Effects of Straw Return and Moisture Condition on Temporal Changes of DOM Composition and Cd Speciation in Polluted Farmland Soil
Abstract
:1. Introduction
2. Materials and Methods
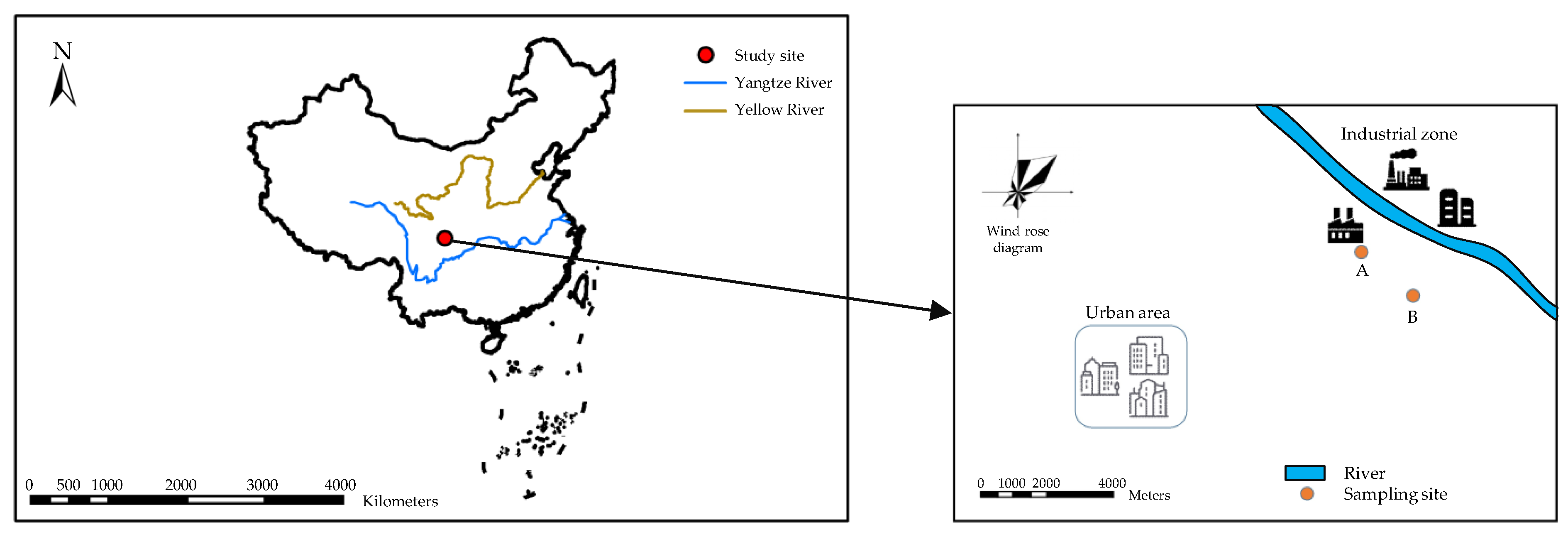
2.1. Material Collection and Sample Preparation
2.2. Laboratory Incubation Experiment
2.3. Characterization of DOM
2.4. Geochemical Fractionation of Cd in Soil
2.5. Fluorescent Indices
2.6. Other Physical and Chemical Analyses
2.7. Statistical Analysis
3. Results
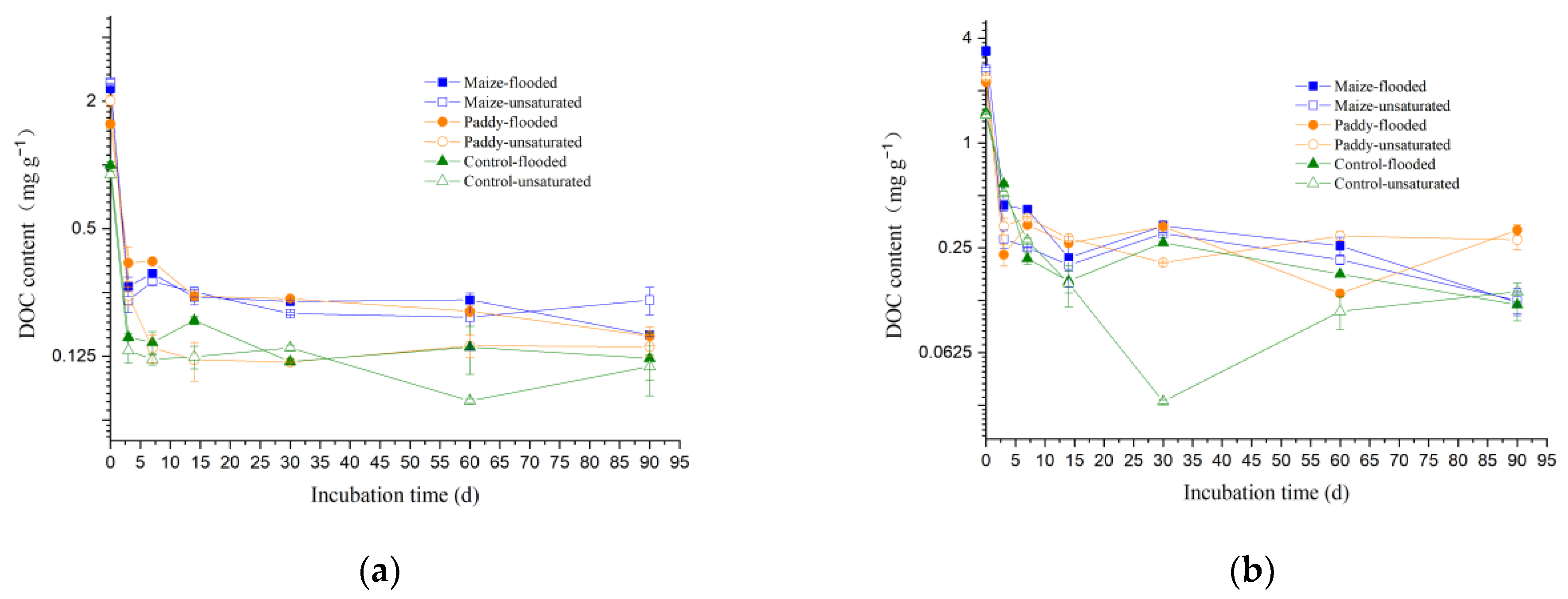
3.1. Dynamics of Total and Dissolved Organic Matter Content in Soil
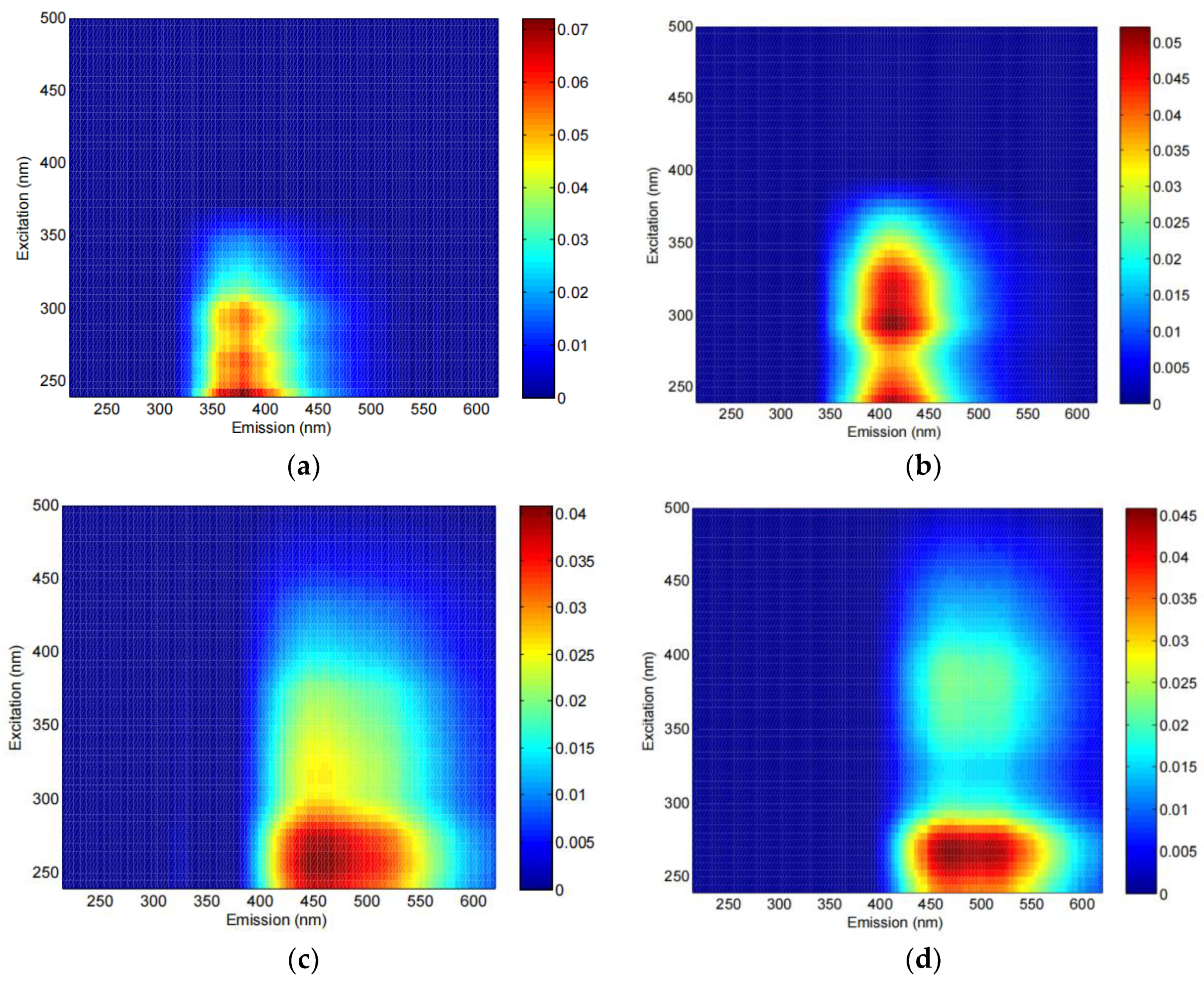
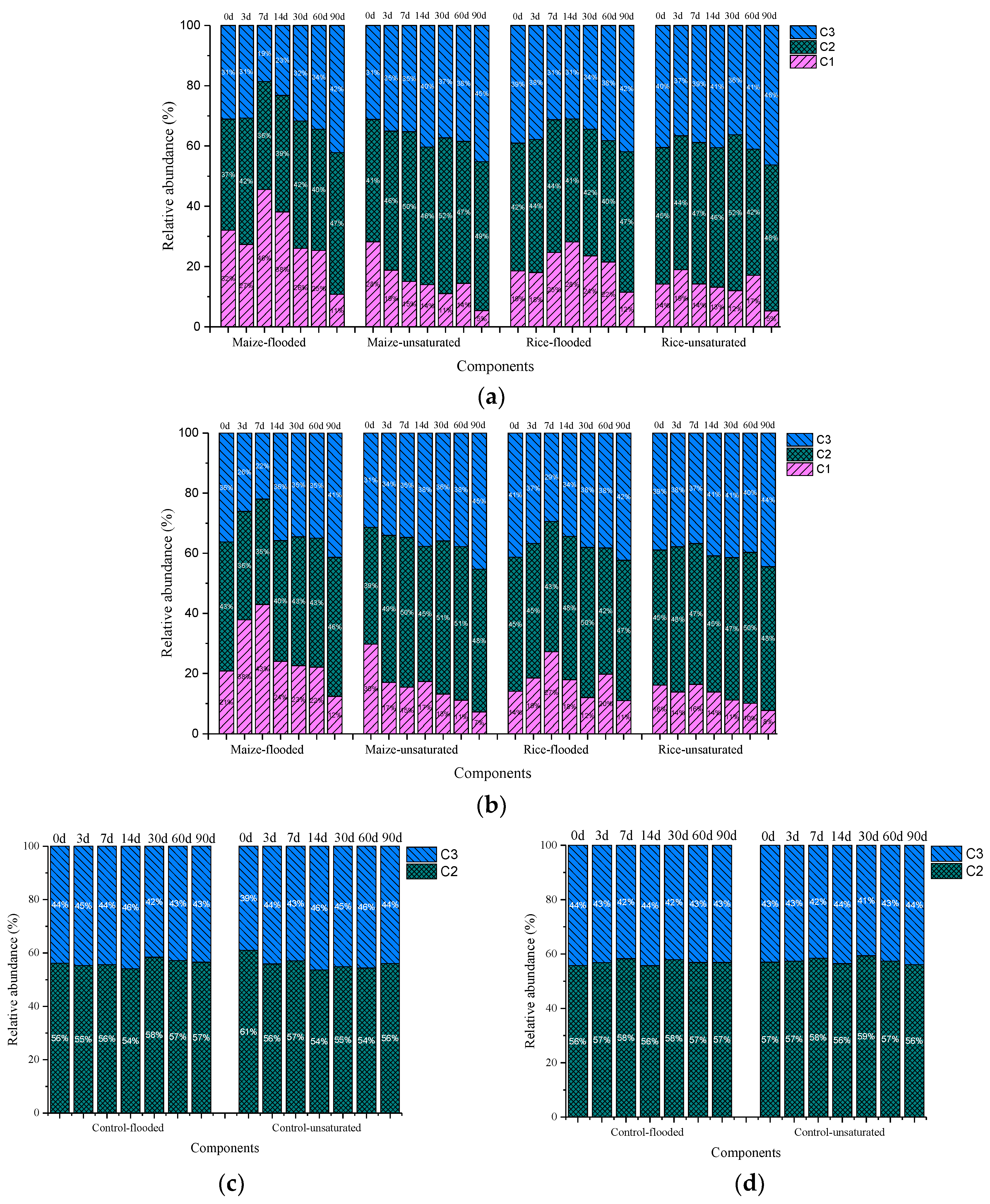
3.2. Composition of DOM Fluorescent Components
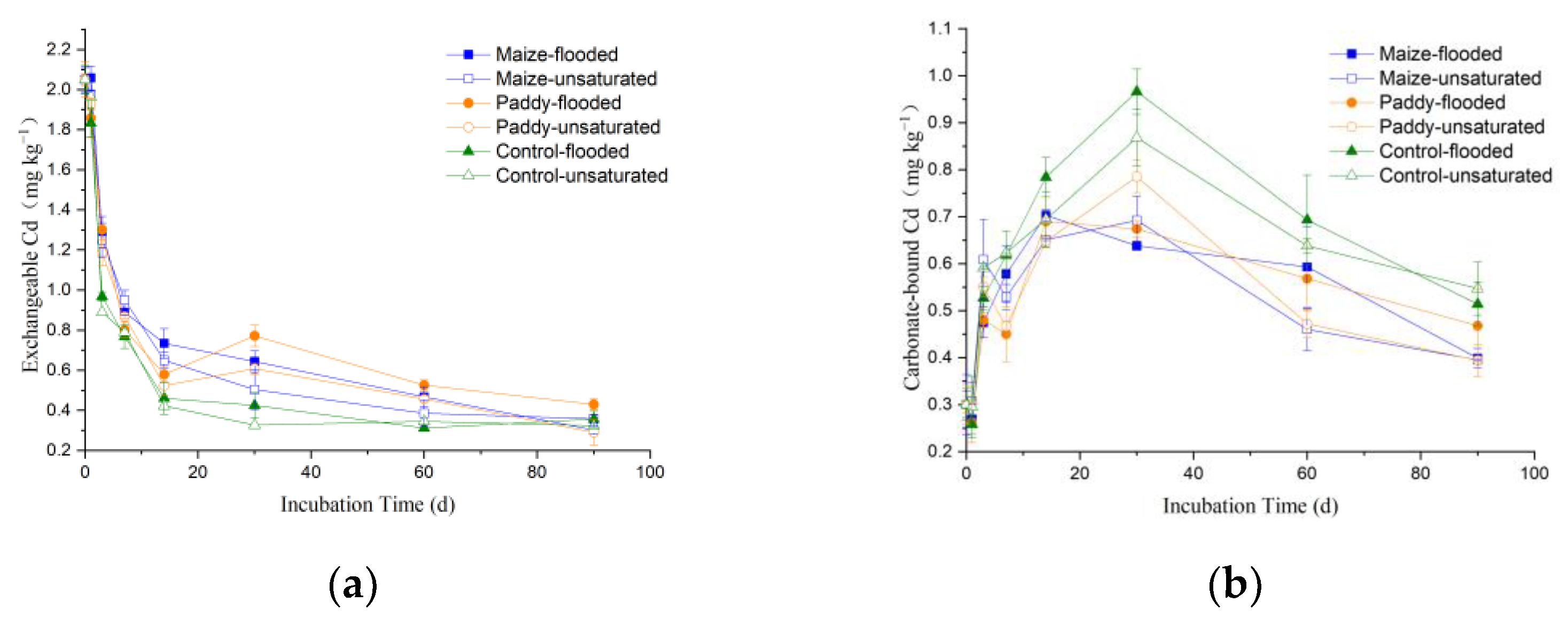
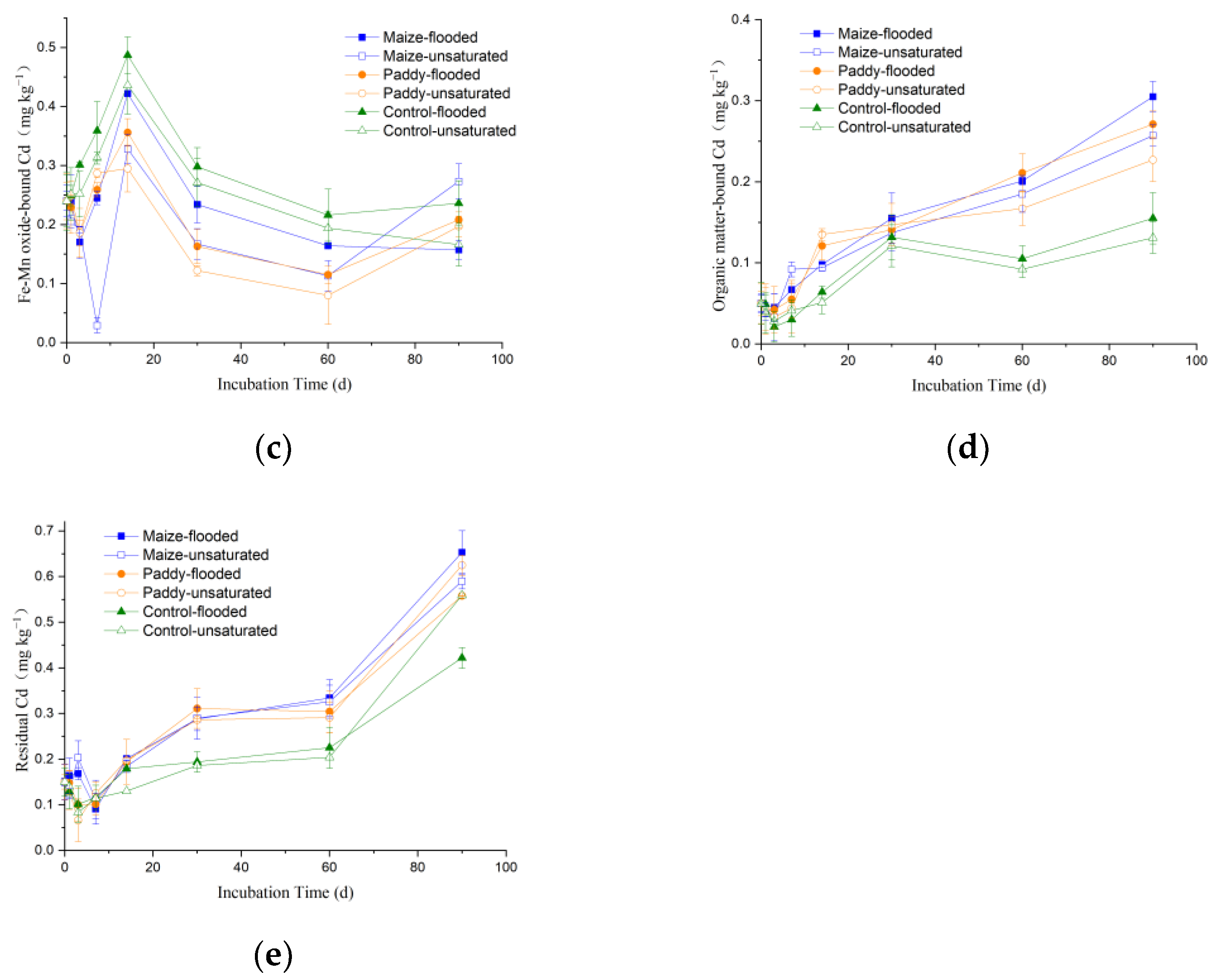
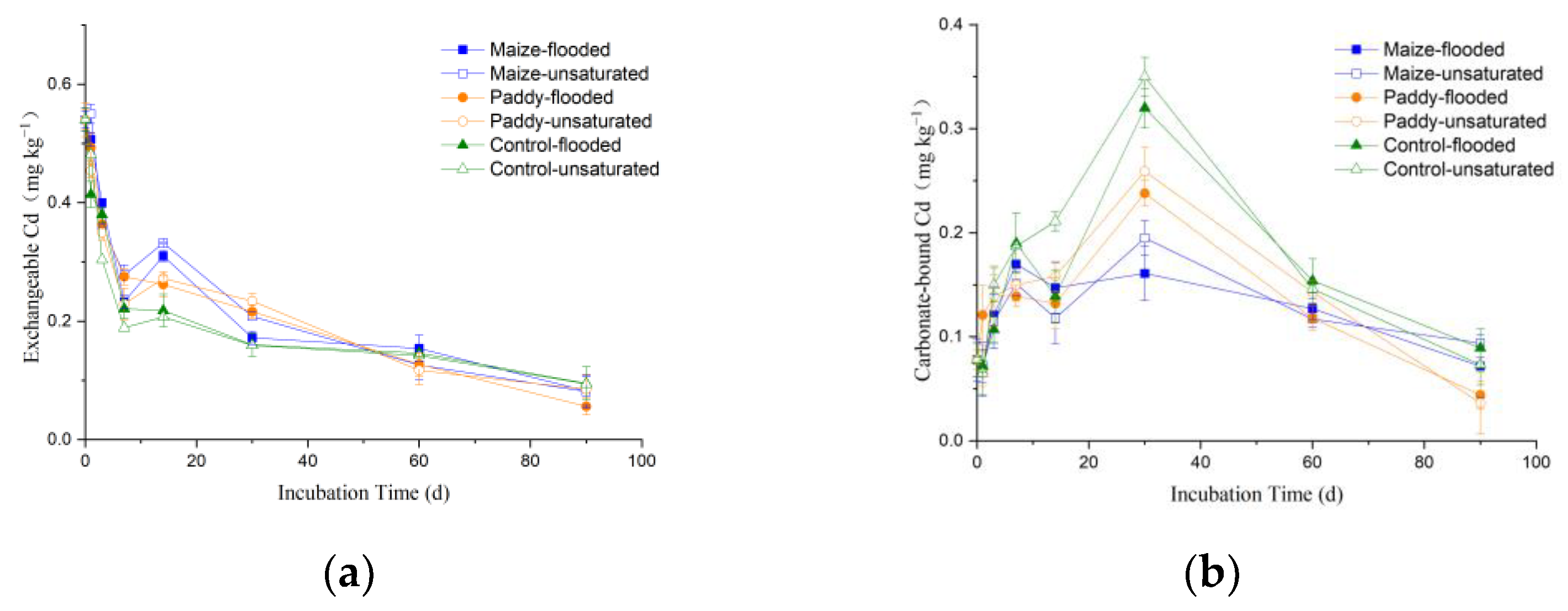
3.3. Dynamics of Cd Speciation in Soil
4. Discussion
4.1. Effects of Straw Return and Soil Moisture on Soil DOM Content
4.2. Effects of Straw Return on Cd Speciation in Soil
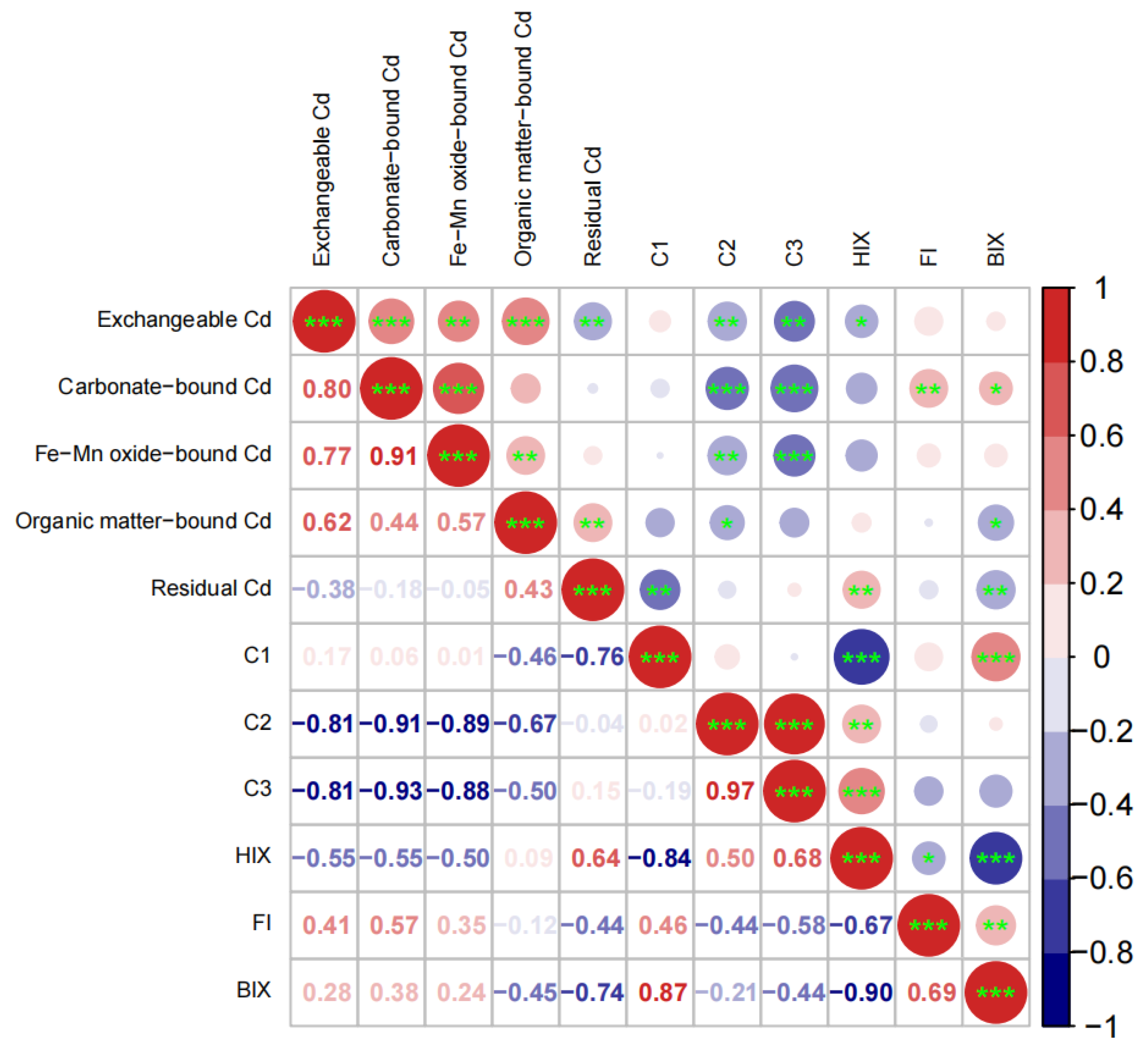
4.3. Correlations among Soil Cd Speciation and DOM Fluorescence Parameters
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Makino, T.; Takano, H.; Kamiya, T.; Itou, T.; Sekiya, N.; Inahara, M.; Sakurai, Y. Restoration of cadmium-contaminated paddy soils by washing with ferric chloride: Cd extraction mechanism and bench-scale verification. Chemosphere 2008, 70, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Y.; Ma, Z.W.; van der Kuijp, T.J.; Yuan, Z.W.; Huang, L. A review of soil heavy metal pollution from mines in China: Pollution and health risk assessment. Sci. Total Environ. 2014, 468, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Six, L.; Smolders, E. Future trends in soil cadmium concentration under current cadmium fluxes to European agricultural soils. Sci. Total Environ. 2014, 485, 319–328. [Google Scholar] [CrossRef]
- Lofts, S.; Spurgeon, D.; Svendsen, C. Fractions affected and probabilistic risk assessment of Cu, Zn, Cd, and Pb in soils using the free ion approach. Environ. Sci. Technol. 2005, 39, 8533–8540. [Google Scholar] [CrossRef]
- Rasool, R.; Kukal, S.S.; Hira, G.S. Soil organic carbon and physical properties as affected by long-term application of FYM and inorganic fertilizers in maize-wheat system. Soil. Till. Res. 2008, 101, 31–36. [Google Scholar] [CrossRef]
- Li, H.; Cao, Y.; Wang, X.M.; Ge, X.; Li, B.Q.; Jin, C.Q. Evaluation on the production of food crop straw in China from 2006 to 2014. Bioenerg. Res. 2017, 10, 949–957. [Google Scholar] [CrossRef]
- Ren, J.Q.; Yu, P.X.; Xu, X.H. Straw utilization in china-status and recommendations. Sustainability 2019, 11, 1762. [Google Scholar] [CrossRef]
- Cui, Y.S.; Du, X.; Weng, L.P.; Zhu, Y.G. Effects of rice straw on the speciation of cadmium (Cd) and copper (Cu) in soils. Geoderma 2008, 146, 370–377. [Google Scholar] [CrossRef]
- Li, P.; Wang, X.X.; Zhang, T.L.; Zhou, D.M.; He, Y.Q. Distribution and accumulation of copper and cadmium in soil-rice system as affected by soil amendments. Water Air Soil Poll. 2009, 196, 29–40. [Google Scholar] [CrossRef]
- Bai, Y.C.; Gu, C.H.; Tao, T.Y.; Chen, G.H.; Shan, Y.H. Straw incorporation increases solubility and uptake of cadmium by rice plants. Acta Agric. Scand. Sect. B–Soil Plant Sci. 2013, 63, 193–199. [Google Scholar] [CrossRef]
- Han, D.F.; Luo, D.; Chen, Y.H.; Wang, G. Transfer of Cd, Pb, and Zn to water spinach from a polluted soil amended with lime and organic materials. J. Soils Sediments 2013, 13, 1360–1368. [Google Scholar] [CrossRef]
- Tang, W.; Zhong, H.; Xiao, L.; Tan, Q.; Zeng, Q.; Wei, Z. Inhibitory effects of rice residues amendment on Cd phytoavailability: A matter of Cd-organic matter interactions? Chemosphere 2017, 186, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.Y.; Tan, Z.X.; Gong, H.B.; Huang, Q.Y. Migration and transformation mechanisms of nutrient elements (N, P, K) within biochar in straw-biochar-soil-plant systems: A review. ACS Sustain. Chem. Eng. 2019, 7, 22–32. [Google Scholar] [CrossRef]
- Yan, F.J.; Sun, Y.J.; Hui, X.; Jiang, M.J.; Xiang, K.H.; Wu, Y.X.; Zhang, Q.; Tang, Y.; Yang, Z.Y.; Sun, Y.Y.; et al. The effect of straw mulch on nitrogen, phosphorus and potassium uptake and use in hybrid rice. Paddy Water Environ. 2019, 17, 23–33. [Google Scholar] [CrossRef]
- Zsolnay, A.; Baigar, E.; Jimenez, M.; Steinweg, B.; Saccomandi, F. Differentiating with fluorescence spectroscopy the sources of dissolved organic matter in soils subjected to drying. Chemosphere 1999, 38, 45–50. [Google Scholar] [CrossRef]
- Wang, B.; Zeng, D.; Chen, Y.W.; Belzile, N.; Bai, Y.C.; Zhu, J.P.; Shu, J.C.; Chen, S. Adsorption behaviors of phenanthrene and bisphenol A in purple paddy soils amended with straw-derived DOM in the West Sichuan Plain of China. Ecotoxicol. Environ. Saf. 2019, 169, 737–746. [Google Scholar] [CrossRef]
- Wang, B.; Liu, C.; Chen, Y.; Dong, F.; Chen, S.; Zhang, D.; Zhu, J. Structural characteristics, analytical techniques and interactions with organic contaminants of dissolved organic matter derived from crop straw: A critical review. RSC Adv. 2018, 8, 36927–36938. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.N.; Gao, S.J.; Zhou, G.P.; Cao, W.D. Spectroscopic characteristics of water-extractable organic matter from different green manures. Environ. Technol. 2021, 42, 3688–3697. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Huang, L.P.; Zhang, J.; Shi, D.Z.; Yang, J.X. Optical properties of straw-derived dissolved organic matter and growth inhibition of Microcystis aeruginosa by straw-derived dissolved organic matter via photo-generated hydrogen peroxide. Environ. Pollut. 2018, 242, 760–768. [Google Scholar] [CrossRef]
- Wang, B.; Li, M.; Zhang, H.Y.; Zhu, J.P.; Chen, S.; Ren, D. Effect of straw-derived dissolved organic matter on the adsorption of sulfamethoxazole to purple paddy soils. Ecotoxicol. Environ. Saf. 2020, 203, 110990. [Google Scholar] [CrossRef]
- Sauve, S.; Norvell, W.A.; McBride, M.; Hendershot, W. Speciation and complexation of cadmium in extracted soil solutions. Environ. Sci. Technol. 2000, 34, 291–296. [Google Scholar] [CrossRef]
- Krishnamurti, G.S.R.; Naidu, R. Solid–solution equilibria of cadmium in soils. Geoderma 2003, 113, 17–30. [Google Scholar] [CrossRef]
- Tipping, E. Modeling the Competition between alkaline-earth cations and trace-metal species for binding by humic substances. Environ. Sci. Technol. 1993, 27, 520–529. [Google Scholar] [CrossRef]
- Kolbl, A.; Kaiser, K.; Winkler, P.; Mosley, L.; Fitzpatrick, R.; Marschner, P.; Wagner, F.E.; Hausler, W.; Mikutta, R. Transformation of jarosite during simulated remediation of a sandy sulfuric soil. Sci. Total Environ. 2021, 773, 145546. [Google Scholar] [CrossRef]
- Honma, T.; Ohba, H.; Kaneko-Kadokura, A.; Makino, T.; Nakamura, K.; Katou, H. Optimal soil Eh, pH, and water management for simultaneously minimizing arsenic and cadmium concentrations in rice grains. Environ. Sci. Technol. 2016, 50, 4178–4185. [Google Scholar] [CrossRef]
- Wang, J.; Wang, P.M.; Gu, Y.; Kopittke, P.M.; Zhao, F.J.; Wang, P. Iron-manganese (oxyhydro)oxides, rather than oxidation of sulfides, determine mobilization of Cd during soil drainage in paddy soil systems. Environ. Sci. Technol. 2019, 53, 2500–2508. [Google Scholar] [CrossRef] [PubMed]
- Fulda, B.; Voegelin, A.; Kretzschmar, R. Redox-controlled changes in cadmium solubility and solid-phase speciation in a paddy soil as affected by reducible sulfate and copper. Environ. Sci. Technol. 2013, 47, 12775–12783. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.K. Analytical Methods of Soil and Agricultural Chemistry; China Agricultural Science and Technology Press: Beijing, China, 2000; pp. 1–9, 12–14. [Google Scholar]
- Ministry of Ecology and Environment of PRC, State Administration for Market Regulation of PRC. Soil Environmental Quality—Risk Control Standard for Soil Contamination of Agricultural Land (GB15618-2018); China Environmental Science Press: Beijing, China, 2018; pp. 1–4. [Google Scholar]
- Kumar, K.V.; Sivanesan, S. Selection of optimum sorption kinetics: Comparison of linear and non-linear method. J. Hazard. Mater. 2006, 134, 277–279. [Google Scholar] [CrossRef]
- Hu, S.H.; Lu, C.; Zhang, C.J.; Zhang, Y.J.; Yao, H.R.; Wu, Y.G. Effects of fresh and degraded dissolved organic matter derived from maize straw on copper sorption onto farmland loess. J. Soils Sediments 2016, 16, 327–338. [Google Scholar] [CrossRef]
- Ohno, T.; Bro, R. Dissolved organic matter characterization using multiway spectral decomposition of fluorescence landscapes. Soil. Sci. Soc. Am. J. 2006, 70, 2028–2037. [Google Scholar] [CrossRef]
- Murphy, K.R.; Stedmon, C.A.; Graeber, D.; Bro, R. Fluorescence spectroscopy and multi-way techniques. PARAFAC. Anal. Methods 2013, 5, 6557–6566. [Google Scholar] [CrossRef]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace-metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- McKnight, D.M.; Boyer, E.W.; Westerhoff, P.K.; Doran, P.T.; Kulbe, T.; Andersen, D.T. Spectrofluorometric characterization of dissolved organic matter for indication of precursor organic material and aromaticity. Limnol. Oceanogr. 2001, 46, 38–48. [Google Scholar] [CrossRef]
- Ohno, T. Fluorescence inner-filtering correction for determining the humification index of dissolved organic matter. Environ. Sci. Technol. 2002, 36, 742–746. [Google Scholar] [CrossRef] [PubMed]
- Huguet, A.; Vacher, L.; Relexans, S.; Saubusse, S.; Froidefond, J.M.; Parlanti, E. Properties of fluorescent dissolved organic matter in the Gironde Estuary. Org. Geochem. 2009, 40, 706–719. [Google Scholar] [CrossRef]
- Cory, R.M.; Miller, M.P.; McKnight, D.M.; Guerard, J.J.; Miller, P.L. Effect of instrument-specific response on the analysis of fulvic acid fluorescence spectra. Limnol. Oceanogr. Methods 2010, 8, 67–78. [Google Scholar]
- Fellman, J.B.; Hood, E.; Spencer, R.G.M. Fluorescence spectroscopy opens new windows into dissolved organic matter dynamics in freshwater ecosystems: A review. Limnol. Oceanogr. 2010, 55, 2452–2462. [Google Scholar] [CrossRef]
- Zhang, H.; Cui, K.P.; Guo, Z.; Li, X.Y.; Chen, J.; Qi, Z.G.; Xu, S.Y. Spatiotemporal variations of spectral characteristics of dissolved organic matter in river flowing into a key drinking water source in China. Sci. Total Environ. 2020, 700, 134360. [Google Scholar] [CrossRef]
- Wilson, H.F.; Xenopoulos, M.A. Effects of agricultural land use on the composition of fluvial dissolved organic matter. Nat. Geosci. 2009, 2, 37–41. [Google Scholar] [CrossRef]
- Wei, M.X.; Wang, B.; Chen, S.; Bai, Y.C.; Dong, F.Q.; Zhu, J.P.; Li, M.; Wei, J. Study on spectral characteristics of dissolved organic matter collected from the decomposing process of crop straw in West Sichuan Plain. Spectrosc. Spect. Anal. 2017, 37, 2861–2868. [Google Scholar]
- Devevre, O.C.; Horwath, W.R. Decomposition of rice straw and microbial carbon use efficiency under different soil temperatures and moistures. Soil Biol. Biochem. 2000, 32, 1773–1785. [Google Scholar] [CrossRef]
- Pal, D.; Broadbent, F.E. Influence of moisture on rice straw decomposition in soils. Soil Sci. Soc. Am. J. 1975, 39, 59–63. [Google Scholar] [CrossRef]
- Roper, M.M. Straw decomposition and nitrogenase activity (C2H2 reduction): Effects of soil moisture and temperature. Soil Biol. Biochem. 1985, 17, 65–71. [Google Scholar] [CrossRef]
- Tulina, A.S.; Semenov, V.M.; Rozanova, L.N.; Kuznetsova, T.V.; Semenova, N.A. Influence of moisture on the stability of soil organic matter and plant residues. Eurasian Soil Sci. 2009, 42, 1241–1248. [Google Scholar] [CrossRef]
- Yao, S.H.; Zhang, B.; Hu, F. Soil biophysical controls over rice straw decomposition and sequestration in soil: The effects of drying intensity and frequency of drying and wetting cycles. Soil Biol. Biochem. 2011, 43, 590–599. [Google Scholar] [CrossRef]
- Miller, A.E.; Schimel, J.P.; Meixner, T.; Sickman, J.O.; Melack, J.M. Episodic rewetting enhances carbon and nitrogen release from chaparral soils. Soil Biol. Biochem. 2005, 37, 2195–2204. [Google Scholar] [CrossRef]
- Kalbitz, K.; Schwesig, D.; Rethemeyer, J.; Matzner, E. Stabilization of dissolved organic matter by sorption to the mineral soil. Soil Biol. Biochem. 2005, 37, 1319–1331. [Google Scholar] [CrossRef]
- Nelson, P.N.; Dictor, M.C.; Soulas, G. Availability of organic-carbon in soluble and particle-size fractions from a soil-profile. Soil Biol. Biochem. 1994, 26, 1549–1555. [Google Scholar] [CrossRef]
- Kaiser, K.; Kaupenjohann, M.; Zech, W. Sorption of dissolved organic carbon in soils: Effects of soil sample storage, soil-to-solution ratio, and temperature. Geoderma 2001, 99, 317–328. [Google Scholar] [CrossRef]
- Zsolnay, A.; Gorlitz, H. Water-extractable organic-matter in arable soils—Effects of drought and long-term fertilization. Soil Biol. Biochem. 1994, 26, 1257–1261. [Google Scholar] [CrossRef]
- Coble, P.G. Characterization of marine and terrestrial DOM in seawater using excitation emission matrix spectroscopy. Mar. Chem. 1996, 51, 325–346. [Google Scholar] [CrossRef]
- Fellman, J.B.; Hood, E.; D’Amore, D.V.; Edwards, R.T.; White, D. Seasonal changes in the chemical quality and biodegradability of dissolved organic matter exported from soils to streams in coastal temperate rainforest watersheds. Biogeochemistry 2009, 95, 277–293. [Google Scholar] [CrossRef]
- Williams, C.J.; Yamashita, Y.; Wilson, H.F.; Jaffe, R.; Xenopoulos, M.A. Unraveling the role of land use and microbial activity in shaping dissolved organic matter characteristics in stream ecosystems. Limnol. Oceanogr. 2010, 55, 1159–1171. [Google Scholar] [CrossRef]
- Coble, P.G.; Del Castillo, C.E.; Avril, B. Distribution and optical properties of CDOM in the Arabian Sea during the 1995 Southwest Monsoon. Deep. Sea Res. Part II Top. Stud. Oceanogr. 1998, 45, 2195–2223. [Google Scholar] [CrossRef]
- Ishii, S.K.L.; Boyer, T.H. Behavior of reoccurring PARAFAC components in fluorescent dissolved organic matter in natural and engineered systems: A critical review. Environ. Sci. Technol. 2012, 46, 2006–2017. [Google Scholar] [CrossRef]
- Gao, J.K.; Liang, C.L.; Shen, G.Z.; Lv, J.; Wu, H.M. Spectral characteristics of dissolved organic matter in various agricultural soils throughout China. Chemosphere 2017, 176, 108–116. [Google Scholar] [CrossRef]
- Wu, D.M.; Li, M.; Du, L.; Ren, D.; Wang, J.J. Straw return in paddy field alters photodegradation of organic contaminants by changing the quantity rather than the quality of water-soluble soil organic matter. Sci. Total Environ. 2022, 821, 153371. [Google Scholar] [CrossRef]
- Davidson, E.A.; Verchot, L.V.; Cattanio, J.H.; Ackerman, I.L.; Carvalho, J.E.M. Effects of soil water content on soil respiration in forests and cattle pastures of eastern Amazonia. Biogeochemistry 2000, 48, 53–69. [Google Scholar] [CrossRef]
- Chen, H.L.; Zhou, J.M.; Xiao, B.H. Characterization of dissolved organic matter derived from rice straw at different stages of decay. J. Soils Sediments 2010, 10, 915–922. [Google Scholar] [CrossRef]
- Gu, B.H.; Schmitt, J.; Chen, Z.H.; Liang, L.Y.; McCarthy, J.F. Adsorption and desorption of natural organic matter on iron oxide: Mechanisms and models. Environ. Sci. Technol. 1994, 28, 38–46. [Google Scholar] [CrossRef]
- Bottner, P. Response of microbial biomass to alternate moist and dry conditions in a soil incubated with C-14-labeled and N-15-labelled plant-material. Soil Biol. Biochem. 1985, 17, 329–337. [Google Scholar] [CrossRef]
- Fierer, N.; Schimel, J.P. A proposed mechanism for the pulse in carbon dioxide production commonly observed following the rapid rewetting of a dry soil. Soil Sci. Soc. Am. J. 2003, 67, 798–805. [Google Scholar] [CrossRef]
- Saetre, P.; Stark, J.M. Microbial dynamics and carbon and nitrogen cycling following re-wetting of soils beneath two semi-arid plant species. Oecologia 2005, 142, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Vangestel, M.; Merckx, R.; Vlassak, K. Microbial biomass responses to soil drying and rewetting—The fate of fast-growing and slow-growing microorganisms in soils from different climates. Soil Biol. Biochem. 1993, 25, 109–123. [Google Scholar] [CrossRef]
- Doerr, S.H.; Shakesby, R.A.; Walsh, R.P.D. Soil water repellency: Its causes, characteristics and hydro-geomorphological significance. Earth-Sci. Rev. 2000, 51, 33–65. [Google Scholar] [CrossRef]
- Jones, D.L.; Edwards, A.C. Influence of sorption on the biological utilization of two simple carbon substrates. Soil Biol. Biochem. 1998, 30, 1895–1902. [Google Scholar] [CrossRef]
- Strom, L.; Owen, A.G.; Godbold, D.L.; Jones, D.L. Organic acid behaviour in a calcareous soil: Sorption reactions and biodegradation rates. Soil Biol. Biochem. 2001, 33, 2125–2133. [Google Scholar] [CrossRef]
- van Hees, P.A.W.; Vinogradoff, S.I.; Edwards, A.C.; Godbold, D.L.; Jones, D.L. Low molecular weight organic acid adsorption in forest soils: Effects on soil solution concentrations and biodegradation rates. Soil Biol. Biochem. 2003, 35, 1015–1026. [Google Scholar] [CrossRef]
- Borggaard, O.K.; Holm, P.E.; Strobel, B.W. Potential of dissolved organic matter (DOM) to extract As, Cd, Co, Cr, Cu, Ni, Pb and Zn from polluted soils: A review. Geoderma 2019, 343, 235–246. [Google Scholar] [CrossRef]
- Connell, W.E.; Patrick, W.H. Sulfate reduction in soil—Effects of redox potential and pH. Science 1968, 159, 86–87. [Google Scholar] [CrossRef]
- McLaughlin, M.J. Ageing of metals in soils changes bioavailability. In ICME Fact Sheet on Environ Risk Assess; International Council on Metals and the Environment: Ottawa, ON, Canada, 2001; Volume 4, pp. 1–6. [Google Scholar]
- Zhang, X.Q.; Li, Y.; Ye, J.; Chen, Z.H.; Ren, D.J.; Zhang, S.Q. The spectral characteristics and cadmium complexation of soil dissolved organic matter in a wide range of forest lands. Environ. Pollut. 2022, 299, 118834. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.K.; Lv, J.L.; Wu, H.M.; Dai, Y.C.; Nasir, M. Impacts of wheat straw addition on dissolved organic matter characteristics in cadmium-contaminated soils: Insights from fluorescence spectroscopy and environmental implications. Chemosphere 2018, 193, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Han, R.X.; Muhammad, A.; Guan, D.X.; Zama, E.; Li, G. Correlative distribution of DOM and heavy metals in the soils of the Zhangxi watershed in Ningbo city, East of China. Environ. Pollut. 2022, 299, 118811. [Google Scholar] [CrossRef] [PubMed]

| Soil Sampling Site | Straw Type | Moisture Condition | DOC Content 1 | R2 |
|---|---|---|---|---|
| Fitted Equation | ||||
| A | Maize | Unsaturated | 0.998 * 1 | |
| Flooded | 0.995 * | |||
| Rice | Unsaturated | 0.999 * | ||
| Flooded | 0.980 * | |||
| Control | Unsaturated | 0.994 * | ||
| Flooded | 0.993 * | |||
| B | Maize | Unsaturated | 0.995 * | |
| Flooded | 0.991 * | |||
| Rice | Unsaturated | 0.994 * | ||
| Flooded | 0.941 * | |||
| Control | Unsaturated | 0.986 * | ||
| Flooded | 0.986 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, G.; Tang, X.; Guan, Z.; Cui, J. Effects of Straw Return and Moisture Condition on Temporal Changes of DOM Composition and Cd Speciation in Polluted Farmland Soil. Int. J. Environ. Res. Public Health 2022, 19, 12128. https://doi.org/10.3390/ijerph191912128
Yang G, Tang X, Guan Z, Cui J. Effects of Straw Return and Moisture Condition on Temporal Changes of DOM Composition and Cd Speciation in Polluted Farmland Soil. International Journal of Environmental Research and Public Health. 2022; 19(19):12128. https://doi.org/10.3390/ijerph191912128
Chicago/Turabian StyleYang, Guang, Xiangyu Tang, Zhuo Guan, and Junfang Cui. 2022. "Effects of Straw Return and Moisture Condition on Temporal Changes of DOM Composition and Cd Speciation in Polluted Farmland Soil" International Journal of Environmental Research and Public Health 19, no. 19: 12128. https://doi.org/10.3390/ijerph191912128
APA StyleYang, G., Tang, X., Guan, Z., & Cui, J. (2022). Effects of Straw Return and Moisture Condition on Temporal Changes of DOM Composition and Cd Speciation in Polluted Farmland Soil. International Journal of Environmental Research and Public Health, 19(19), 12128. https://doi.org/10.3390/ijerph191912128








