Distribution and Risk Factors of Malaria in the Greater Accra Region in Ghana
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Data Sources
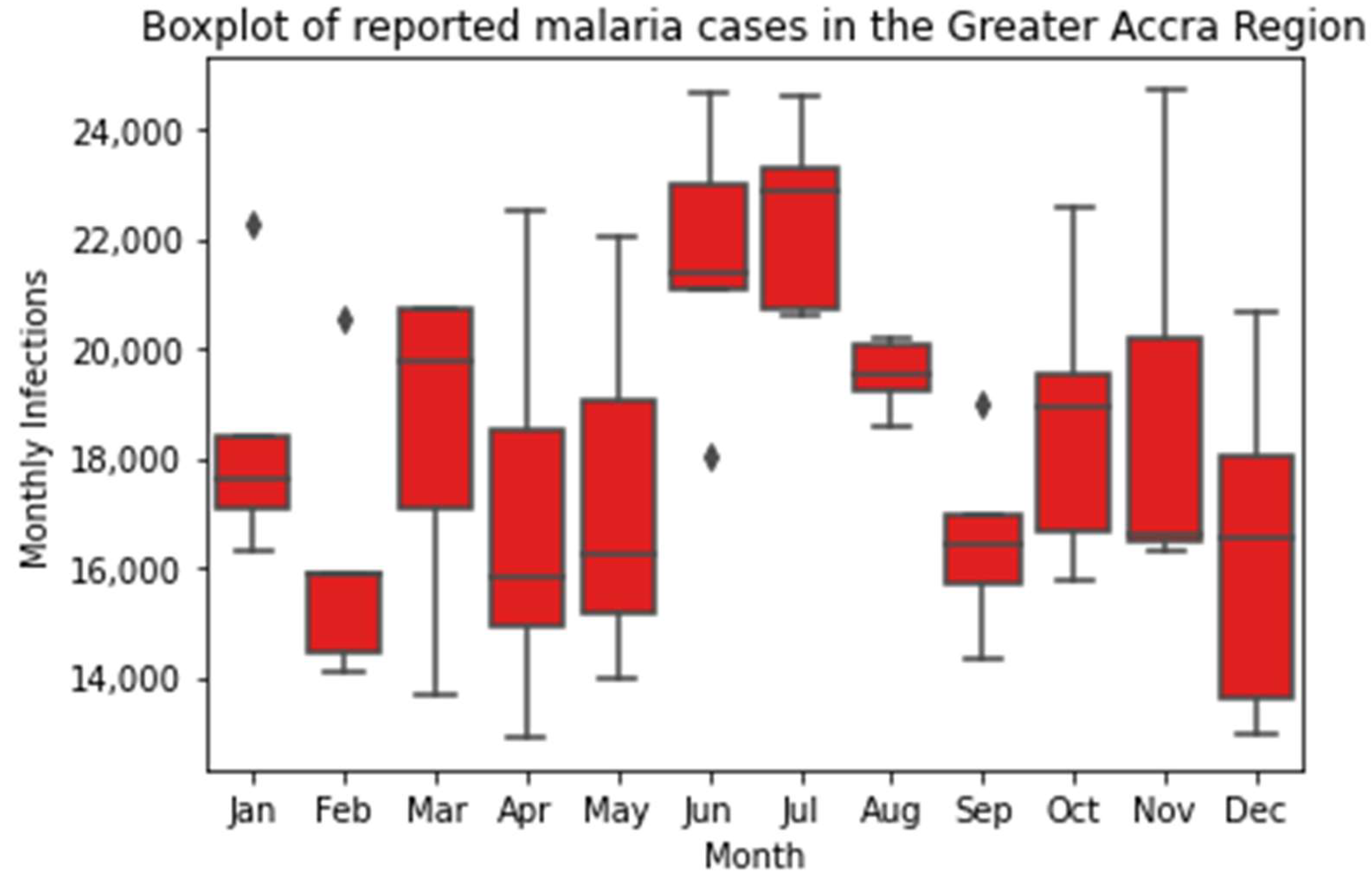
2.3. Descriptive Statistics
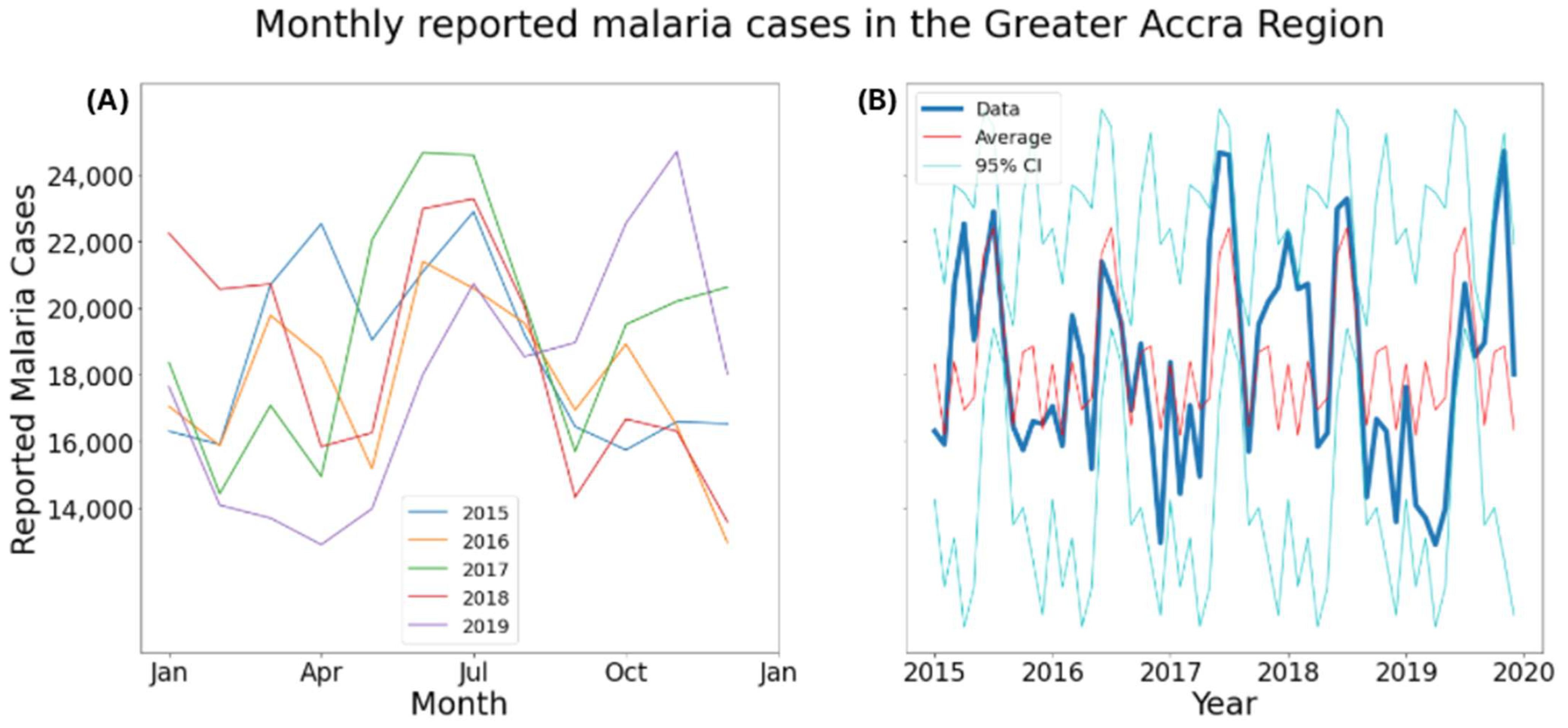
2.4. Temporal Analysis
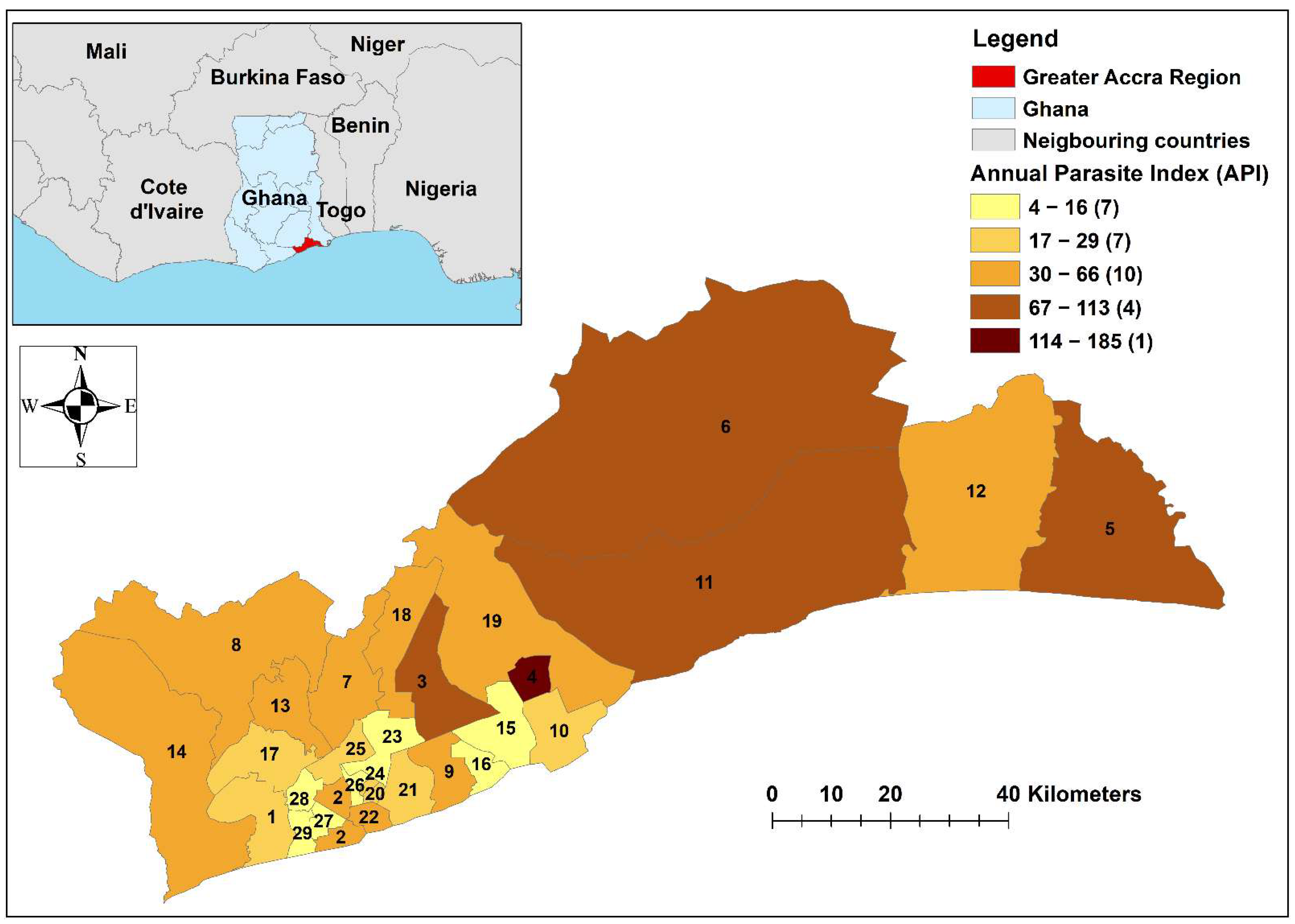
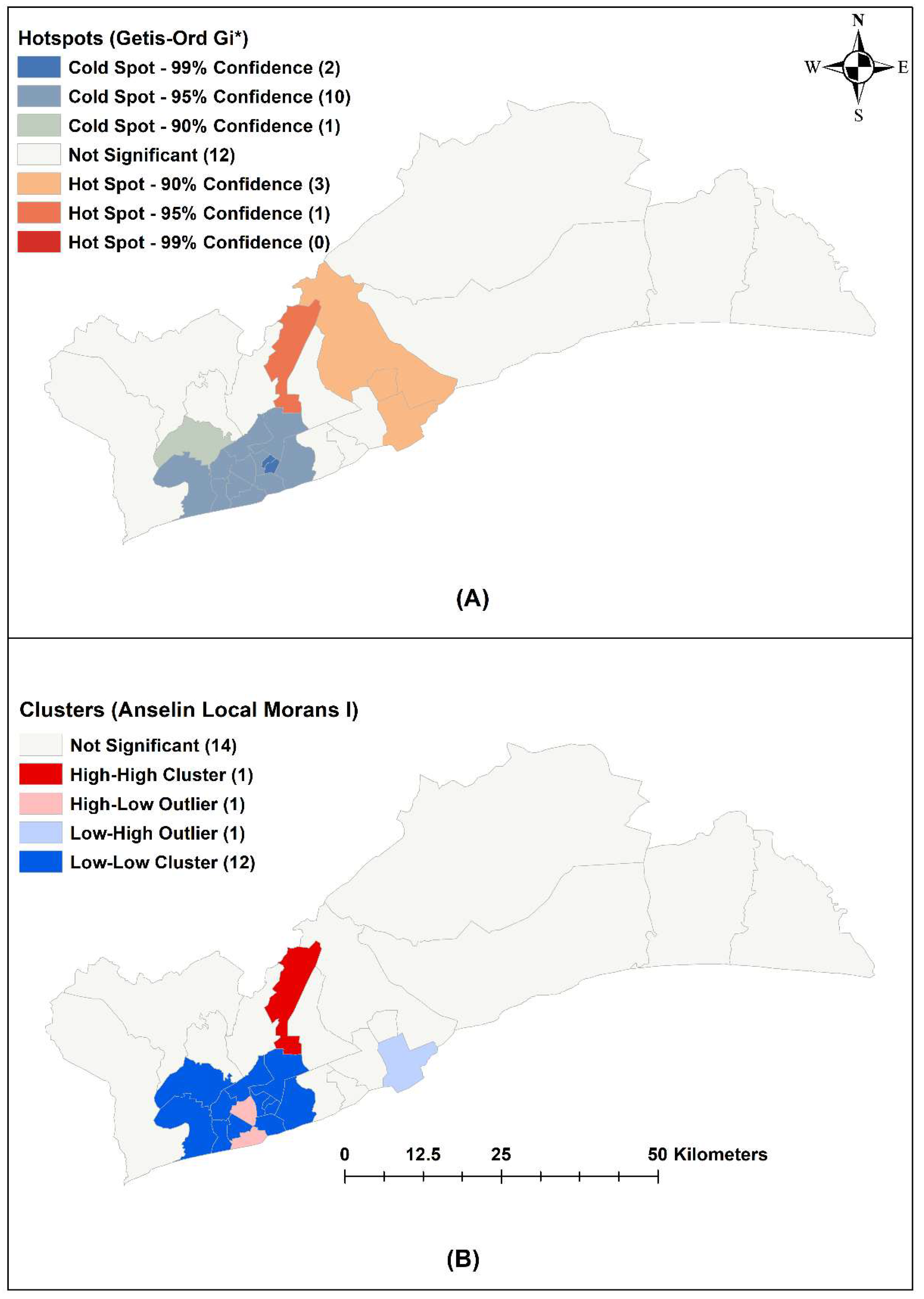
2.5. Spatial Analysis
2.6. Space–Time Analysis
2.7. Regression Analysis
3. Results
3.1. Descriptive Statistics
3.2. Temporal Analysis
3.3. Spatial Analysis
3.4. Space–Time Analysis
3.5. Negative-Binomial Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. World Malaria Report 2021; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- GBD 2017 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1859–1922. [Google Scholar] [CrossRef]
- WHO. Malaria. Available online: https://www.who.int/news-room/fact-sheets/detail/malaria (accessed on 18 September 2022).
- Ashley, E.A.; Pyae Phyo, A.; Woodrow, C.J. Malaria. Lancet 2018, 391, 1608–1621. [Google Scholar] [CrossRef]
- Talapko, J.; Skrlec, I.; Alebic, T.; Jukic, M.; Vcev, A. Malaria: The Past and the Present. Microorganisms 2019, 7, 179. [Google Scholar] [CrossRef] [PubMed]
- Kipruto, E.K.; Ochieng, A.O.; Anyona, D.N.; Mbalanya, M.; Mutua, E.N.; Onguru, D.; Nyamongo, I.K.; Estambale, B.B.A. Effect of climatic variability on malaria trends in Baringo County, Kenya. Malar. J. 2017, 16, 220. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, B.M. The epidemiology of malaria. Ann. Trop. Med. Parasitol. 1997, 91, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Langhorne, J.; Ndungu, F.M.; Sponaas, A.M.; Marsh, K. Immunity to malaria: More questions than answers. Nat. Immunol. 2008, 9, 725–732. [Google Scholar] [CrossRef]
- Woeld Health Organization. World Malaria Report 2020: 20 Years of Global Progress and Challenges; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Awine, T.; Malm, K.; Bart-Plange, C.; Silal, S.P. Towards malaria control and elimination in Ghana: Challenges and decision making tools to guide planning. Glob. Health Action 2017, 10, 1381471. [Google Scholar] [CrossRef]
- Krefis, A.C.; Schwarz, N.G.; Kruger, A.; Fobil, J.; Nkrumah, B.; Acquah, S.; Loag, W.; Sarpong, N.; Adu-Sarkodie, Y.; Ranft, U.; et al. Modeling the relationship between precipitation and malaria incidence in children from a holoendemic area in Ghana. Am. J. Trop. Med. Hyg. 2011, 84, 285–291. [Google Scholar] [CrossRef]
- Mattah, P.A.; Futagbi, G.; Amekudzi, L.K.; Mattah, M.M.; de Souza, D.K.; Kartey-Attipoe, W.D.; Bimi, L.; Wilson, M.D. Diversity in breeding sites and distribution of Anopheles mosquitoes in selected urban areas of southern Ghana. Parasit Vectors 2017, 10, 25. [Google Scholar] [CrossRef]
- Sachs, J.; Malaney, P. The economic and social burden of malaria. Nature 2002, 415, 680–685. [Google Scholar] [CrossRef]
- Blanford, J.I.; Blanford, S.; Crane, R.G.; Mann, M.E.; Paaijmans, K.P.; Schreiber, K.V.; Thomas, M.B. Implications of temperature variation for malaria parasite development across Africa. Sci. Rep. 2013, 3, 1300. [Google Scholar] [CrossRef] [PubMed]
- Godfray, H.C. Mosquito ecology and control of malaria. J. Anim. Ecol. 2013, 82, 15–25. [Google Scholar] [CrossRef]
- Rossati, A.; Bargiacchi, O.; Kroumova, V.; Zaramella, M.; Caputo, A.; Garavelli, P.L. Climate, environment and transmission of malaria. Infez. Med. 2016, 24, 93–104. [Google Scholar] [PubMed]
- The World Bank. Incidence of Malaria (per 1000 Population at Risk); The World Bank: Washington, DC, USA, 2022. [Google Scholar]
- Donkor, E.; Kelly, M.; Eliason, C.; Amotoh, C.; Gray, D.J.; Clements, A.C.A.; Wangdi, K. A Bayesian Spatio-Temporal Analysis of Malaria in the Greater Accra Region of Ghana from 2015 to 2019. Int. J. Environ. Res. Public Health 2021, 18, 80. [Google Scholar] [CrossRef]
- Frank, C.; Krumkamp, R.; Sarpong, N.; Sothmann, P.; Fobil, J.N.; Foli, G.; Jaeger, A.; Ehlkes, L.; Owusu-Dabo, E.; Adu-Sarkodie, Y.; et al. Spatial heterogeneity of malaria in Ghana: A cross-sectional study on the association between urbanicity and the acquisition of immunity. Malar. J. 2016, 15, 84. [Google Scholar] [CrossRef] [PubMed]
- Kursah, M.B. Modelling malaria susceptibility using geographic information system. GeoJournal 2017, 82, 1101–1111. [Google Scholar] [CrossRef]
- Ghana Statistical Service. Ghana Malaria Indicator Survey 2019; Ghana Statistical Service: Accra, Ghana, 2020. [Google Scholar]
- Clements, A.C.A.; Reid, H.L.; Kelly, G.C.; Hay, S.I. Further shrinking the malaria map: How can geospatial science help to achieve malaria elimination? Lancet Infect. Dis. 2013, 13, 709–718. [Google Scholar] [CrossRef]
- Wangdi, K.; Kaewkungwal, J.; Singhasivanon, P.; Silawan, T.; Lawpoolsri, S.; White, N.J. Spatio-temporal patterns of malaria infection in Bhutan: A country embarking on malaria elimination. Malar. J. 2011, 10, 89. [Google Scholar] [CrossRef]
- Wangdi, K.; Penjor, K.; Lawpoolsri, S.; Price, R.N.; Gething, P.W.; Gray, D.J.; Da Silva Fonseca, E.; Clements, A.C.A. Space-Time Clustering Characteristics of Malaria in Bhutan at the End Stages of Elimination. Int. J. Environ. Res. Public Health 2021, 18, 5553. [Google Scholar] [CrossRef]
- Wangdi, K.; Canavati, S.E.; Ngo, T.D.; Nguyen, T.M.; Tran, L.K.; Kelly, G.C.; Martin, N.J.; Clements, A.C.A. Spatial and Temporal Patterns of Malaria in Phu Yen Province, Vietnam, from 2005 to 2016. Am. J. Trop. Med. Hyg. 2020, 103, 1540–1548. [Google Scholar] [CrossRef]
- Wangdi, K.; Wetzler, E.; Cox, H.; Marchesini, P.; Villegas, L.; Canavati, S. Spatial patterns and climate drivers of malaria in three border areas of Brazil, Venezuela and Guyana, 2016–2018. Sci. Rep. 2022, 12, 10995. [Google Scholar] [CrossRef] [PubMed]
- About GAR—Greater Accra Region. Available online: http://www.gtarcc.gov.gh/index.php/about-gar (accessed on 1 December 2021).
- Ghana Statistical Service. Ghana Statistical Service. Ghana 2021 Population and Housing Census. In Population of Regions and Districts; Ghana Statistical Service: Accra, Ghana, 2021; Volume 3A. [Google Scholar]
- Greater Accra Region. Available online: https://lgs.gov.gh/index.php/greater-accra (accessed on 3 December 2021).
- Worldclim. Global Climate and Weather Data. Available online: https://worldclim.org (accessed on 7 December 2021).
- Relation: Greater Accra Region. Available online: https://www.openstreetmap.org/relation/1991849 (accessed on 7 December 2021).
- World Health Organization. Indicator Metadata Registry List—Annual Parasite Index; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Getis, A.; Ord, J.K. The Analysis of Spatial Association by Use of Distance Statistics. Geogr. Anal. 1992, 24, 189–206. [Google Scholar] [CrossRef]
- Moran, P.A.P. Notes on Continuous Stochastic Phenomena. Biometrika 1950, 37, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Anselin, L. Local Indicators of Spatial Association—LISA. Geogr. Anal. 1995, 27, 93–115. [Google Scholar] [CrossRef]
- Kulldorff, M. A spatial scan statistic. Commun. Stat.-Theory Methods 1997, 26, 1481–1496. [Google Scholar] [CrossRef]
- Craig, M.H.; Snow, R.W.; le Sueur, D. A climate-based distribution model of malaria transmission in sub-Saharan Africa. Parasitol. Today 1999, 15, 105–111. [Google Scholar] [CrossRef]
- Gunda, R.; Chimbari, M.J.; Shamu, S.; Sartorius, B.; Mukaratirwa, S. Malaria incidence trends and their association with climatic variables in rural Gwanda, Zimbabwe, 2005–2015. Malar. J. 2017, 16, 393. [Google Scholar] [CrossRef]
- Thomson, M.C.; Mason, S.J.; Phindela, T.; Connor, S.J. Use of rainfall and sea surface temperature monitoring for malaria early warning in Botswana. Am. J. Trop. Med. Hyg. 2005, 73, 214–221. [Google Scholar] [CrossRef]
- Bi, P.; Tong, S.; Donald, K.; Parton, K.A.; Ni, J. Climatic variables and transmission of malaria: A 12-year data analysis in Shuchen County, China. Public Health Rep. 2003, 118, 65–71. [Google Scholar] [CrossRef]
- Dolo, G.; Briët, O.J.; Dao, A.; Traoré, S.F.; Bouaré, M.; Sogoba, N.; Niaré, O.; Bagayogo, M.; Sangaré, D.; Teuscher, T.; et al. Malaria transmission in relation to rice cultivation in the irrigated Sahel of Mali. Acta Trop. 2004, 89, 147–159. [Google Scholar] [CrossRef]
- Meyrowitsch, D.W.; Pedersen, E.M.; Alifrangis, M.; Scheike, T.H.; Malecela, M.N.; Magesa, S.M.; Derua, Y.A.; Rwegoshora, R.T.; Michael, E.; Simonsen, P.E. Is the current decline in malaria burden in sub-Saharan Africa due to a decrease in vector population? Malar. J. 2011, 10, 188. [Google Scholar] [CrossRef] [PubMed]
- Wubishet, M.K.; Berhe, G.; Adissu, A.; Tafa, M.S. Effectiveness of long-lasting insecticidal nets in prevention of malaria among individuals visiting health centres in Ziway-Dugda District, Ethiopia: Matched case-control study. Malar. J. 2021, 20, 301. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Technical Strategy for Malaria 2016–2030; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Meibalan, E.; Marti, M. Biology of Malaria Transmission. Cold Spring Harb. Perspect. Med. 2017, 7, a025452. [Google Scholar] [CrossRef]
- Mohammadkhani, M.; Khanjani, N.; Bakhtiari, B.; Sheikhzadeh, K. The relation between climatic factors and malaria incidence in Kerman, South East of Iran. Parasite Epidemiol. Control. 2016, 1, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Dabaro, D.; Birhanu, Z.; Negash, A.; Hawaria, D.; Yewhalaw, D. Effects of rainfall, temperature and topography on malaria incidence in elimination targeted district of Ethiopia. Malar. J. 2021, 20, 104. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Y.; Li, Y.; Wang, G.; Lourenço, J.; Kraemer, M.; He, Q.; Cazelles, B.; Li, Y.; Wang, R.; et al. The relationship between rising temperatures and malaria incidence in Hainan, China, from 1984 to 2010: A longitudinal cohort study. Lancet Planet. Health 2022, 6, e350–e358. [Google Scholar] [CrossRef]
- Brooker, S.; Clarke, S.; Njagi, J.K.; Polack, S.; Mugo, B.; Estambale, B.; Muchiri, E.; Magnussen, P.; Cox, J. Spatial clustering of malaria and associated risk factors during an epidemic in a highland area of western Kenya. Trop. Med. Int. Health 2004, 9, 757–766. [Google Scholar] [CrossRef]
- Noden, B.H.; Kent, M.D.; Beier, J.C. The impact of variations in temperature on early Plasmodium falciparum development in Anopheles stephensi. Parasitology 1995, 111 Pt 5, 539–545. [Google Scholar] [CrossRef]
- Pathak, A.K.; Shiau, J.C.; Thomas, M.B.; Murdock, C.C. Field Relevant Variation in Ambient Temperature Modifies Density-Dependent Establishment of Plasmodium falciparum Gametocytes in Mosquitoes. Front. Microbiol. 2019, 10, 2651. [Google Scholar] [CrossRef]
- Patz, J.A.; Olson, S.H. Malaria risk and temperature: Influences from global climate change and local land use practices. Proc. Natl. Acad. Sci. USA 2006, 103, 5635–5636. [Google Scholar] [CrossRef]
- Shapiro, L.L.M.; Whitehead, S.A.; Thomas, M.B. Quantifying the effects of temperature on mosquito and parasite traits that determine the transmission potential of human malaria. PLoS Biol. 2017, 15, e2003489. [Google Scholar] [CrossRef] [PubMed]
- Tompkins, A.M.; Ermert, V. A regional-scale, high resolution dynamical malaria model that accounts for population density, climate and surface hydrology. Malar. J. 2013, 12, 65. [Google Scholar] [CrossRef] [PubMed]
- Paaijmans, K.P.; Blanford, S.; Bell, A.S.; Blanford, J.I.; Read, A.F.; Thomas, M.B. Influence of climate on malaria transmission depends on daily temperature variation. Proc. Natl. Acad. Sci. USA 2010, 107, 15135–15139. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, C.; Biggar, R.J.; Collins, W.E.; Nkrumah, F.K. Malaria in urban and rural areas of southern Ghana: A survey of parasitaemia, antibodies, and antimalarial practices. Bull. World Health Organ. 1984, 62, 607–613. [Google Scholar] [PubMed]
- Mbouna, A.D.; Tompkins, A.M.; Lenouo, A.; Asare, E.O.; Yamba, E.I.; Tchawoua, C. Modelled and observed mean and seasonal relationships between climate, population density and malaria indicators in Cameroon. Malar. J. 2019, 18, 359. [Google Scholar] [CrossRef]
- Kabaria, C.W.; Gilbert, M.; Noor, A.M.; Snow, R.W.; Linard, C. The impact of urbanization and population density on childhood Plasmodium falciparum parasite prevalence rates in Africa. Malar. J. 2017, 16, 49. [Google Scholar] [CrossRef]
- Ghana Statistical Service. Ghana Statistical Service. Ghana 2021 Population and Housing Census. In Housing Characteristics; Ghana Statistical Service: Accra, Ghana, 2021; Volume 3K. [Google Scholar]
- De Silva, P.M.; Marshall, J.M. Factors Contributing to Urban Malaria Transmission in Sub-Saharan Africa: A Systematic Review. J. Trop. Med. 2012, 2012, 819563. [Google Scholar] [CrossRef]
- Fobil, J.N.; Kraemer, A.; Meyer, C.G.; May, J. Neighborhood Urban Environmental Quality Conditions Are Likely to DriveMalaria and Diarrhea Mortality in Accra, Ghana. J. Environ. Public Health 2011, 2011, 10. [Google Scholar] [CrossRef]
- Deressa, W.; Ali, A.; Berhane, Y. Review of the interplay between population dynamics and malaria transmission in Ethiopia. Ethiop. J. Health Dev. 2016, 20, 52653. [Google Scholar] [CrossRef]
- Padi, M. Food Crop Farming and the Climate in Southern Ghana. Acta Sci. Agric. 2018, 2, 23–26. [Google Scholar]
- Mohino, E.; Janicot, S.; Bader, J. Sahel rainfall and decadal to multi-decadal sea surface temperature variability. Clim. Dyn. 2011, 37, 419–440. [Google Scholar] [CrossRef]
- Grassly, N.C.; Fraser, C. Seasonal infectious disease epidemiology. Proc. Biol. Sci. 2006, 273, 2541–2550. [Google Scholar] [CrossRef] [PubMed]

| Time Period (Month Year) | Latitude | Longitude | Radius (km) | Population | No. of Cases | Expected Cases | No. of Districts | RR | LLR | p-Value * |
|---|---|---|---|---|---|---|---|---|---|---|
| 15 February–17 July | 5.8126 | 0.1730 | 26.6 | 483,466 | 216,521 | 54,874.3 | 4 | 4.66 | 148,688.1 | <0.001 |
| 15 February–17 July | 5.6967 | 0.1308 | 0 | 88,105 | 41,972 | 10,000.12 | 1 | 4.32 | 28,704.2 | <0.001 |
| 15 May–16 November | 5.6060 | 0.1218 | 0 | 156,336 | 15,921 | 11,208.48 | 1 | 1.43 | 885.4 | <0.001 |
| 15 July | 5.5386 | 0.2269 | 0 | 509,868 | 2243 | 1918.16 | 1 | 1.17 | 26.1 | <0.001 |
| 17 June–19 November | 5.7000 | 0.2196 | 7.9 | 411,736 | 94,709 | 50,348.11 | 3 | 1.96 | 16,426.8 | <0.001 |
| 17 June–19 November | 5.6169 | 0.3065 | 0 | 133,495 | 22,665 | 16,324.08 | 1 | 1.40 | 1115.8 | <0.001 |
| 18 April | 5.5591 | 0.3128 | 0 | 180,058 | 849 | 714.82 | 1 | 1.19 | 11.9 | <0.05 |
| 19 August–19 November | 5.6212 | 0.2249 | 0 | 236,067 | 8296 | 3990.12 | 1 | 2.09 | 1774.8 | <0.001 |
| Covariates | Coefficient | 95% CI | Risk Ratio | 95% CI |
|---|---|---|---|---|
| Intercept | ||||
| Rainfall (cm) | ||||
| Min temp (°C) | ||||
| Malaria cases * | ||||
| Population density ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawaguchi, K.; Donkor, E.; Lal, A.; Kelly, M.; Wangdi, K. Distribution and Risk Factors of Malaria in the Greater Accra Region in Ghana. Int. J. Environ. Res. Public Health 2022, 19, 12006. https://doi.org/10.3390/ijerph191912006
Kawaguchi K, Donkor E, Lal A, Kelly M, Wangdi K. Distribution and Risk Factors of Malaria in the Greater Accra Region in Ghana. International Journal of Environmental Research and Public Health. 2022; 19(19):12006. https://doi.org/10.3390/ijerph191912006
Chicago/Turabian StyleKawaguchi, Koh, Elorm Donkor, Aparna Lal, Matthew Kelly, and Kinley Wangdi. 2022. "Distribution and Risk Factors of Malaria in the Greater Accra Region in Ghana" International Journal of Environmental Research and Public Health 19, no. 19: 12006. https://doi.org/10.3390/ijerph191912006
APA StyleKawaguchi, K., Donkor, E., Lal, A., Kelly, M., & Wangdi, K. (2022). Distribution and Risk Factors of Malaria in the Greater Accra Region in Ghana. International Journal of Environmental Research and Public Health, 19(19), 12006. https://doi.org/10.3390/ijerph191912006







