Association between Severity of Freezing of Gait and Turning Characteristics in People with Parkinson’s Disease
Abstract
:1. Introduction
2. Materials and Methods
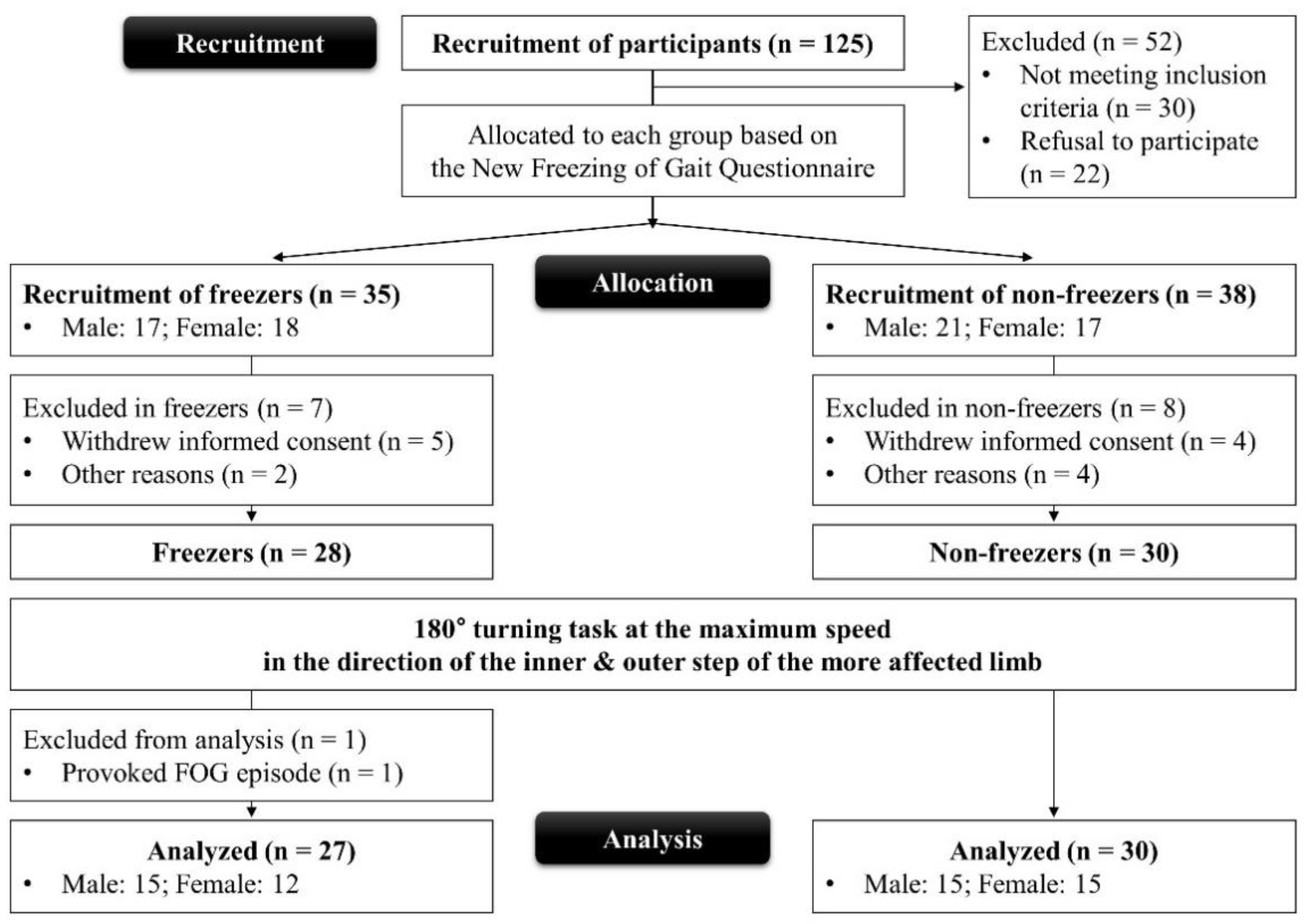
2.1. Participants
2.2. Experiment Procedures
2.3. Data Acquisition and Analyses
2.4. Statistical Analyses
3. Results
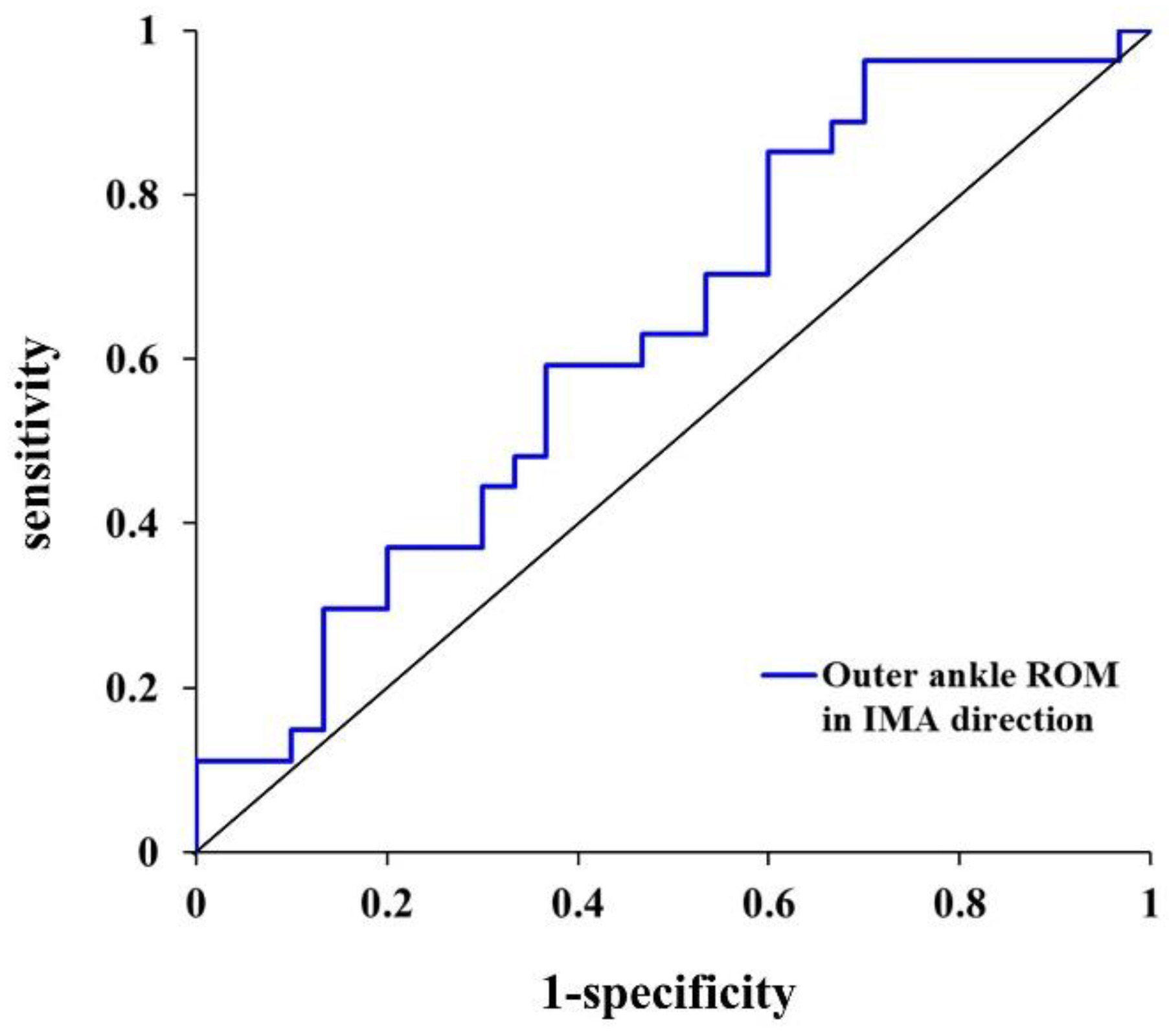
3.1. Classifier Variable for Freezers and Non-Freezers
3.2. Association between NFOGQ Score and 180° Turning Characteristics in Freezers
4. Discussion
4.1. Classifier Variables According to 180° Turning Characteristics for Freezers and Non-Freezers
4.2. 180° Turning Characteristics Associated with NFOGQ Score in Freezers
4.3. Clinical Implications and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bertoli, M.; Croce, U.D.; Cereatti, A.; Mancini, M. Objective Measures to Investigate Turning Impairments and Freezing of Gait in People with Parkinson’s Disease. Gait Posture 2019, 74, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Mancini, M.; Shah, V.V.; Stuart, S.; Curtze, C.; Horak, F.B.; Safarpour, D.; Nutt, J.G. Measuring Freezing of Gait During Daily-Life: An Open-Source, Wearable Sensors Approach. J. Neuroeng. Rehabil. 2021, 18, 1. [Google Scholar] [CrossRef] [PubMed]
- Steinbach, M.J.; Campbell, R.W.; DeVore, B.B.; Harrison, D.W. Laterality in Parkinson’s Disease: A Neuropsychological Review. Appl. Neuropsychol. Adult 2021, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Nieuwboer, A.; Rochester, L.; Herman, T.; Vandenberghe, W.; Emil, G.E.; Thomaes, T.; Giladi, N. Reliability of the New Freezing of Gait Questionnaire: Agreement Between Patients with Parkinson’s Disease and Their Carers. Gait Posture 2009, 30, 459–463. [Google Scholar] [CrossRef]
- Goetz, C.G.; Poewe, W.; Rascol, O.; Sampaio, C.; Stebbins, G.T.; Counsell, C.; Giladi, N.; Holloway, R.G.; Moore, C.G.; Wenning, G.K.; et al. Movement Disorder Society Task Force Report on the Hoehn and Yahr Staging Scale: Status and Recommendations. Mov. Disord. 2004, 19, 1020–1028. [Google Scholar] [CrossRef]
- Moore, S.T.; MacDougall, H.G.; Gracies, J.M.; Cohen, H.S.; Ondo, W.G. Long-Term Monitoring of Gait in Parkinson’s Disease. Gait Posture 2007, 26, 200–207. [Google Scholar] [CrossRef]
- Frazzitta, G.; Pezzoli, G.; Bertotti, G.; Maestri, R. Asymmetry and Freezing of Gait in Parkinsonian Patients. J. Neurol. 2013, 260, 71–76. [Google Scholar] [CrossRef]
- Pelizzari, L.; Di Tella, S.; Laganà, M.M.; Bergsland, N.; Rossetto, F.; Nemni, R.; Baglio, F. White Matter Alterations in Early Parkinson’s Disease: Role of Motor Symptom Lateralization. Neurol. Sci. 2020, 41, 357–364. [Google Scholar] [CrossRef]
- Park, H.; Youm, C.; Lee, M.; Noh, B.; Cheon, S.M. Turning Characteristics of the More-Affected Side in Parkinson’s Disease Patients with Freezing of Gait. Sensors 2020, 20, 3098. [Google Scholar] [CrossRef]
- Spildooren, J.; Vinken, C.; Van Baekel, L.; Nieuwboer, A. Turning Problems and Freezing of Gait in Parkinson’s Disease: A Systematic Review and Meta-analysis. Disabil. Rehabil. 2019, 41, 2994–3004. [Google Scholar] [CrossRef]
- Son, M.; Youm, C.; Cheon, S.; Kim, J.; Lee, M.; Kim, Y.; Kim, J.; Sung, H. Evaluation of the Turning Characteristics According to the Severity of Parkinson Disease During the Timed up and Go Test. Aging Clin. Exp. Res. 2017, 29, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Spildooren, J.; Vercruysse, S.; Meyns, P.; Vandenbossche, J.; Heremans, E.; Desloovere, K.; Vandenberghe, W.; Nieuwboer, A. Turning and Unilateral Cueing in Parkinson’s Disease Patients with and Without Freezing of Gait. Neuroscience 2012, 207, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Nieuwboer, A.; Giladi, N. Characterizing Freezing of Gait in Parkinson’s Disease: Models of an Episodic Phenomenon. Mov. Disord. 2013, 28, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Spildooren, J.; Vercruysse, S.; Desloovere, K.; Vandenberghe, W.; Kerckhofs, E.; Nieuwboer, A. Freezing of Gait in Parkinson’s Disease: The Impact of Dual-Tasking and Turning. Mov. Disord. 2010, 25, 2563–2570. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Shin, S.; Youm, C.; Cheon, S.M.; Lee, M.; Noh, B. Classification of Parkinson’s Disease with Freezing of Gait Based on 360° Turning Analysis Using 36 Kinematic Features. J. Neuroeng. Rehabil. 2021, 18, 177. [Google Scholar] [CrossRef] [PubMed]
- Bengevoord, A.; Vervoort, G.; Spildooren, J.; Heremans, E.; Vandenberghe, W.; Bloem, B.R.; Nieuwboer, A. Center of Mass Trajectories During Turning in Patients with Parkinson’s Disease with and Without Freezing of Gait. Gait Posture 2016, 43, 54–59. [Google Scholar] [CrossRef]
- Peterson, D.S.; Van Liew, C.; Stuart, S.; Carlson-Kuhta, P.; Horak, F.B.; Mancini, M. Relating Parkinson Freezing and Balance Domains: A Structural Equation Modeling Approach. Parkinsonism Relat. Disord. 2020, 79, 73–78. [Google Scholar] [CrossRef]
- Coelho, D.B.; de Souza, C.R.; de Lima-Pardini, A.C.; Treza, R.C.; Shida, T.K.F.; Silva-Batista, C.; Teixeira, L.A. Is Freezing of Gait Correlated with Postural Control in Patients with Moderate-to-Severe Parkinson’s Disease? Eur. J. Neurosci. 2021, 53, 1189–1196. [Google Scholar] [CrossRef]
- Hughes, A.J.; Daniel, S.E.; Lees, A.J. Improved Accuracy of Clinical Diagnosis of Lewy Body Parkinson’s Disease. Neurology 2001, 57, 1497–1499. [Google Scholar] [CrossRef]
- Kang, Y.; Na, D.-L.; Hahn, S. A Validity Study on the Korean Mini-Mental State Examination (K-MMSE) in Dementia Patients. J. Korean Neurol. Assoc. 1997, 15, 300–308. [Google Scholar]
- Vicon Motion Systems Ltd. Plug-in Gait Reference Guide. 2017. Available online: https://docs.vicon.com/download/attachments/42696722/Plug-in%20Gait%20Reference%20Guide.pdf?version=1&modificationDate=1502364735000&api=v2 (accessed on 22 July 2022).
- Plotnik, M.; Giladi, N.; Balash, Y.; Peretz, C.; Hausdorff, J.M. Is Freezing of Gait in Parkinson’s Disease Related to Asymmetric Motor Function? Ann. Neurol. 2005, 57, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.-G. Statistical Power Analyses Using G*Power 3.1: Tests for Correlation and Regression Analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A Flexible Statistical Power Analysis Program for the Social, Behavioral, and Biomedical Sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.E.; Bachmann, L.M.; Jaeschke, R. A Readers’ Guide to the Interpretation of Diagnostic Test Properties: Clinical Example of Sepsis. Intensive Care Med. 2003, 29, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Marquez, J.S.; Hasan, S.M.S.; Siddiquee, M.R.; Luca, C.C.; Mishra, V.R.; Mari, Z.; Bai, O. Neural Correlates of Freezing of Gait in Parkinson’s Disease: An Electrophysiology Mini-Review. Front. Neurol. 2020, 11, 571086. [Google Scholar] [CrossRef]
- Chou, P.-Y.; Lee, S.-C. Turning Deficits in People with Parkinson’s Disease. Tzu Chi Med. J. 2013, 25, 200–202. [Google Scholar] [CrossRef]
- Wang, Y.; Witchalls, J.; Preston, E.; Wang, Z.; Zhuang, J.; Waddington, G.; Adams, R.; Han, J. The Relationship Between Ankle Proprioception and Functional Mobility in People with Parkinson’s Disease: A Cross-Sectional Study. Front. Neurol. 2021, 11, 1859. [Google Scholar] [CrossRef]
- Valsky, D.; Heiman Grosberg, S.H.; Israel, Z.; Boraud, T.; Bergman, H.; Deffains, M. What Is the True Discharge Rate and Pattern of the Striatal Projection Neurons in Parkinson’s Disease and Dystonia? eLife 2020, 9, e57445. [Google Scholar] [CrossRef]
- Conradsson, D.; Paquette, C.; Franzén, E. Turning Stability in Individuals with Parkinson Disease. J. Neurol. Phys. Ther. 2018, 42, 241–247. [Google Scholar] [CrossRef]
- Contreras, A.; Grandas, F. Risk Factors for Freezing of Gait in Parkinson’s Disease. J. Neurol. Sci. 2012, 320, 66–71. [Google Scholar] [CrossRef]
- Mancini, M.; Smulders, K.; Cohen, R.G.; Horak, F.B.; Giladi, N.; Nutt, J.G. The Clinical Significance of Freezing while Turning in Parkinson’s Disease. Neuroscience 2017, 343, 222–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pardoel, S.; Kofman, J.; Nantel, J.; Lemaire, E.D. Wearable-Sensor-Based Detection and Prediction of Freezing of Gait in Parkinson’s Disease: A Review. Sensors 2019, 19, 5141. [Google Scholar] [CrossRef] [PubMed]
- Toosizadeh, N.; Mohler, J.; Lei, H.; Parvaneh, S.; Sherman, S.; Najafi, B. Motor Performance Assessment in Parkinson’s Disease: Association Between Objective in-Clinic, Objective in-Home, and Subjective/Semi-objective Measures. PLoS ONE 2015, 10, e0124763. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Characteristics | People with PD | p-Value | |
|---|---|---|---|
| Freezers (n = 27) | Non-Freezers (n = 30) | ||
| Sex (male/female) | 15/12 | 15/15 | 0.792 a |
| Age (years) | 68.05 ± 5.21 | 68.06 ± 4.62 | 0.991 b |
| Height (cm) | 157.99 ± 9.30 | 157.32 ± 8.38 | 0.776 b |
| Body weight (kg) | 59.71 ± 8.86 | 59.44 ± 7.53 | 0.902 b |
| BMI (kg/m2) | 23.88 ± 2.61 | 24.03 ± 2.61 | 0.831 b |
| MMSE (scores) | 28.00 ± 1.78 | 27.27 ± 1.80 | 0.105 c |
| Disease duration (years) | 9.00 ± 6.67 | 4.24 ± 4.04 | 0.001 c |
| LED (mg/day) | 745.83 ± 349.89 | 460.00 ± 252.03 | 0.001 c |
| NFOGQ (scores) | 13.22 ± 8.02 | - | - |
| Hoehn and Yahr scale (stages) | 2.65 ± 0.41 | 2.33 ± 0.44 | 0.008 c |
| UPDRS total (scores) | 53.30 ± 12.77 | 46.38 ± 12.35 | 0.043 b |
| UPDRS Part Ⅰ (scores) | 3.37 ± 1.91 | 2.88 ± 1.31 | 0.262 b |
| UPDRS Part Ⅱ (scores) | 11.94 ± 5.41 | 7.63 ± 4.77 | 0.002 b |
| UPDRS Part Ⅲ (scores) | 34.81 ± 7.30 | 33.90 ± 8.00 | 0.655 b |
| UPDRS Part Ⅳ (scores) | 3.17 ± 2.64 | 1.97 ± 1.88 | 0.105 c |
| More affected limb (left/right) | 16/11 | 21/9 | 0.420 a |
| Parameters | Variables | Description |
|---|---|---|
| Spatiotemporal parameter | Total steps and duration | Total steps and duration were calculated within the analysis phase. |
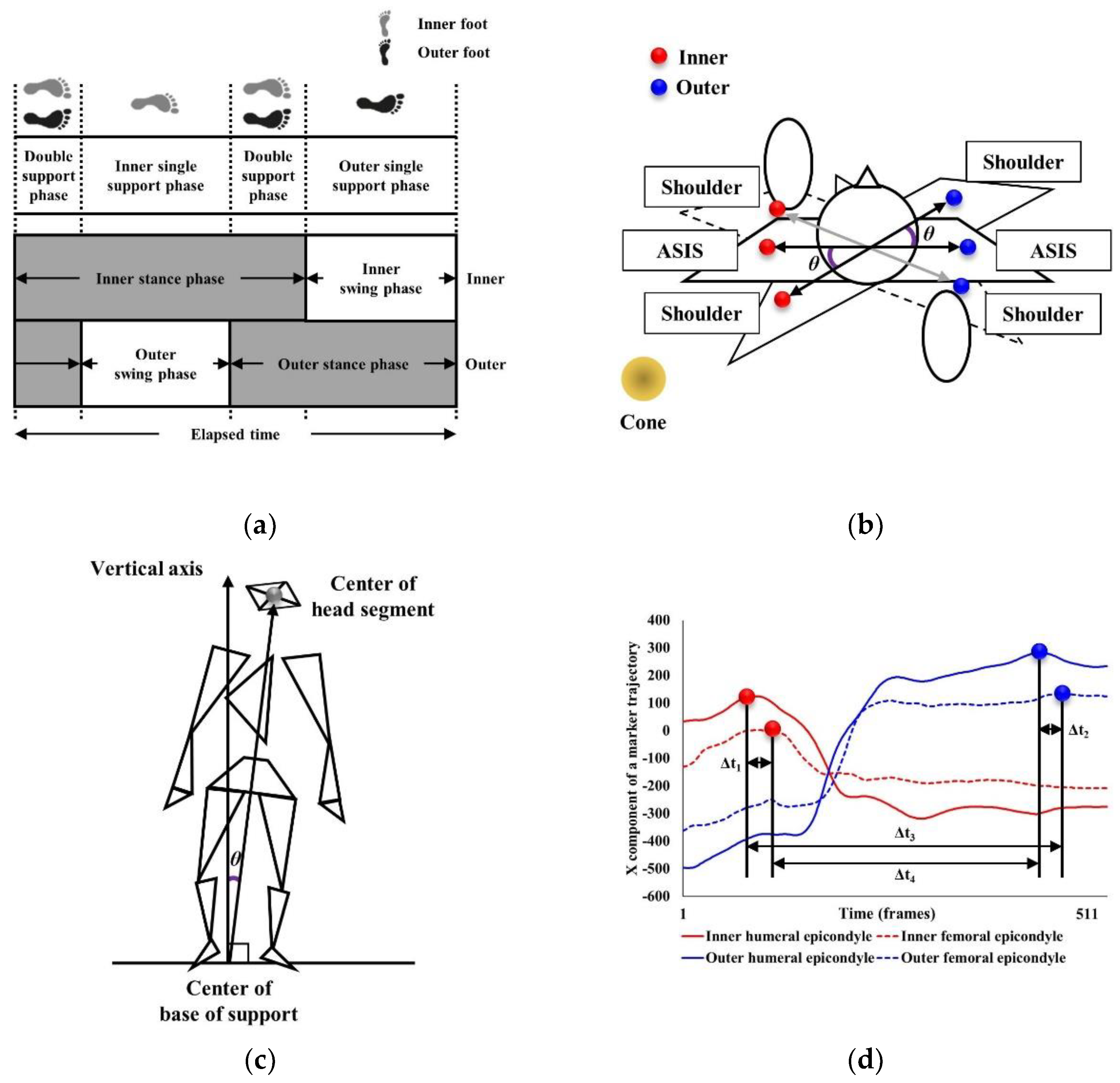
| Step width | Step width was defined as the perpendicular distance in the lateral plane to the initial heel contact of one lower limb and the initial heel contact of the opposite lower limb (Figure 2b). | |
| Inner and outer step lengths | Inner and outer step lengths were defined as the perpendicular distance in the AP plane between the initial heel contact of the inner/outer lower limb and the initial heel contact of the other lower limb, respectively (Figure 2b). | |
| Inner and outer single support phases | Inner and outer single support phases were defined as when either the inner or outer foot was in contact with the ground (Figure 3a). | |
| Inner and outer double support phases | Inner and outer double support phases were defined as when both inner/outer feet were in contact with the ground (Figure 3a). | |
| Inner and outer stance phases | Inner and outer stance phases were defined as the inner/outer foot contacting the ground, moving from initial heel contact to toe-off (Figure 3a). | |
| Kinematic parameter | ROM | ROM was calculated from the maximum and minimum joint (inner and outer hip, knee, ankle, shoulder, pelvis, and thorax) angles during 180° turning. |
| Inner and outer toe clearance height | Inner and outer toe clearance height was selected as the maximum vertical height of the toe marker in the swing phase after toe-off for each step. | |
| Maximum anti-phase | Maximum anti-phase was calculated as the maximum angle (θ) between the lateral ASIS vector and lateral acromion processes vector in the horizontal plane during 180° turning (Figure 3b) [11]. | |
| Incline angle | Incline angle indicated the degree of body tilt during turning and was calculated as the maximum angle (θ) on the lateral plane between the vector from the center of the base of support to the center of the head segment and the vertical vector of the global coordinate system located on the cone (Figure 3c) [15]. | |
| Inner/outer ipsilateral and contralateral tempo | Inner/outer ipsilateral and contralateral tempo were calculated using the lateral humeral epicondyle and lateral femoral epicondyle markers to determine the time difference (Δt) reaching the peak position in the AP plane of the inner-inner (Δt1)/outer-outer (Δt2) (ipsilateral) and inner-outer (Δt3)/outer-inner (Δt4) (contralateral) limbs (Figure 3d) [15]. | |
| COM parameter | AP and ML RMS distances | The area of 95% CI was calculated using the trajectory of the COM on the horizontal plane during the 180° turning tasks, followed by the AP and ML RMS distances, total distance, and average speed [9]. |
| Total distance and average speed |
| Variable | Cutoff Value | β (SE) | OR (95% CI) | p-Value | RN2 | |
|---|---|---|---|---|---|---|
| IMA | Outer ankle ROM | 31.0° | −0.221 | 0.802 | 0.026 | 0.735 |
| (0.100) | (0.659–0.974) | |||||
| Variables | β | SE | t-Value | p-Value | VIF | Tolerance Limit | |
|---|---|---|---|---|---|---|---|
| Constant | 160.710 | 58.697 | 2.738 | 0.013 | |||
| IMA | AP RMS distance of the COM | 61.272 | 28.589 | 2.143 | 0.045 | 1.405 | 0.712 |
| OMA | Inner stance phase | 1.027 | 0.399 | 2.571 | 0.018 | 1.390 | 0.720 |
| Adjusted R2 = 0.501, F = 5.353, p = 0.002 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, H.; Youm, C.; Park, H.; Kim, B.; Cheon, S.-M.; Lee, M. Association between Severity of Freezing of Gait and Turning Characteristics in People with Parkinson’s Disease. Int. J. Environ. Res. Public Health 2022, 19, 12131. https://doi.org/10.3390/ijerph191912131
Choi H, Youm C, Park H, Kim B, Cheon S-M, Lee M. Association between Severity of Freezing of Gait and Turning Characteristics in People with Parkinson’s Disease. International Journal of Environmental Research and Public Health. 2022; 19(19):12131. https://doi.org/10.3390/ijerph191912131
Chicago/Turabian StyleChoi, Hyejin, Changhong Youm, Hwayoung Park, Bohyun Kim, Sang-Myung Cheon, and Myeounggon Lee. 2022. "Association between Severity of Freezing of Gait and Turning Characteristics in People with Parkinson’s Disease" International Journal of Environmental Research and Public Health 19, no. 19: 12131. https://doi.org/10.3390/ijerph191912131
APA StyleChoi, H., Youm, C., Park, H., Kim, B., Cheon, S.-M., & Lee, M. (2022). Association between Severity of Freezing of Gait and Turning Characteristics in People with Parkinson’s Disease. International Journal of Environmental Research and Public Health, 19(19), 12131. https://doi.org/10.3390/ijerph191912131










