Passability of Chironomid Larvae in Granular Activated Carbon
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Species
2.2. Experimental Procedures
2.3. Data Analysis
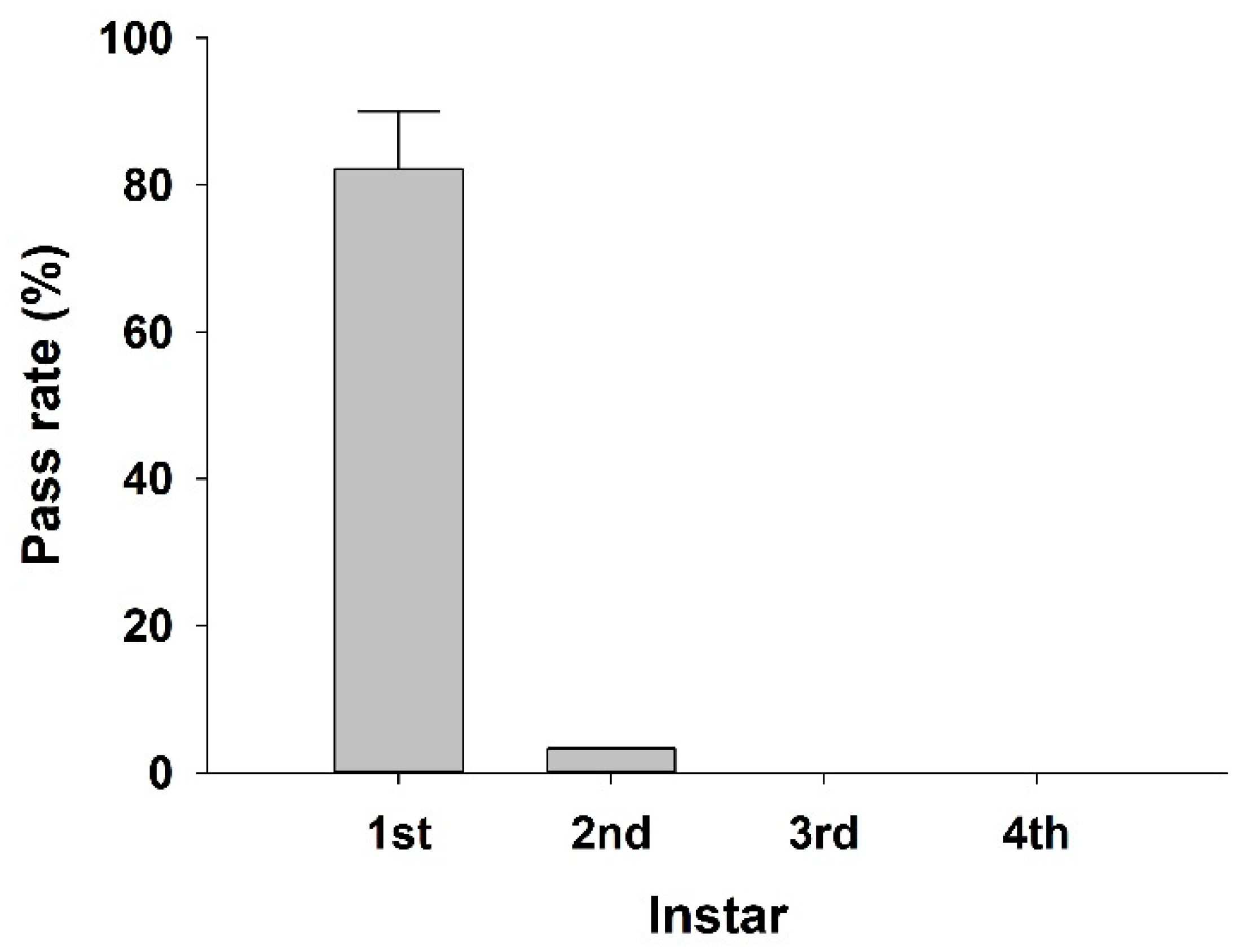
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ministry of Environment (MOE). Drinking Water Management Act. Available online: https://www.law.go.kr/LSW/lsInfoP.do?efYd=20210401&lsiSeq=216343#0000 (accessed on 1 October 2021).
- Nishijima, W.; Speitel, G.E., Jr. Fate of biodegradable dissolved organic carbon produced by ozonation on biological activated Carbon. Chemosphere 2004, 56, 113–119. [Google Scholar] [CrossRef]
- Yapsakli, K.; Çeçen, F. Effect of type of granular activated carbon on DOC biodegradation in biological activated carbon filters. Process Biochem. 2010, 45, 355–362. [Google Scholar] [CrossRef]
- Ministry of Environment (MOE). Criteria for Waterworks Facilities (2010) (In Korean). Available online: http://www.me.go.kr/home/web/policy_data/read.do?menuId=10264&seq=4769 (accessed on 31 December 2021).
- National Assembly Research Service. Occurrence of Larvae in Tap Water and Improvement Tasks. Available online: https://www.nars.go.kr/index.do (accessed on 18 October 2021).
- Ashe, P.; Murray, D.A.; Reiss, F. The zoogeographical distribution of Chironomidae (Insecta: Diptera). Ann. Limnol. -Int. J. Lim. 1987, 23, 27–60. [Google Scholar] [CrossRef]
- Baek, M.J.; Yoon, T.J.; Bae, Y.J. Development of Glyptotendipes tokunagai (Diptera: Chironomidae) under different temperature conditions. Environ. Entomol. 2012, 41, 950–958. [Google Scholar] [CrossRef]
- Kwak, I.-S.; Park, J.-W.; Kim, W.-S.; Park, K. Morphological and genetic species identification in the Chironomus larvae (Diptera: Chironomidae) found in domestic tap water purification plants. Korean J. Ecol. Environ. 2020, 53, 286–294. [Google Scholar] [CrossRef]
- Wu, Z.; Tang, X.; Chen, H. Seasonal and treatment-process variations in invertebrates in drinking water treatment plants. Front. Environ. Sci. Eng. 2021, 15, 62. [Google Scholar] [CrossRef]
- van Lieverloo, J.H.M.; Bosboom, D.W.; Bakker, G.L.; Brouwer, A.J.; Voogt, R.; De Roos, J.E.M. Sampling and quantifying invertebrates from drinking water distribution mains. Water Res. 2004, 38, 1101–1112. [Google Scholar] [CrossRef] [PubMed]
- Gunkel, G.; Michels, U.; Scheideler, M. Water lice and other macroinvertebrates in drinking water pipes: Diversity, abundance and health risk. Water 2021, 13, 276. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, H. Comparison of invertebrate removal by traditional-BAC and pre-BAC treatment processes: Verification in a full-scale drinking water treatment plant. Water Supply 2018, 18, 1261–1269. [Google Scholar] [CrossRef]
- Wotton, R.S.; Hirabayashi, K. Midge larvae (Diptera: Chironomidae) as engineers in slow sand filter beds. Water Res. 1999, 33, 1509–1515. [Google Scholar] [CrossRef]
- Alexander, M.K.; Merritt, R.W.; Berg, M.B. New strategies for the control of the parthenogenetic chironomid (Paratanytarsus grimmii) infecting water systems. J. Am. Mosq. Control Assoc. 1997, 13, 189–192. [Google Scholar]
- Christensen, S.C.B.; Nissen, E.; Arvin, E.; Albrechtsen, H.J. Distribution of Asellus aquaticus and microinvertebrates in a non-chlorinated drinking water supply system - effects of pipe material and sedimentation. Water Res. 2011, 45, 3215–3224. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Yin, W.; Yang, J.; Zhang, J.; Jiang, C. Risk assessment and inactivation of invertebrate-internalized bacteria in pilot-scale biological activated carbon filtration. Sci. Total Environ. 2019, 676, 321–332. [Google Scholar] [CrossRef]
- Weeks, M.A.; Leadbeater, B.S.C.; Callow, M.E.; Bale, J.S.; Barrie Holden, J. Effects of backwashing on the prosobranch Snail Potamopyrgus Jenkinsi Smith in granular activated carbon (GAC) adsorbers. Water Res. 2007, 41, 2690–2696. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, H.; Schoenen, D.; Traunspurger, W. Invertebrate colonization of granular activated carbon filters. Water Res. 1997, 31, 743–748. [Google Scholar] [CrossRef]
- Na, K.B. Taxonomy of the Chironmidae (Diptera, Insecta) in Seoul-Gyeonggi Area, Korea. Master’s Thesis, Seoul Women’s University, Seoul, Korea, 2004. [Google Scholar]
- Choi, S.; Kim, S.; Bae, Y.-J.; Park, J.-W.; Jung, J. Size-dependent toxicity of silver nanoparticles to Glyptotendipes tokunagai. Environ. Health Toxicol. 2015, 30, e2015003. [Google Scholar] [CrossRef] [PubMed]
- Baek, M.J.; Yoon, T.J.; Kim, D.G.; Lee, C.Y.; Cho, K.; Bae, Y.J. Effects of road deicer runoff on benthic macroinvertebrate communities in Korean freshwaters with toxicity tests of Calcium chloride (CaCl2). Water Air Soil Pollut. 2014, 225, 1961. [Google Scholar] [CrossRef]
- Baek, M.J.; Yoon, T.J.; Kang, H.J.; Bae, Y.J. Biological and genetic characteristics of Glyptotendipes tokunagai (Diptera: Chironomidae) on the basis of successive rearing of forty-two generations over seven years under laboratory conditions. Environ. Entomol. 2014, 43, 1406–1418. [Google Scholar] [CrossRef]
- Baek, M.J. Temperature-dependent development and successive rearing of Glyptotendipse tokunagai (Dipera: Chironomidae). Master’s Thesis, Seoul Women’s University, Seoul, Korea, 2009. [Google Scholar]
- Hooper, H.L.; Sibly, R.M.; Hutchinson, T.H.; Maund, S.J. The influence of larval density, food availability and habitat longevity on the life history and population growth rate of the midge Chironomus riparius. Oikos 2003, 102, 515–524. [Google Scholar] [CrossRef]
- de Winter, J.C.F. Using the Student’s t-test with extremely small sample sizes. Pract. Assess. Res. Eval. 2013, 18, 10. [Google Scholar] [CrossRef]
- Ahmad, R.; Amirtharajah, A.; Al-Shawwa, A.; Huck, P.M. Effects of backwashing on biological filters. J. Am. Water Works Assoc. 1998, 90, 62–73. [Google Scholar] [CrossRef]
- Kim, S.-G.; Park, H.-G.; Son, H.-J.; Yoom, H.-S.; Ryu, D.-C. Evaluation of influence factors for determination of proper backwashing time of biological activated carbon (BAC) process in drinking water treatment process. J. Environ. Sci. Int. 2015, 24, 1551–1558. [Google Scholar] [CrossRef][Green Version]
- Niedrist, G.H.; Füreder, L. Trophic ecology of alpine stream invertebrates: Current status and future research needs. Freshw. Sci. 2017, 36, 466–478. [Google Scholar] [CrossRef]
- Oliver, D.R. Life history of the Chironomidae. Annu. Rev. Entomol. 1971, 16, 211–230. [Google Scholar] [CrossRef]
- Mckie, B.G. Disturbance and investment: Developmental responses of tropical lotic midges to repeated tube destruction in the juvenile stages. Ecol. Entomol. 2004, 29, 457–466. [Google Scholar] [CrossRef]
- Hershey, A.E. Tubes and foraging behavior in larval Chironomidae: Implications for predator avoidance. Oecologia 1987, 73, 236–241. [Google Scholar] [CrossRef]
- Begon, M.; Townsend, C.R.; Harper, J.L. Ecology: From Individuals to Ecosystem, 4th ed.; Wiley-Blackwell: Oxford, UK, 2006; pp. 132–185. [Google Scholar]
- van Lieverloo, J.H.M.; Hoogenboezem, W.; Veenendaal, G.; van der Kooij, D. Variability of invertebrate abundance in drinking water distribution systems in the Netherlands in relation to biostability and sediment volumes. Water Res. 2012, 46, 4918–4932. [Google Scholar] [CrossRef]
- Evins, C. Small animals in drinking-water distribution systems. In Safe Piped Water: Managing Microbial Water Quality in Piped Distribution Systems; Ainsworth, R., Ed.; World Health Organization. IWA Publishing: London, UK, 2004; pp. 101–120. ISBN 1843390396. [Google Scholar]
- Dettinger-Klemm, P.M.A. Chironomids (Diptera, Nematocera) of Temporary Pools—An Ecological Case Study. Ph.D. Thesis, Philipps-Universität Marburg, Marburg, Germany, 2003. [Google Scholar]
- Figueras, M.J.; Beaz-Hidalgo, R.; Senderovich, Y.; Laviad, S.; Halpern, M. Re-identification of Aeromonas isolates from chironomid egg masses as the potential pathogenic bacteria Aeromonas aquariorum. Environ. Microbiol. Rep. 2011, 3, 239–244. [Google Scholar] [CrossRef]


| Instar | Kruskal-Wallis | Jonckheere–Terpstra |
|---|---|---|
| Second | NS 1 | NS |
| Third | NS | 0.009 |
| Fourth | 0.05 | 0.034 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.Y.; Byeon, J.; Kim, M.K.; Lee, T.-g.; Kim, D.G. Passability of Chironomid Larvae in Granular Activated Carbon. Int. J. Environ. Res. Public Health 2022, 19, 1005. https://doi.org/10.3390/ijerph19021005
Lee CY, Byeon J, Kim MK, Lee T-g, Kim DG. Passability of Chironomid Larvae in Granular Activated Carbon. International Journal of Environmental Research and Public Health. 2022; 19(2):1005. https://doi.org/10.3390/ijerph19021005
Chicago/Turabian StyleLee, Cha Young, Jinseok Byeon, Min Kyung Kim, Tae-gwan Lee, and Dong Gun Kim. 2022. "Passability of Chironomid Larvae in Granular Activated Carbon" International Journal of Environmental Research and Public Health 19, no. 2: 1005. https://doi.org/10.3390/ijerph19021005
APA StyleLee, C. Y., Byeon, J., Kim, M. K., Lee, T.-g., & Kim, D. G. (2022). Passability of Chironomid Larvae in Granular Activated Carbon. International Journal of Environmental Research and Public Health, 19(2), 1005. https://doi.org/10.3390/ijerph19021005






