, R. Estimating the number of quit attempts it takes to quit smoking successfully in a longitudinal cohort of smokers. BMJ Open 2016, 6, e011045. [Google Scholar] [CrossRef] [PubMed]
- Buczkowski, K.; Dachtera-Frąckiewicz, M.; Luszkiewicz, D.; Klucz, K.; Sawicka-Powierza, J.; Marcinowicz, L. Reasons for and Scenarios Associated with Failure to Cease Smoking: Results from a Qualitative Study Among Polish Smokers Who Had Unsuccessfully Attempted to Quit. Patient Prefer. Adherence 2021, 15, 2071–2084. [Google Scholar] [CrossRef]
- Babb, S.; Malarcher, A.; Schauer, G.; Asman, K.; Jamal, A. Quitting smoking among adults-United States, 2000–2015. Morb. Mortal. Wkly. Rep. 2017, 65, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Borland, R.; Partos, T.R.; Yong, H.H.; Cummings, K.M.; Hyland, A. How much unsuccessful quitting activity is going on among adult smokers? Data from the International Tobacco Control Four Country cohort survey. Addiction 2012, 107, 673–682. [Google Scholar] [CrossRef]
- Gravely, S.; Cummings, K.M.; Hammond, D.; Borland, R.; McNeill, A.; East, K.A.; Loewen, R.; Martin, N.; Yong, H.-H.; Li, L.; et al. Self-Reported Quit Aids and Assistance Used By Smokers At Their Most Recent Quit Attempt: Findings from the 2020 International Tobacco Control Four Country Smoking and Vaping Survey. Nicotine Tob. Res. 2021, 23, 1699–1707. [Google Scholar] [CrossRef] [PubMed]
- Abrams, D.B.; Glasser, A.M.; Pearson, J.L.; Villanti, A.C.; Collins, L.K.; Niaura, R.S. Harm Minimization and Tobacco Control: Reframing Societal Views of Nicotine Use to Rapidly Save Lives. Annu. Rev. Public Health 2018, 39, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Public Health Consequences of E-Cigarettes; Stratton, K., Kwan, L.Y., Eaton, D.L., Eds.; National Academies of Sciences, Engineering, and Medicine (NASEM); Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on the Review of the Health Effects of Electronic Nicotine Delivery Systems: Washington, DC, USA, 2018; Available online: https://nap.nationalacademies.org/catalog/24952/public-health-consequences-of-e-cigarettes (accessed on 26 September 2022).
- McNeill, A.; Brose, L.S.; Calder, R.; Simonavicius, E.; Robson, D. Vaping in England: An Evidence Update including Vaping for Smoking Cessation, February 2021: A Report Commissioned by PHE; PHE: London, UK, 2021. [Google Scholar]
- McNeill, A.; Simonavičius, E.; Brose, L.S.; Taylor, E.; East, K.; Zuikova, E.; Calder, R.; Robson, D. Nicotine Vaping in England: An Evidence Update including Health Risks and Perceptions, September 2022. A Report Commissioned by the Office for Health Improvement and Disparities; Office for Health Improvement and Disparities: London, UK, 2022. [Google Scholar]
- Hartmann-Boyce, J.; McRobbie, H.; Butler, A.R.; Lindson, N.; Bullen, C.; Begh, R.; Theodoulou, A.; Notley, C.; Rigotti, N.A.; Turner, T.; et al. Electronic cigarettes for smoking cessation. Cochrane Database Syst. Rev. 2021, 9, CD010216. [Google Scholar]
- Gravely, S.; Meng, G.; Hammond, D.; Hyland, A.; Cummings, K.M.; Borland, R.; Kasza, K.A.; Yong, H.-H.; Thompson, M.E.; Quah, A.C.; et al. Differences in cigarette smoking quit attempts and cessation between adults who did and did not take up nicotine vaping: Findings from the ITC four country smoking and vaping surveys. Addict. Behav. 2022, 132, 107339. [Google Scholar] [CrossRef] [PubMed]
- Glasser, A.M.; Vojjala, M.; Cantrell, J.; Levy, D.T.; Giovenco, D.P.; Abrams, D.; Niaura, R. Patterns of E-cigarette Use and Subsequent Cigarette Smoking Cessation Over 2 Years (2013/2014–2015/2016) in the Population Assessment of Tobacco and Health Study. Nicotine Tob. Res. 2021, 23, 669–677. [Google Scholar] [CrossRef] [PubMed]
- McDermott, M.S.; East, K.A.; Brose, L.S.; McNeill, A.; Hitchman, S.C.; Partos, T.R. The effectiveness of using e-cigarettes for quitting smoking compared to other cessation methods among adults in the United Kingdom. Addiction 2021, 116, 2825–2836. [Google Scholar] [CrossRef] [PubMed]
- Hummel, K.; Nagelhout, G.E.; Fong, G.T.; Vardavas, C.I.; Papadakis, S.; Herbec, A.; Mons, U.; Putte, B.V.D.; Borland, R.; Fernández, E.; et al. Quitting activity and use of cessation assistance reported by smokers in eight European countries: Findings from the EUREST-PLUS ITC Europe Surveys. Tob. Induc. Dis. 2018, 16 (Suppl. 2), A6. [Google Scholar] [CrossRef] [PubMed]
- Benmarhnia, T.; Pierce, J.P.; Leas, E.; White, M.M.; Strong, D.R.; Noble, M.L.; Trinidad, D.R. Can E-Cigarettes and Pharmaceutical Aids Increase Smoking Cessation and Reduce Cigarette Consumption? Findings From a Nationally Representative Cohort of American Smokers. Am. J. Epidemiol. 2018, 187, 2397–2404. [Google Scholar] [CrossRef] [PubMed]
- Yong, H.H.; Borland, R.; Cummings, K.M.; Gravely, S.; Thrasher, J.F.; McNeill, A.; Hitchman, S.; Greenhalgh, E.; Thompson, M.E.; Fong, G.T. Reasons for regular vaping and for its discontinuation among smokers and recent ex-smokers: Findings from the 2016 ITC Four Country Smoking and Vaping Survey. Addiction 2019, 114 (Suppl. 1), 35–48. [Google Scholar] [CrossRef]
- Glasser, A.M.; Collins, L.; Pearson, J.L.; Abudayyeh, H.; Niaura, R.S.; Abrams, D.B.; Villanti, A.C. Overview of electronic nicotine delivery systems: A systematic review. Am. J. Prev. Med. 2017, 52, e33–e66. [Google Scholar] [CrossRef] [PubMed]
- Soule, E.K.; Rosas, S.R.; Nasim, A. Reasons for electronic cigarette use beyond cigarette smoking cessation: A concept mapping approach. Addict. Behav. 2016, 56, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Bedi, M.K.; Bedi, D.K.; Ledgerwood, D.M. Gender differences in reasons for using e-cigarettes: A systematic review. Nicotine Tob. Res. 2022, 24, 1355–1362. [Google Scholar] [CrossRef] [PubMed]
- Government of Canada. Tobacco and Vaping Products Act. 2018. Available online: https://www.canada.ca/en/health-canada/services/health-concerns/tobacco/legislation/federal-laws/tobacco-act.html (accessed on 26 September 2022).
- Health Canada. Canada’s Tobacco Strategy. (7 February 2022). Available online: https://www.canada.ca/en/health-canada/services/publications/healthy-living/canada-tobacco-strategy.html (accessed on 26 September 2022).
- US Food and Drug Administration. FDA Finalizes Enforcement Policy on Unauthorized Flavored Cartridge-Based E-Cigarettes That Appeal to Children, including Fruit and Mint. 2020. Available online: https://www.fda.gov/news-events/press-announcements/fda-finalizes-enforcement-policy-unauthorized-flavored-cartridge-based-e-cigarettes-appeal-children (accessed on 26 September 2022).
- Australian Government. The Office of Drug Control. Australian Government Proposes Strengthening Its Stance Against E-Cigarettes Containing Vaporiser Nicotine 2020. Available online: https://www.odc.gov.au/news-media/news/australian-government-proposes-strengthening-its-stance-against-e-cigarettes (accessed on 26 September 2022).
- Gov.UK. Public Health England. E-Cigarettes and Vaping: Policy, Regulation and Guidance. 2022. Available online: https://www.gov.uk/government/collections/e-cigarettes-and-vaping-policy-regulation-and-guidance (accessed on 26 September 2022).
- National Institute for Health and Care Excellence (NICE). Stop Smoking Interventions and Services. NICE Guideline [NG92]. Advice on E Cigarettes. 2018. Available online: https://www.nice.org.uk/guidance/ng92/chapter/Recommendations#advice-on-ecigarettes (accessed on 26 September 2022).
- ITC Project. ITC Four Country Smoking and Vaping Survey Wave 1 (2016) Technical Report; University of Waterloo: Waterloo, ON, Canada; Medical University of South Carolina: Charleston, SC, USA; Cancer Council Victoria: Melbourne, Australia; King’s College London: London, UK, 2018; Available online: https://itcproject.org/methods/technical-reports/itc-four-country-smoking-and-vaping-survey-4cv1-technical-report-wave-1-2016-november-2018/ (accessed on 20 October 2022).
- ITC Project. ITC Four Country Smoking and Vaping Survey, Wave 2 (2018) Technical Report; University of Waterloo: Waterloo, ON, Canada; Medical University of South Carolina: Charleston, SC, USA; Cancer Council Victoria: Melbourne, Australia; The University of Queensland: Brisbane, Australia; King’s College London: London, UK, 2020; Available online: https://itcproject.org/methods/technical-reports/itc-four-country-smoking-and-vaping-survey-wave-2-4cv2-technical-report/ (accessed on 20 October 2022).
- ITC Project. ITC Four Country Smoking and Vaping Survey, Wave 3 (4CV3, 2020). Technical Report; University of Waterloo: Waterloo, ON, Canada; Medical University of South Carolina: Charleston, SC, USA; Cancer Council Victoria: Melbourne, Australia; The University of Queensland: Brisbane, Australia; King’s College London: London, UK, 2021; Available online: https://itcproject.s3.amazonaws.com/uploads/documents/4CV3_Technical_Report_23Jul2021_2.pdf (accessed on 26 September 2022).
- Thompson, M.E.; Fong, G.T.; Boudreau, C.; Driezen, P.; Li, G.; Gravely, S.; Cummings, K.M.; Heckman, B.W.; O’Connor, R.; Thrasher, J.F.; et al. Methods of the ITC Four Country Smoking and Vaping Survey, wave 1 (2016). Addiction 2019, 114, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, C.; Thompson, M.E.; Li, Y. Sampling Weights and Design of the International Tobacco Control (ITC) 4CV Wave 1 Survey. Available online: https://itcproject.s3.amazonaws.com/uploads/documents/4CV1_Sampling_Weights_Info-Jul20.pdf (accessed on 20 October 2022).
- Bandi, P.; Asare, S.; Majmundar, A.; Nargis, N.; Jemal, A.; Fedewa, S.A. Relative Harm Perceptions of E-Cigarettes Versus Cigarettes, U.S. Adults, 2018–2020. Am. J. Prev. Med. 2022, 63, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Malt, L.; Verron, T.; Cahours, X.; Guo, M.; Weaver, S.; Walele, T.; O’Connell, G. Perception of the relative harm of electronic cigarettes compared to cigarettes amongst US adults from 2013 to 2016: Analysis of the Population Assessment of Tobacco and Health (PATH) study data. Harm. Reduct. J. 2020, 17, 65. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Feng, B.; Weaver, S.R.; Pechacek, T.F.; Slovic, P.; Eriksen, M.P. Changing perceptions of harm of e-cigarette vs cigarette use among adults in 2 US national surveys from 2012 to 2017. JAMA Netw. Open 2019, 2, e191047. [Google Scholar] [CrossRef] [PubMed]
- Notley, C.; Ward, E.; Dawkins, L.; Holland, R. The unique contribution of e-cigarettes for tobacco harm reduction in supporting smoking relapse prevention. Harm. Reduct. J. 2018, 15, 31. [Google Scholar] [CrossRef] [PubMed]
| All Respondents N = 3070 | Daily NVP Use n = 1824 (59.4%) | Weekly NVP Use n = 1246 (40.6%) | ||
|---|---|---|---|---|
| n | % | % | % | |
| Country of residence | ||||
| Australia | 202 | 6.6 | 7.0 | 5.9 |
| Canada | 927 | 30.2 | 25.8 | 36.6 |
| England | 1300 | 42.4 | 44.4 | 39.4 |
| United States | 641 | 20.9 | 22.8 | 18.1 |
| Sex | ||||
| Male | 1741 | 56.7 | 55.8 | 58.0 |
| Female | 1329 | 43.3 | 44.2 | 42.0 |
| Age | ||||
| 18–39 | 1882 | 61.3 | 57.7 | 66.5 |
| 40+ | 1188 | 38.7 | 42.3 | 33.5 |
| Income | ||||
| Low | 723 | 23.6 | 24.6 | 22.0 |
| Moderate | 861 | 28.1 | 27.8 | 28.4 |
| High | 1390 | 45.3 | 44.4 | 46.6 |
| Not reported | 96 | 3.1 | 3.2 | 3.1 |
| Education | ||||
| Low | 593 | 19.3 | 21.5 | 16.1 |
| Moderate | 1350 | 44.0 | 43.5 | 44.7 |
| High | 1100 | 35.8 | 33.9 | 38.6 |
| Not reported | 27 | 0.9 | 1.1 | 0.6 |
| Smoking status | ||||
| Daily smoking | 1828 | 59.5 | 57.3 | 62.8 |
| Weekly smoking | 639 | 20.8 | 14.3 | 30.3 |
| Former smoking (quit < 2 years) | 275 | 9.0 | 11.7 | 5.0 |
| Former smoking (quit ≥ 2 years) | 328 | 10.7 | 16.7 | 1.9 |
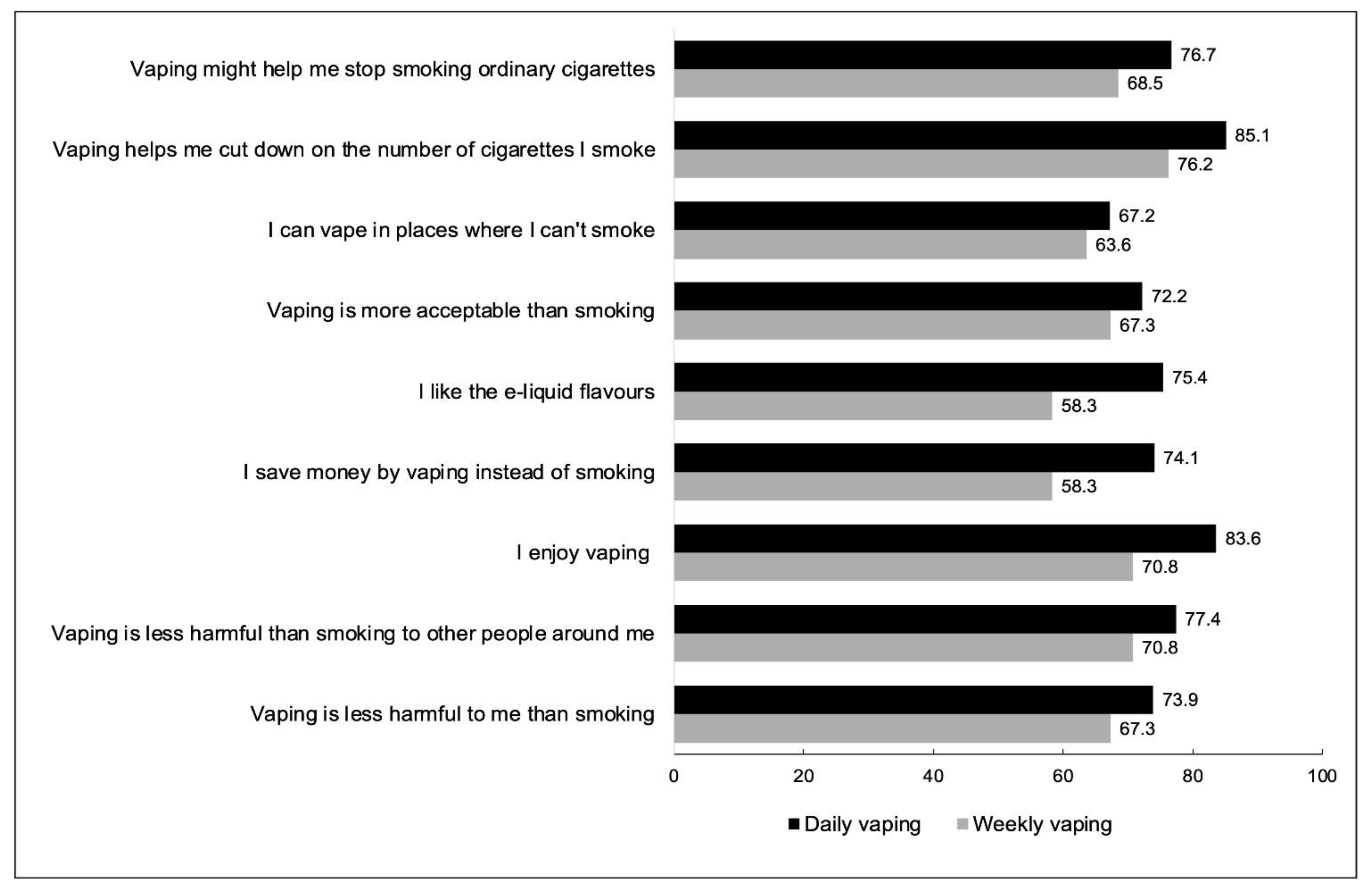
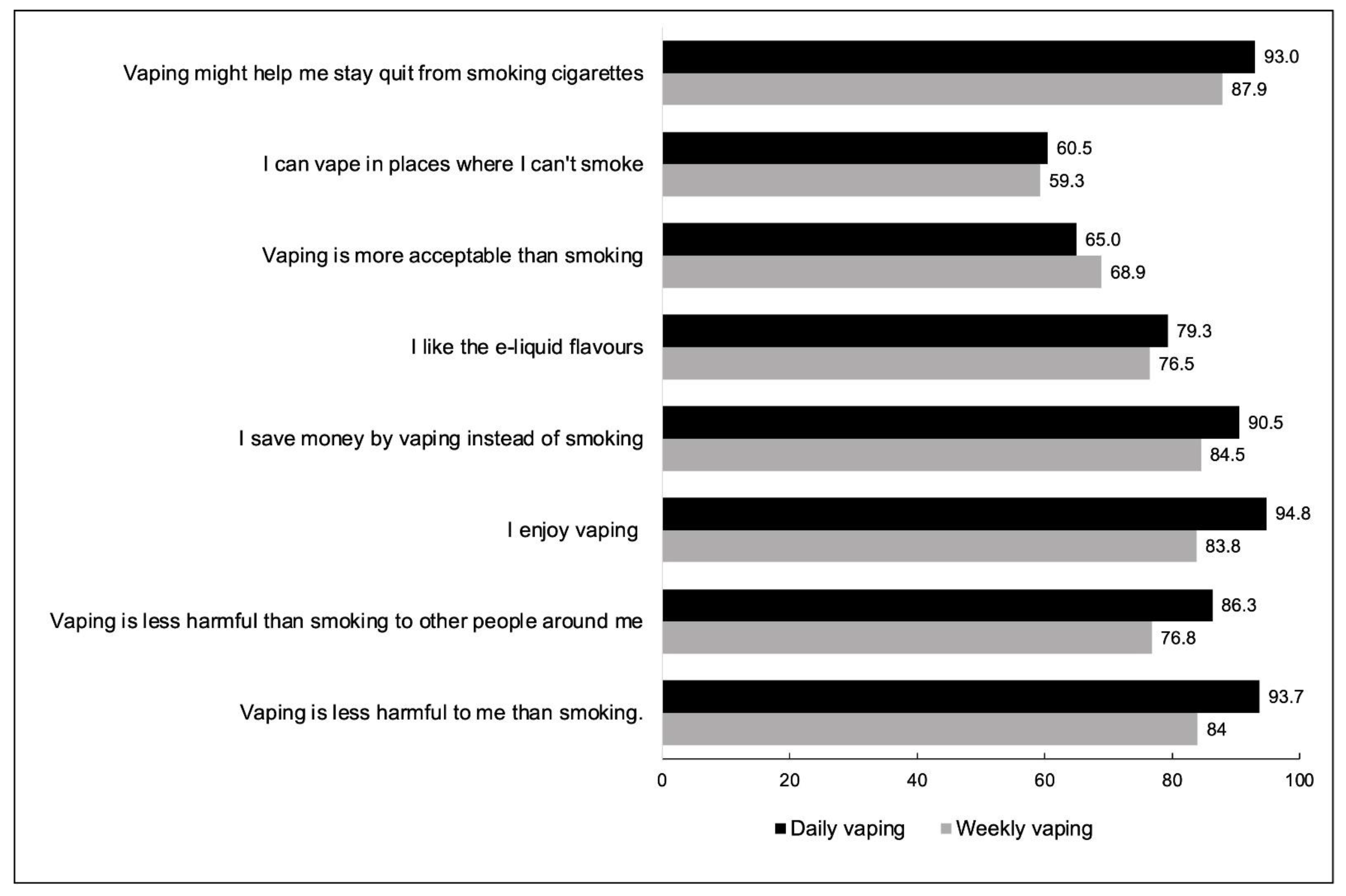
| Current Smokers | Ex-Smokers | |||||
|---|---|---|---|---|---|---|
| Reasons for Vaping, % Yes Weighted (95%CI) | Daily Vaping (n = 1306) | Weekly Vaping (n = 1161) | Total (n = 2467) | Daily Vaping (n = 518) | Weekly Vaping (n = 85) | Total (n = 603) |
| Vaping is less harmful to me than smoking | 73.9 (70.2–77.3) | 67.3 (63.5–70.9) | 70.9 (68.2–73.4) | 93.7 (90.1–96.1) | 84.0 (71.2–91.7) | 92.7 (89.1–95.1) |
| aOR (95% CI) | 1.37 (1.07–1.77) | Reference | 2.85 (1.20–6.76) | Reference | ||
| Vaping is less harmful than smoking to other people around me | 77.4 (74.0–80.6) | 70.8 (67.0–74.4) | 74.5 (71.9–76.9) | 86.3 (80.3–90.6) | 76.8 (62.6–86.7) | 85.0 (79.8–89.1) |
| aOR (95% CI) | 1.41 (1.09–1.83) | Reference | 1.90 (0.81–4.47) | Reference | ||
| I enjoy vaping | 83.6 (80.5–86.4) | 70.8 (67.0–74.4) | 78.2 (75.8–80.5) | 94.8 (92.0–96.6) | 83.8 (69.4–92.2) | 93.7 (90.9–95.7) |
| aOR (95% CI) | 2.10 (1.58–2.80) | Reference | 3.50 (1.34–9.10) | Reference | ||
| I save money by vaping instead of smoking | 74.1 (70.6–77.2) | 58.3 (54.2–62.3) | 67.0 (64.3–69.6) | 90.5 (86.3–93.6) | 84.5 (72.5–91.9) | 89.7 (85.7–92.7) |
| aOR (95% CI) | 2.04 (1.61–2.60) | Reference | 1.76 (0.78–3.96) | Reference | ||
| I like the e-liquid flavours | 75.4 (71.9–78.6) | 67.5 (63.6–71.3) | 71.9 (69.3–74.3) | 79.3 (73.8–84.0) | 76.5 (60.3–87.4) | 78.9 (73.8–83.3) |
| aOR (95% CI) | 1.48 (1.14–1.90) | Reference | 1.18 (0.52–2.69) | Reference | ||
| Vaping is more acceptable than smoking | 72.2 (68.6–75.6) | 67.3 (63.4–71.0) | 69.9 (67.3–72.5) | 65.0 (58.5–70.9) | 68.9 (53.3–81.1) | 65.6 (59.8–70.9) |
| aOR (95% CI) | 1.26 (0.99–1.61) | Reference | 0.84 (0.40–1.75) | Reference | ||
| I can vape in places where I can’t smoke | 67.2 (63.5–70.8) | 63.6 (59.6–67.4) | 65.5 (62.8–68.2) | 60.5 (54.1–66.5) | 59.3 (43.5–73.3) | 60.3 (54.4–65.9) |
| aOR (95% CI) | 1.17 (0.93–1.48) | Reference | 1.05 (0.53–2.10) | Reference | ||
| Smoking Management | ||||||
| Vaping helps me cut down on the number of cigarettes I smoke | 85.1 (82.2–87.6) | 76.2 (72.3–79.6) | 81.3 (78.9–83.5) | — | — | — |
| aOR (95% CI) | 1.79 (1.34–2.38) | Reference | ||||
| Vaping might help me stop smoking cigarettes | 76.7 (73.2–79.8) | 68.5 (64.5–72.2) | 73.0 (70.4–75.4) | |||
| aOR (95% CI) | 1.51 (1.17–1.96) | Reference | — | — | — | |
| Vaping might help me stay quit from smoking cigarettes | — | — | — | 93.0 (89.3–95.4) | 87.9 (71.4–95.5) | 92.3 (88.5–94.9) |
| aOR (95% CI) | 1.82 (0.60–5.52) | Reference | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gravely, S.; Yong, H.-H.; Reid, J.L.; East, K.A.; Gartner, C.E.; Levy, D.T.; Cummings, K.M.; Borland, R.; Quah, A.C.K.; Bansal-Travers, M.; et al. Do Current Smokers and Ex-Smokers Who Use Nicotine Vaping Products Daily Versus Weekly Differ on Their Reasons for Vaping? Findings from the 2020 ITC Four Country Smoking and Vaping Survey. Int. J. Environ. Res. Public Health 2022, 19, 14130. https://doi.org/10.3390/ijerph192114130
Gravely S, Yong H-H, Reid JL, East KA, Gartner CE, Levy DT, Cummings KM, Borland R, Quah ACK, Bansal-Travers M, et al. Do Current Smokers and Ex-Smokers Who Use Nicotine Vaping Products Daily Versus Weekly Differ on Their Reasons for Vaping? Findings from the 2020 ITC Four Country Smoking and Vaping Survey. International Journal of Environmental Research and Public Health. 2022; 19(21):14130. https://doi.org/10.3390/ijerph192114130
Chicago/Turabian StyleGravely, Shannon, Hua-Hie Yong, Jessica L. Reid, Katherine A. East, Coral E. Gartner, David T. Levy, K. Michael Cummings, Ron Borland, Anne C. K. Quah, Maansi Bansal-Travers, and et al. 2022. "Do Current Smokers and Ex-Smokers Who Use Nicotine Vaping Products Daily Versus Weekly Differ on Their Reasons for Vaping? Findings from the 2020 ITC Four Country Smoking and Vaping Survey" International Journal of Environmental Research and Public Health 19, no. 21: 14130. https://doi.org/10.3390/ijerph192114130
APA StyleGravely, S., Yong, H.-H., Reid, J. L., East, K. A., Gartner, C. E., Levy, D. T., Cummings, K. M., Borland, R., Quah, A. C. K., Bansal-Travers, M., Ouimet, J., & Fong, G. T. (2022). Do Current Smokers and Ex-Smokers Who Use Nicotine Vaping Products Daily Versus Weekly Differ on Their Reasons for Vaping? Findings from the 2020 ITC Four Country Smoking and Vaping Survey. International Journal of Environmental Research and Public Health, 19(21), 14130. https://doi.org/10.3390/ijerph192114130










