Impact of Constraint-Induced Movement Therapy (CIMT) on Functional Ambulation in Stroke Patients—A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection Criteria of Studies for This Review
2.2. Literature Search and Study Selection
2.3. Data Extraction
2.4. Evaluation of Methodological Quality and Level of Evidence
2.5. Risk of Bias Assessment
2.6. Data Synthesis
3. Results
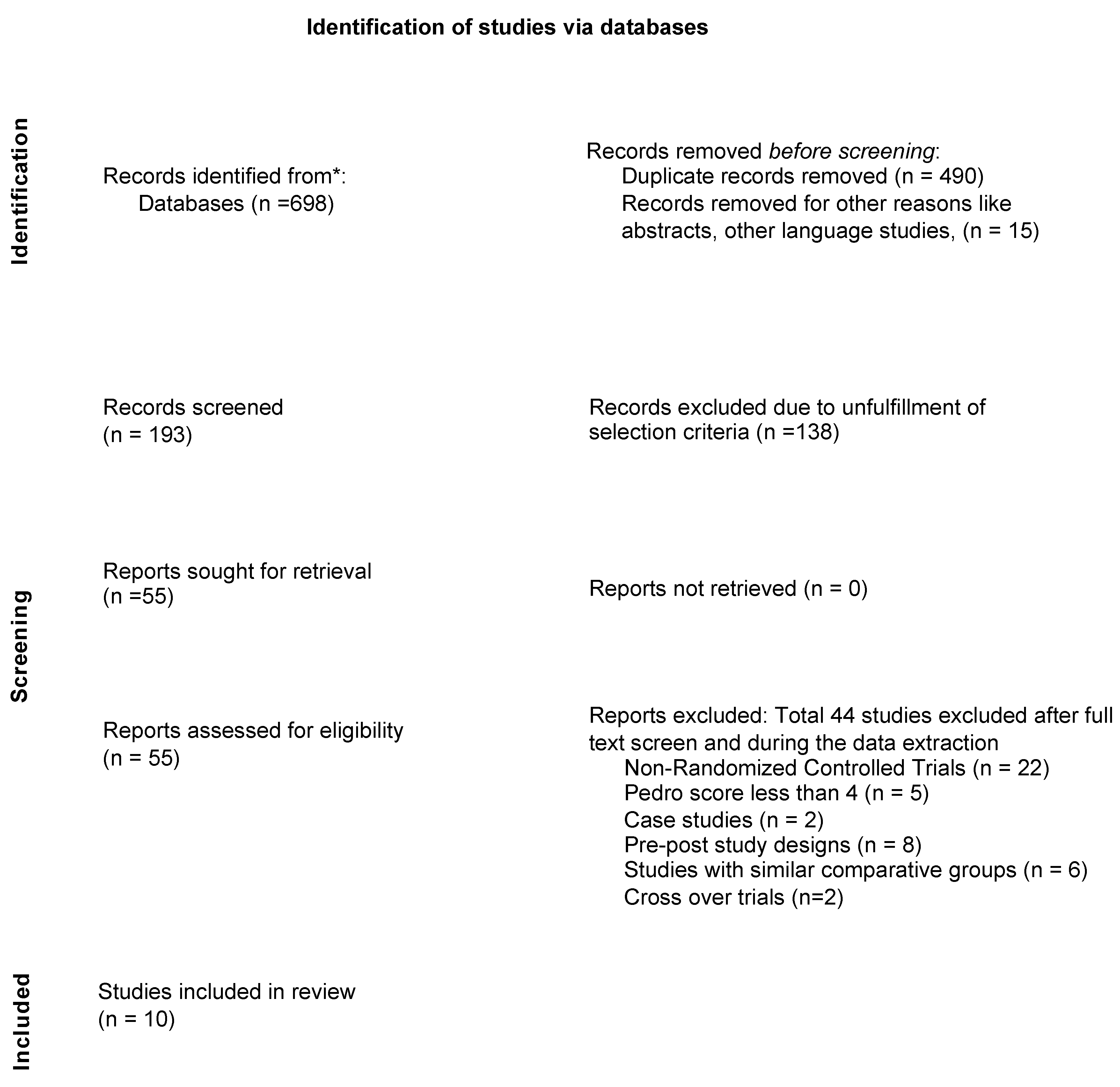
3.1. Search Results
3.2. Characteristics of Included Studies
3.3. Outcome Measures
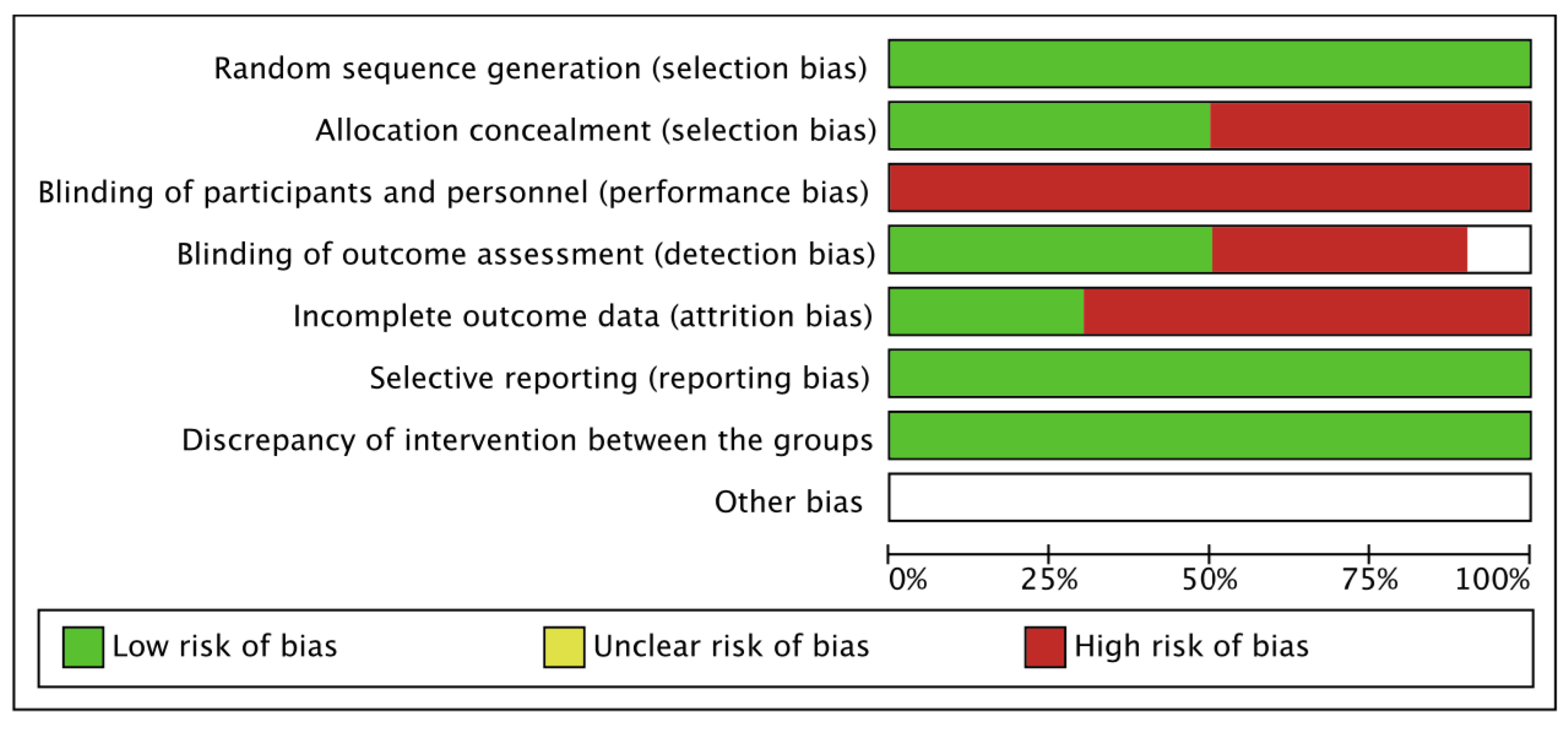
3.4. Methodological Quality and Level of Evidence
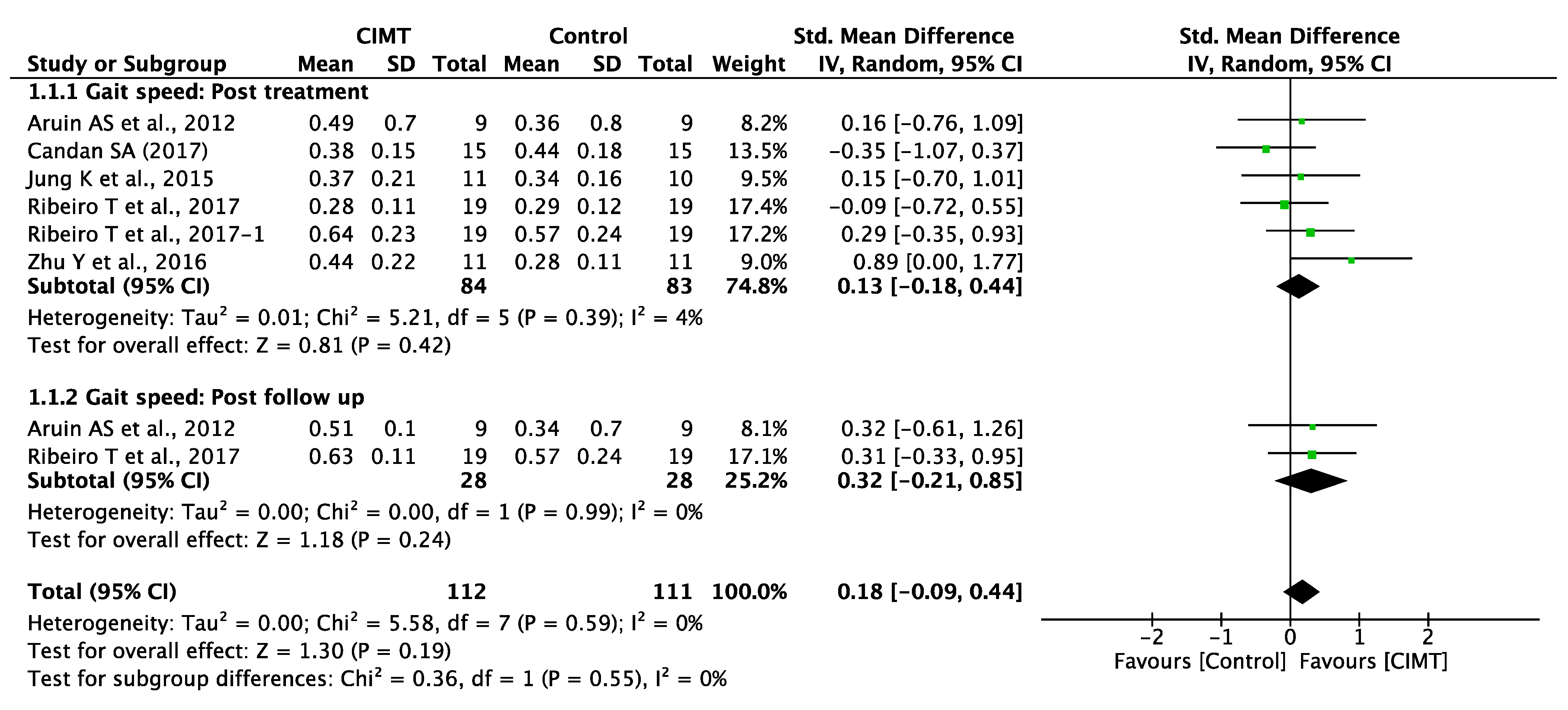
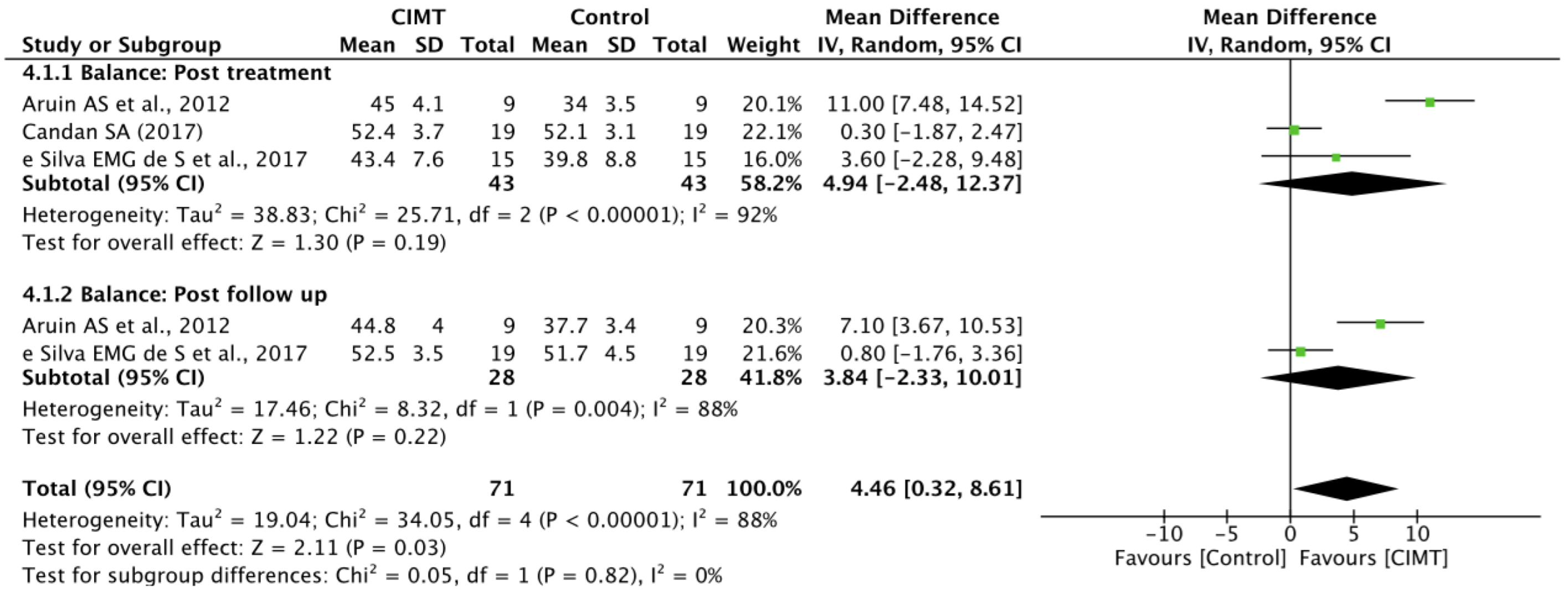
3.5. Quantitative Synthesis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krishnamurthi, R.V.; Ikeda, T.; Feigin, V.L. Global, regional and country-specific burden of ischaemic stroke, intracerebral haemorrhage and subarachnoid haemorrhage: A systematic analysis of the global burden of disease study 2017. Neuroepidemiology 2020, 54, 171–179. [Google Scholar] [CrossRef]
- Johnson, W.; Onuma, O.; Owolabi, M.; Sachdev, S. Stroke: A global response is needed. Bull. World Health Organ. 2016, 94, 634. [Google Scholar] [CrossRef]
- Lanas, F.; Seron, P. Facing the stroke burden worldwide. Lancet Glob. Health 2021, 9, e235–e236. [Google Scholar] [CrossRef]
- Harris, J.; Eng, J. Goal priorities identified by individuals with chronic stroke: Implications for rehabilitation professionals. Physiother. Can. 2004, 56, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Griffin, J.; Reddin, G. Shoulder pain in patients with hemiplegia: A literature review. Phys. Ther. 1981, 61, 1041–1045. [Google Scholar] [CrossRef] [PubMed]
- Michael, K.M.; Allen, J.K.; Macko, R.F. Reduced ambulatory activity after stroke: The role of balance, gait, and cardiovascular fitness. Arch. Phys. Med. Rehabil. 2005, 86, 1552–1556. [Google Scholar] [CrossRef] [PubMed]
- Hessam, M.; Salehi, R.; Yazdi, M.J.S.; Negahban, H.; Rafie, S.; Mehravar, M. Relationship between functional balance and walking ability in individuals with chronic stroke. J. Phys. Ther. Sci. 2018, 30, 993–996. [Google Scholar] [CrossRef]
- Lee, B.J.; Joo, N.-Y.; Kim, S.H.; Kim, C.R.; Yang, D.; Park, D. Evaluation of balance functions using temporo-spatial gait analysis parameters in patients with brain lesions. Sci. Rep. 2021, 11, 1–7. [Google Scholar]
- Norvang, O.P.; Askim, T.; Egerton, T.; Dahl, A.E.; Thingstad, P. Associations between changes in gait parameters, balance, and walking capacity during the first 3 months after stroke: A prospective observational study. Physiother. Theory Pract. 2022, 38, 534–542. [Google Scholar] [CrossRef]
- Chang, M.C.; Lee, B.J.; Joo, N.-Y.; Park, D. The parameters of gait analysis related to ambulatory and balance functions in hemiplegic stroke patients: A gait analysis study. BMC Neurol. 2021, 21, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Krasovsky, T.; Levin, M.F. Toward a better understanding of coordination in healthy and poststroke gait. Neurorehabil. Neural Repair 2010, 24, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Tok, F.; Ozcakar, L.; Safaz, I.; Alaca, R. Effects of botulinum toxin-A on the muscle architecture of stroke patients: An ultrasonographic study. J. Rehabilitation Med. 2011, 43, 1016–1019. [Google Scholar] [CrossRef] [PubMed]
- Perry, J.; Garrett, M.; Gronley, J.K.; Mulroy, S.J. Classification of walking handicap in the stroke population. Stroke 1995, 26, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.; Galna, B.; Morris, M.E.; Olver, J. Spatiotemporal deficits and kinematic classification of gait following a traumatic brain injury: A systematic review. J. Head Trauma Rehabil. 2010, 25, 366–374. [Google Scholar] [CrossRef]
- Bensoussan, L.; Mesure, S.; Viton, J.-M.; Delarque, A. Kinematic and kinetic asymmetries in hemiplegic patients’ gait initiation patterns. J. Rehabil. Med. 2006, 38, 287–294. [Google Scholar] [CrossRef]
- Baert, I.; Daly, D.; Dejaeger, E.; Vanroy, C.; Vanlandewijck, Y.; Feys, H. Evolution of cardiorespiratory fitness after stroke: A 1-year follow-up study. Influence of prestroke patients’ characteristics and stroke-related factors. Arch. Phys. Med. Rehabil. 2012, 93, 669–676. [Google Scholar] [CrossRef]
- Pase, M.P.; Beiser, A.; Enserro, D.; Xanthakis, V.; Aparicio, H.; Satizabal, C.L.; Himali, J.J.; Kase, C.S.; Vasan, R.S.; De Carli, C. Association of ideal cardiovascular health with vascular brain injury and incident dementia. Stroke 2016, 47, 1201–1206. [Google Scholar] [CrossRef]
- Billinger, S.A.; Kluding, P.M. Use of doppler ultrasound to assess femoral artery adaptations in the hemiparetic limb in people with stroke. Cerebrovasc. Dis. 2009, 27, 552–558. [Google Scholar] [CrossRef]
- Ivey, F.M.; Gardner, A.W.; Dobrovolny, C.L.; Macko, R.F. Unilateral impairment of leg blood flow in chronic stroke patients. Cerebrovasc. Dis. 2004, 18, 283–289. [Google Scholar]
- Landin, S.; Hagenfeldt, L.; Saltin, B.; Wahren, J. Muscle metabolism during exercise in hemiparetic patients. Clin. Sci. Mol. Med. 1977, 53, 257–269. [Google Scholar] [CrossRef]
- Gordon, N.F.; Gulanick, M.; Costa, F.; Fletcher, G.; Franklin, B.A.; Roth, E.J.; Shephard, T. Physical activity and exercise recommendations for stroke survivors: An American Heart Association scientific statement from the Council on Clinical Cardiology, Subcommittee on Exercise, Cardiac Rehabilitation, and Prevention; the Council on Cardiovascular Nursing; the Council on Nutrition, Physical Activity, and Metabolism; and the Stroke Council. Circulation 2004, 109, 2031–2041. [Google Scholar] [PubMed]
- MacKay-Lyons, M.J.; Makrides, L. Exercise capacity early after stroke. Arch. Phys. Med. Rehabil. 2002, 83, 1697–1702. [Google Scholar] [CrossRef] [PubMed]
- Raimundo, R.D.; de Abreu, L.C.; Adami, F.; Vanderlei, F.M.; de Carvalho, T.D.; Moreno, I.L.; Pereira, V.X.; Valenti, V.E.; Sato, M.A. Heart rate variability in stroke patients submitted to an acute bout of aerobic exercise. Transl. Stroke Res. 2013, 4, 488–499. [Google Scholar] [CrossRef] [PubMed]
- Nadeau, S.; Arsenault, A.B.; Gravel, D.; Bourbonnais, D. Analysis of the clinical factors determining natural and maximal gait speeds in adults with A Stroke1. Am. J. Phys. Med. Rehabil. 1999, 78, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Jonsdottir, J.; Recalcati, M.; Rabuffetti, M.; Casiraghi, A.; Boccardi, S.; Ferrarin, M. Functional resources to increase gait speed in people with stroke: Strategies adopted compared to healthy controls. Gait Posture 2009, 29, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.O.; Kilbreath, S.L.; Davis, G.M.; Zeman, B.; Raymond, J. Cardiorespiratory fitness and walking ability in subacute stroke patients. Arch. Phys. Med. Rehabil. 2003, 84, 1780–1785. [Google Scholar] [CrossRef]
- Tang, A.; Sibley, K.M.; Bayley, M.T.; McIlroy, W.E.; Brooks, D. Do functional walk tests reflect cardiorespiratory fitness in sub-acute stroke? J. Neuroeng. Rehabil. 2006, 3, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.H.; Kim, M.S.; Huh, J.P.; Lee, P.K.; Kim, Y.-H. Effects of robot-assisted gait training on cardiopulmonary fitness in subacute stroke patients: A randomized controlled study. Neurorehabil. Neural Repair 2012, 26, 318–324. [Google Scholar] [CrossRef]
- Yu, J.; Kang, H.; Jung, J. Effects of modified constraint-induced movement therapy on hand dexterity, grip strength and activities of daily living of children with cerebral palsy: A randomized control trial. J. Phys. Ther. Sci. 2012, 24, 1029–1031. [Google Scholar] [CrossRef]
- Shaw, S.E.; Morris, D.M.; Uswatte, G.; McKay, S.; Meythaler, J.M.; Taub, E. Constraint-induced movement therapy for recovery of upper-limb function following traumatic brain injury. J. Rehabil. Res. Dev. 2005, 42, 769–778. [Google Scholar] [CrossRef]
- Balardin, J.B.; Miotto, E.C. A review of Constraint-Induced Therapy applied to aphasia rehabilitation in stroke patients. Dement. Neuropsychol. 2009, 3, 275–282. [Google Scholar] [CrossRef][Green Version]
- Kim, Y.-J.; Kim, J.-K.; Park, S.-Y. Effects of modified constraint-induced movement therapy and functional bimanual training on upper extremity function and daily activities in a patient with incomplete spinal cord injury: A case study. J. Phys. Ther. Sci. 2015, 27, 3945–3946. [Google Scholar] [CrossRef] [PubMed][Green Version]
- de Sire, A.; Bigoni, M.; Priano, L.; Baudo, S.; Solaro, C.; Mauro, A. Constraint-induced movement therapy in multiple sclerosis: Safety and three-dimensional kinematic analysis of upper limb activity. A randomized single-blind pilot study. NeuroRehabilitation 2019, 45, 247–254. [Google Scholar] [CrossRef]
- Kim, J.-H.; Chang, M.-Y. Effects of modified constraint-induced movement therapy on upper extremity function and occupational performance of stroke patients. J. Phys. Ther. Sci. 2018, 30, 1092–1094. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kwakkel, G.; Veerbeek, J.M.; van Wegen, E.E.; Wolf, S.L. Constraint-induced movement therapy after stroke. Lancet Neurol. 2015, 14, 224–234. [Google Scholar] [CrossRef]
- Taub, E. Somatosensory deafferetation research with monkeys: Implications for rehabilitation medicine. Behav. Psychol. Rehabil. Med. Clin. Appl. 1980, 13, 26–35. [Google Scholar]
- Taub, E.; Miller, N.E.; Novack, T.A.; Cook, E.W.; Fleming, W.C.; Nepomuceno, C.S.; Connell, J.S.; Crago, J. Technique to improve chronic motor deficit after stroke. Arch. Phys. Med. Rehabil. 1993, 74, 347–354. [Google Scholar] [PubMed]
- Mark, V.W.; Taub, E. Constraint-induced movement therapy for chronic stroke hemiparesis and other disabilities. Restor. Neurol. Neurosci. 2004, 22, 317–336. [Google Scholar] [PubMed]
- Titianova, E.B.; Pitkänen, K.; Pääkkönen, A.; Sivenius, J.; Tarkka, I.M. Gait characteristics and functional ambulation profile in patients with chronic unilateral stroke. Am. J. Phys. Med. Rehabil. 2003, 82, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Woolley, S.M. Characteristics of gait in hemiplegia. Top. Stroke Rehabil. 2001, 7, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Liepert, J.; Miltner, W.; Bauder, H.; Sommer, M.; Dettmers, C.; Taub, E.; Weiller, C. Motor cortex plasticity during constraint-induced movement therapy in stroke patients. Neurosci. Lett. 1998, 250, 5–8. [Google Scholar] [CrossRef]
- Eng, J.J.; Tang, P.-F. Gait training strategies to optimize walking ability in people with stroke: A synthesis of the evidence. Expert Rev. Neurother. 2007, 7, 1417–1436. [Google Scholar] [CrossRef]
- Wang, W.; Wang, A.; Yu, L.; Han, X.; Jiang, G.; Weng, C.; Zhang, H.; Zhou, Z. Constraint-induced movement therapy promotes brain functional reorganization in stroke patients with hemiplegia. Neural Regen. Res. 2012, 7, 2548. [Google Scholar]
- Könönen, M.; Tarkka, I.; Niskanen, E.; Pihlajamäki, M.; Mervaala, E.; Pitkänen, K.; Vanninen, R. Functional MRI and motor behavioral changes obtained with constraint-induced movement therapy in chronic stroke. Eur. J. Neurol. 2012, 19, 578–586. [Google Scholar] [CrossRef]
- Dixit, S.; Gular, K.; Asiri, F. Effect of diverse physical rehabilitative interventions on static postural control in diabetic peripheral neuropathy: A systematic review. Physiother. Theory Pract. 2018, 36, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Ades, A.; Lu, G.; Higgins, J. The interpretation of random-effects meta-analysis in decision models. Med. Decis. Mak. 2005, 25, 646–654. [Google Scholar] [CrossRef]
- Wu, C.-y.; Lin, K.-c.; Chen, H.-c.; Chen, I.-h.; Hong, W.-h. Effects of modified constraint-induced movement therapy on movement kinematics and daily function in patients with stroke: A kinematic study of motor control mechanisms. Neurorehabil. Neural Repair 2007, 21, 460–466. [Google Scholar] [CrossRef]
- Ribeiro, T.S.; Silva, E.M.; Silva, I.A.; Costa, M.F.; Cavalcanti, F.A.; Lindquist, A.R. Effects of treadmill training with load addition on non-paretic lower limb on gait parameters after stroke: A randomized controlled clinical trial. Gait Posture 2017, 54, 229–235. [Google Scholar] [CrossRef]
- Lin, K.-C.; Wu, C.-Y.; Wei, T.-H.; Gung, C.; Lee, C.-Y.; Liu, J.-S. Effects of modified constraint-induced movement therapy on reach-to-grasp movements and functional performance after chronic stroke: A randomized controlled study. Clin. Rehabil. 2007, 21, 1075–1086. [Google Scholar] [CrossRef]
- Ribeiro, T.S.; Chaves da Silva, T.C.; Carlos, R.; de Souza e Silva, E.M.G.; Lacerda, M.O.; Spaniol, A.P.; Lindquist, A.R.R. Is there influence of the load addition during treadmill training on cardiovascular parameters and gait performance in patients with stroke? A randomized clinical trial. NeuroRehabilitation 2017, 40, 345–354. [Google Scholar] [CrossRef]
- Ribeiro, T.S.; Regalado, I.C.R.; da Silva, S.T.; de Oliveira Sousa, C.; de Figueiredo, K.M.O.B.; Lindquist, A.R.R. Effects of Load Addition During Gait Training on Weight-Bearing and Temporal Asymmetry After Stroke: A Randomized Clinical Trial. Am. J. Phys. Med. Rehabil. 2020, 99, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Kim, Y.; Cha, Y.; In, T.-S.; Hur, Y.-G.; Chung, Y. Effects of gait training with a cane and an augmented pressure sensor for enhancement of weight bearing over the affected lower limb in patients with stroke: A randomized controlled pilot study. Clin. Rehabil. 2015, 29, 135–142. [Google Scholar] [CrossRef] [PubMed]
- de Souza E Silva, E.M.G.; Ribeiro, T.S.; da Silva, T.C.C.; Costa, M.F.P.; da Costa Cavalcanti, F.A.; Lindquist, A.R.R. Effects of constraint-induced movement therapy for lower limbs on measurements of functional mobility and postural balance in subjects with stroke: A randomized controlled trial. Top. Stroke Rehabil. 2017, 24, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Bonnyaud, C.; Pradon, D.; Zory, R.; Bussel, B.; Bensmail, D.; Vuillerme, N.; Roche, N. Effects of a gait training session combined with a mass on the non-paretic lower limb on locomotion of hemiparetic patients: A randomized controlled clinical trial. Gait Posture 2013, 37, 627–630. [Google Scholar] [CrossRef] [PubMed]
- Bonnyaud, C.; Pradon, D.; Boudarham, J.; Robertson, J.; Vuillerme, N.; Roche, N. Effects of gait training using a robotic constraint (Lokomat®) on gait kinematics and kinetics in chronic stroke patients. J. Rehabil. Med. 2014, 46, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Aruin, A.S.; Rao, N.; Sharma, A.; Chaudhuri, G. Compelled body weight shift approach in rehabilitation of individuals with chronic stroke. Top. Stroke Rehabil. 2012, 19, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Bang, D.-H.; Shin, W.-S.; Choi, H.-S. Effects of modified constraint-induced movement therapy with trunk restraint in early stroke patients: A single-blinded, randomized, controlled, pilot trial. NeuroRehabilitation 2018, 42, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhou, C.; Liu, Y.; Liu, J.; Jin, J.; Zhang, S.; Bai, Y.; Huang, D.; Zhu, B.; Xu, Y. Effects of modified constraint-induced movement therapy on the lower extremities in patients with stroke: A pilot study. Disabil. Rehabil. 2016, 38, 1893–1899. [Google Scholar] [CrossRef] [PubMed]
- Candan, S.A.; Livanelioglu, A. Effects of modified constraint-ınduced movement therapy for lower limb on motor function in stroke patients: A randomized controlled study. Int J Physiother 2017, 4, 269–277. [Google Scholar] [CrossRef]
- Abdullahi, A.; Truijen, S.; Umar, N.A.; Useh, U.; Egwuonwu, V.A.; Van Criekinge, T.; Saeys, W. Effects of Lower Limb Constraint Induced Movement Therapy in People With Stroke: A Systematic Review and Meta-Analysis. Front. Neurol. 2021, 12, 343. [Google Scholar] [CrossRef]
- Bernhardt, J.; Hayward, K.S.; Kwakkel, G.; Ward, N.S.; Wolf, S.L.; Borschmann, K.; Krakauer, J.W.; Boyd, L.A.; Carmichael, S.T.; Corbett, D. Agreed definitions and a shared vision for new standards in stroke recovery research: The stroke recovery and rehabilitation roundtable taskforce. Int. J. Stroke 2017, 12, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, M.; Hassan, T.M. Cerebral damage after stroke: The role of neuroplasticity as key for recovery. In Cerebral and Cerebellar Cortex–Interaction and Dynamics in Health and Disease; IntechOpen: London, UK, 2021. [Google Scholar]
- Page, S.J.; Gater, D.R.; Bach-y-Rita, P. Reconsidering the motor recovery plateau in stroke rehabilitation. Arch. Phys. Med. Rehabil. 2004, 85, 1377–1381. [Google Scholar] [CrossRef] [PubMed]
- Dos Anjos, S.; Morris, D.; Taub, E. Constraint-induced movement therapy for lower extremity function: Describing the LE-CIMT protocol. Phys. Ther. 2020, 100, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Sterr, A.; Freivogel, S. Motor-improvement following intensive training in low-functioning chronic hemiparesis. Neurology 2003, 61, 842–844. [Google Scholar] [CrossRef]
- Elbert, T.; Rockstroh, B.; Bulach, D.; Meinzer, M.; Taub, E. New developments in stroke rehabilitation based on behavioral and neuroscientific principles: Constraint-induced therapy. Der Nervenarzt 2003, 74, 334–342. [Google Scholar]
- Danlami, K.A.; Abdullahi, A. Remodelling the protocol of lower limb constraint-induced movement therapy: A pilot randomized controlled trial. Arch. Physiother. Glob. Res. 2017, 21, 21–27. [Google Scholar]
- Abdullahi, A.; Aliyu, N.U.; Useh, U.; Abba, M.A.; Akindele, M.O.; Truijen, S.; Saeys, W. Comparing Two Different Modes of Task Practice during Lower Limb Constraint-Induced Movement Therapy in People with Stroke: A Randomized Clinical Trial. Neural Plast. 2021, 2021, 1–9. [Google Scholar] [CrossRef]
- Classen, J.; Liepert, J.; Wise, S.P.; Hallett, M.; Cohen, L.G. Rapid plasticity of human cortical movement representation induced by practice. J. Neurophysiol. 1998, 79, 1117–1123. [Google Scholar] [CrossRef]
- Bütefisch, C.; Hummelsheim, H.; Denzler, P.; Mauritz, K.-H. Repetitive training of isolated movements improves the outcome of motor rehabilitation of the centrally paretic hand. J. Neurol. Sci. 1995, 130, 59–68. [Google Scholar] [CrossRef]
- Pang, M.Y.; Charlesworth, S.A.; Lau, R.W.; Chung, R.C. Using aerobic exercise to improve health outcomes and quality of life in stroke: Evidence-based exercise prescription recommendations. Cerebrovasc. Dis. 2013, 35, 7–22. [Google Scholar] [CrossRef]
- Stoller, O.; de Bruin, E.D.; Knols, R.H.; Hunt, K.J. Effects of cardiovascular exercise early after stroke: Systematic review and meta-analysis. BMC Neurol. 2012, 12, 45. [Google Scholar] [CrossRef] [PubMed]
- de Havenon, A.; Fino, N.F.; Johnson, B.; Wong, K.-H.; Majersik, J.J.; Tirschwell, D.; Rost, N. Blood pressure variability and cardiovascular outcomes in patients with prior stroke: A secondary analysis of PRoFESS. Stroke 2019, 50, 3170–3176. [Google Scholar] [CrossRef] [PubMed]
- Rhyu, I.; Bytheway, J.; Kohler, S.; Lange, H.; Lee, K.; Boklewski, J.; McCormick, K.; Williams, N.; Stanton, G.; Greenough, W. Effects of aerobic exercise training on cognitive function and cortical vascularity in monkeys. Neuroscience 2010, 167, 1239–1248. [Google Scholar] [CrossRef] [PubMed]
- Hassan, T.M.; Yarube, I.U. Peripheral brain-derived neurotrophic factor is reduced in stroke survivors with cognitive impairment. Pathophysiology 2018, 25, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Holleran, C.L.; Rodriguez, K.S.; Echauz, A.; Leech, K.A.; Hornby, T.G. Potential contributions of training intensity on locomotor performance in individuals with chronic stroke. J. Neurol. Phys. Ther. 2015, 39, 95–102. [Google Scholar] [CrossRef]
- Macko, R.; Ivey, F.; Forrester, L. Task-oriented aerobic exercise in chronic hemiparetic stroke: Training protocols and treatment effects. Top. Stroke Rehabil. 2005, 12, 45–57. [Google Scholar] [CrossRef]
- Taylor, J.L.; Holland, D.J.; Spathis, J.G.; Beetham, K.S.; Wisløff, U.; Keating, S.E.; Coombes, J.S. Guidelines for the delivery and monitoring of high intensity interval training in clinical populations. Prog. Cardiovasc. Dis. 2019, 62, 140–146. [Google Scholar] [CrossRef]
- Munari, D.; Pedrinolla, A.; Smania, N.; Picelli, A.; Gandolfi, M.; Saltuari, L.; Schena, F. High-intensity treadmill training improves gait ability, VO2peak and cost of walking in stroke survivors: Preliminary results of a pilot randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2018, 54, 408–418. [Google Scholar] [CrossRef]
| Databases | PICO Format Search with Bullion Keywords (And) (OR) | |||
|---|---|---|---|---|
| Patient | Intervention | Comparison | Outcome | |
| EBSCO, PubMed, PEDro, Science Direct, Scopus, MEDLINE, CINAHL, and Web of Science | Stroke OR Hemiplegia OR Hemiparesis OR Cerebrovascular accident | CIMT OR Constraint Induced Movement therapy OR Restricted Limb/Extremity OR Forced use | Proprioceptive Neuromuscular Facilitation OR PNF OR Neuro-Developmental Treatment R NDT OR Conventional Physical Therapy OR CPT OR Physiotherapy OR Exercise OR Or traditional rehabilitation OR Standard Physical Therapy | Gait speed OR Gait velocity OR Balance OR Center of Gravity OR Base of Support OR Center of Pressure OR Cardiovascular parameters OR Blood pressure OR percentage of heart rate maximum. |
| Author/Year | Age | Chronicity | Intervention | Outcome Measures | Inferences | ||
|---|---|---|---|---|---|---|---|
| Experimental | Control | Duration | |||||
| Aruin AS et al., 2012 | 57.7 ± 11.9 | Chronic | A shoe insert is provided on the unaffected side to shift body weight onto the affected side to promote muscle strength and weight-bearing capability. | The treatment encompasses the promotion of weight-bearing towards the affected side to promote balance and muscle strength. | 60 min per session, one session per week, six sessions in total, 6 h. | Symmetrical weight bearing, gait speed (m/s), BBS, Fugl-Meyer for lower extremity. | Post and follow-up retention were observed in the experimental group for symmetrical weight bearing, gait speed, and BBS in the experimental group. |
| Bonnyaud C et al., 2013 | 50.03 ± 13.1 | Chronic | Treadmill training with ankle mass on non-paretic lower extremity. | Treadmill training. | 20 min, single session. | Cadence (steps/min), step length (cm), peak hip and knee flexion and dorsiflexion, vertical GRF (N/Kg), peak propulsion (N/Kg), peak breaking (N/Kg) gait speed (m/s). | The experimental and control group showed similar effects for gait variables. |
| Bonnyaud C et al., 2014 | 50.6 5 ± 11.65 | Chronic | Asymmetrical gait training group: RAGT providing negative kinematic restraint applied to non-paretic lower extremity. | Symmetrical RAGT gait-training group. | 20 min, single session. | Symmetry ratio, stance time, double support time, static and dynamic GRF. | Peak knee flexion range was improved in the asymmetrical robotic raining group, and other gait variables improved equally among symmetrical and asymmetrical RAGT groups. |
| Jung K et al., 2015 | 56.35 ± 14.1 | Subacute/chronic | Auditory feedback provided while walking with a cane in addition to standard therapy. | Walking with a cane in addition to standard therapy. | Gait training: 5 days per week for four weeks, 30 min per session. Standard therapy: Five days per week for four weeks, 30 min per session. | Gluteus medius and vastus medialis oblique muscle activity, single support phase of the affected side (% GC) vertical peak force of the cane (% BW) and gait speed (m/s). | The experimental group showed significant improvement in muscle activation and gait speed. |
| Zhu Y et al., 2016 | 58.71 ± 6.02 | Subacute | Gait training consists of 2 h of sit-to-stand transfers, indoor walking, climbing up and down stairs, balance training and one-leg weight training with more repetitions in addition to this standard comprehensive rehabilitation. | Standard comprehensive rehabilitation treatment includes passive and active ROM exercises, stretching, balance and gait training. | Four weeks five days per week. | Step length (m), COM displacements, swing time (%gait cycle) step width(m), and gait speed(m/s). | m-CIMT gait training improved both COM displacements and spatio-temporal gait parameters. |
| Ribeiro T et al., 2017 | 57.75 ± 3.75 | Subacute/chronic | Gait training on a treadmill, applying weight on the unaffected side. | Gait training on a treadmill. | The nine training sessions, 30 min, two consecutive weeks. | Step length, hip, knee and ankle ROM, and gait speed(m/s). | Spatio-temporal and kinematic gait parameters improved in both groups equally. |
| e Silva EMG de S et al., 2017 | 57.75 ± 3.75 | Subacute/chronic | Gait training on a treadmill, applying weight on the unaffected side. | Gait training on a treadmill. | The nine training sessions, 30 min, two consecutive weeks. | BBS, stride time(s), TUG, symmetry ratio, stride width(m), turn speed(m/s), and stride length(m). | Spatio-temporal gait parameters balance and functional mobility improved in both groups equally. |
| Candan SA et al., 2017 | 56.4 ± 13.45 | Subacute/chronic | m-CIMT includes intensive practice, restrain of non-paretic lower extremity and transfer package. | NDT program. | 120 min per session, five sessions per week for two weeks. | BBS, step length ratio, cadence (steps/min), postural symmetry FAC, and gait speed. | The m-CIMT group showed significant improvements on all variables when compared to the NDT group. |
| Ribeiro T et al., 2017 | 57.75 ± 3.75 | Subacute/chronic | Gait training on a treadmill, applying weight on the unaffected side. | Gait training on a treadmill. | 30 min per session, nine training sessions for two consecutive weeks. | SPB (mmHg), DPB (mmHg), % of HR max, distance covered (m), gait speed (m/s). | Kinetic gait parameters improved in both groups equally. Restraint of a non-paretic limb did not show any effect. No changes have been observed in cardiovascular parameters between pre and post sessions. |
| Ribeiro T et al., 2020 | 57.75 ± 3.75 | Subacute/chronic | Gait training on a treadmill, applying weight on the unaffected side. | Gait training on a treadmill. | The nine sessions, 30 min, two consecutive weeks. | Stance time(s), static and dynamic (GRF), double support time (s), symmetrical weight bearing, and symmetry ratio. | The experimental and control group showed similar effects for gait variables. |
| Study ID | Eligibility Criteria | Random Allocation | Concealed Allocation | Baseline Comparability | Blinding of Participants | Blinding of Therapist | Blinding of Assessor | Adequate Follow-Up (>85%) | Intention to Treat | Between-Group Comparison | Point Estimates and Variability | Pedro Score (10) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aruin AS et al., 2012 | Y | Y | N | Y | N | N | N | × | N | Y | Y | 4 |
| Bonnyaud C et al., 2013 | N | Y | N | Y | N | N | N | N | N | Y | Y | 4 |
| Bonnyaud C et al., 2014 | N | Y | N | Y | N | N | N | N | N | Y | Y | 4 |
| Jung K et al., 2015 | Y | Y | Y | Y | N | N | Y | Y | N | Y | Y | 7 |
| Zhu Y et al., 2016 | Y | Y | N | Y | N | N | Y | N | N | Y | Y | 5 |
| Ribeiro T et al., 2017 | Y | Y | Y | Y | N | N | Y | Y | Y | Y | Y | 8 |
| e Silva EMG de S et al., 2017 | Y | Y | Y | Y | N | N | Y | Y | Y | Y | Y | 8 |
| Candan SA et al., 2017 | Y | Y | N | Y | N | N | Y | Y | N | Y | Y | 6 |
| Ribeiro T et al., 2017 | N | Y | N | Y | N | N | N | Y | N | Y | Y | 5 |
| Ribeiro T et al., 2020 | Y | Y | Y | Y | N | N | Y | Y | Y | Y | Y | 8 |
| Outcome Measures | Level of Evidence | Quality of the Studies | |
|---|---|---|---|
| Gait parameters | Gait speed | Level 1b | Good |
| Cardiovascular parameters |
| Level 2 | Fair |
| Balance |
| Level 1b | Good |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reddy, R.S.; Gular, K.; Dixit, S.; Kandakurti, P.K.; Tedla, J.S.; Gautam, A.P.; Sangadala, D.R. Impact of Constraint-Induced Movement Therapy (CIMT) on Functional Ambulation in Stroke Patients—A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 12809. https://doi.org/10.3390/ijerph191912809
Reddy RS, Gular K, Dixit S, Kandakurti PK, Tedla JS, Gautam AP, Sangadala DR. Impact of Constraint-Induced Movement Therapy (CIMT) on Functional Ambulation in Stroke Patients—A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health. 2022; 19(19):12809. https://doi.org/10.3390/ijerph191912809
Chicago/Turabian StyleReddy, Ravi Shankar, Kumar Gular, Snehil Dixit, Praveen Kumar Kandakurti, Jaya Shanker Tedla, Ajay Prashad Gautam, and Devika Rani Sangadala. 2022. "Impact of Constraint-Induced Movement Therapy (CIMT) on Functional Ambulation in Stroke Patients—A Systematic Review and Meta-Analysis" International Journal of Environmental Research and Public Health 19, no. 19: 12809. https://doi.org/10.3390/ijerph191912809
APA StyleReddy, R. S., Gular, K., Dixit, S., Kandakurti, P. K., Tedla, J. S., Gautam, A. P., & Sangadala, D. R. (2022). Impact of Constraint-Induced Movement Therapy (CIMT) on Functional Ambulation in Stroke Patients—A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health, 19(19), 12809. https://doi.org/10.3390/ijerph191912809








