Worker Protection Scenarios for General Analytical Testing Facility under Several Infection Propagation Risks: Scoping Review, Epidemiological Model and ISO 31000
Abstract
:1. Introduction
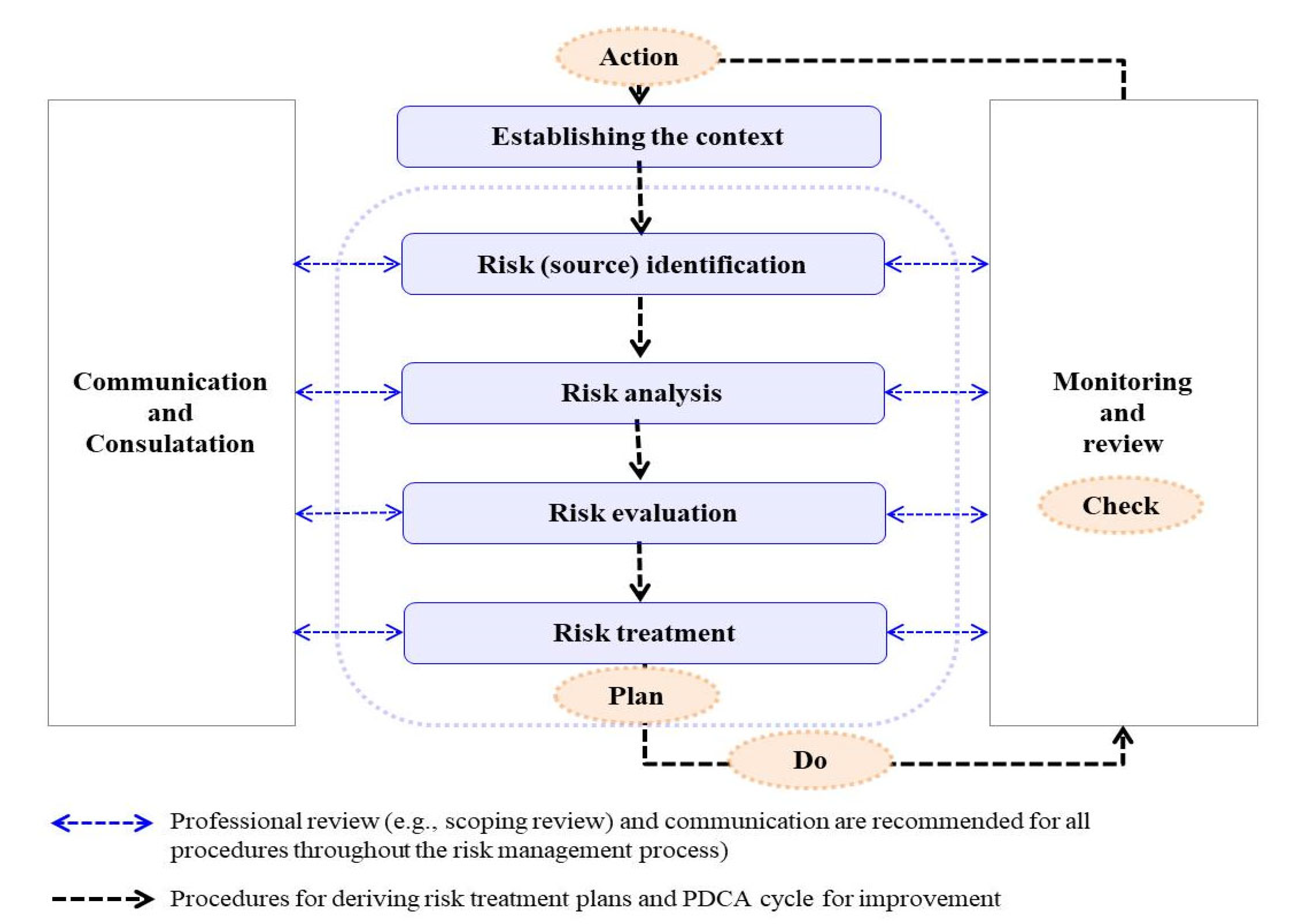
2. Materials and Methods
2.1. Identification of Risk Sources (Step 1 of Risk Management)
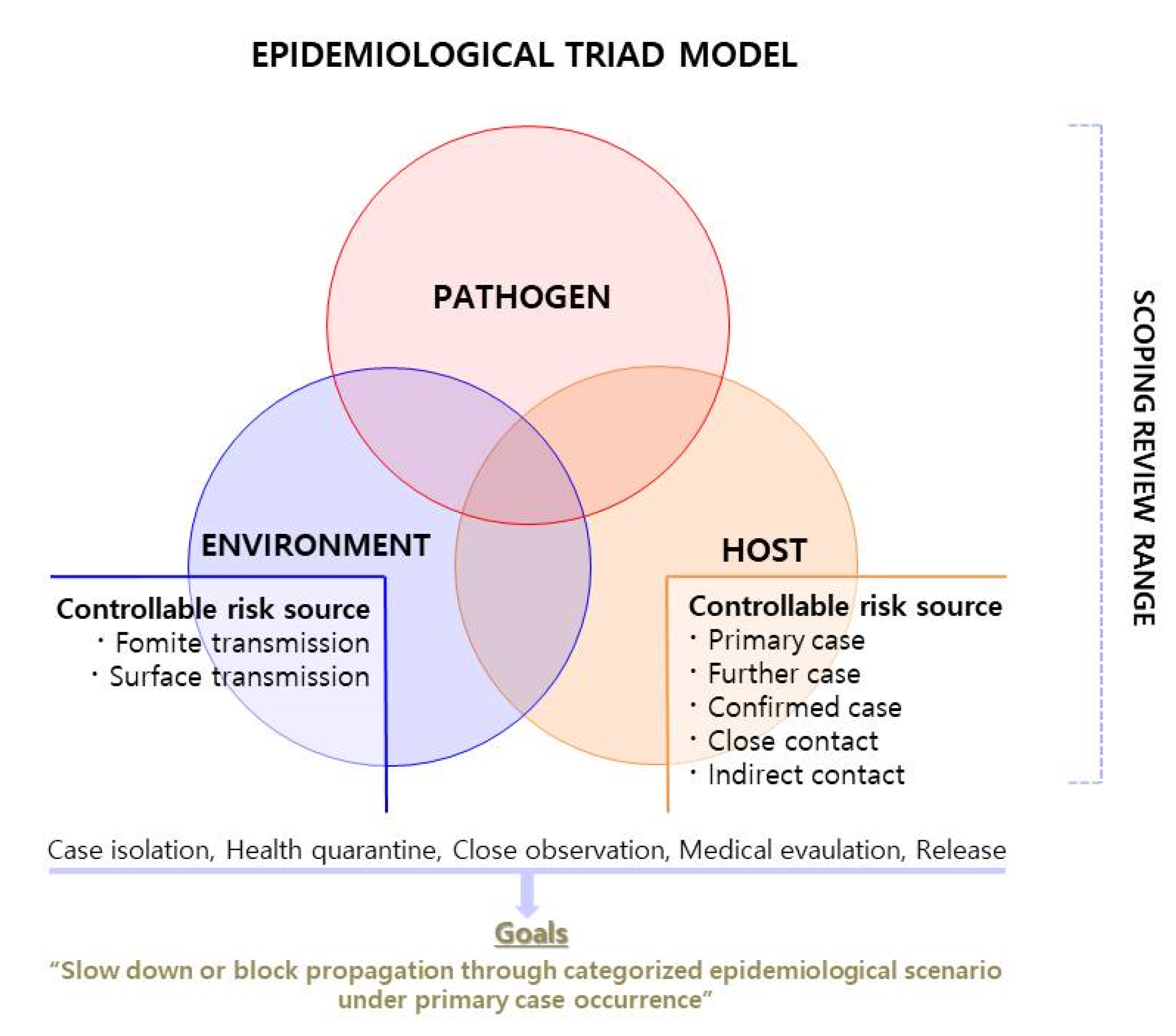
2.1.1. Epidemiological Triad Model and Scoping Review
2.1.2. Deduction of Environmental Risk Sources
2.2. Risk Analysis (Step 2)
2.3. Deduction of Risk Treatment Option (Step 3)
2.4. Establishment of Risk Treatment Plans (Step 4)
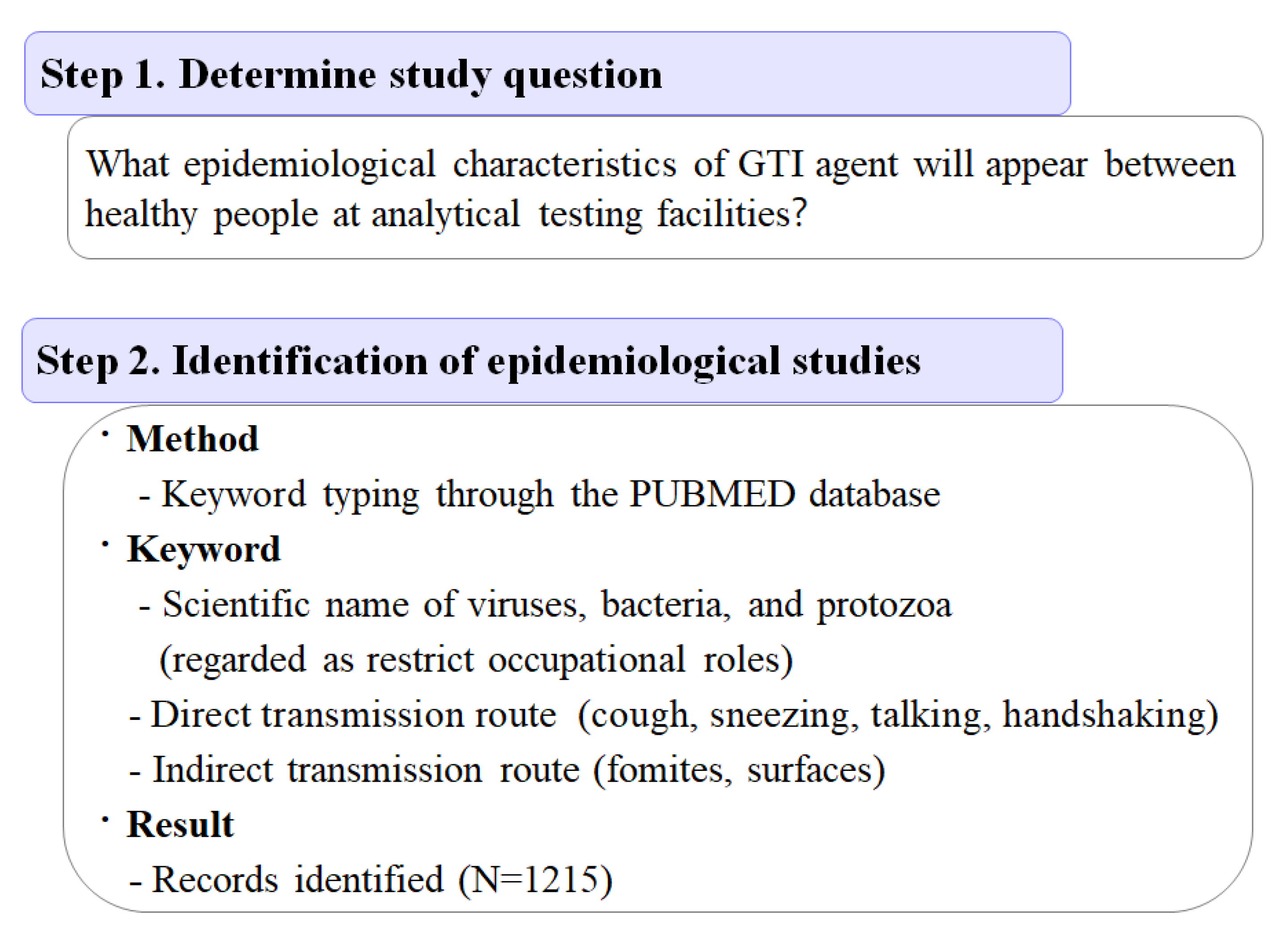
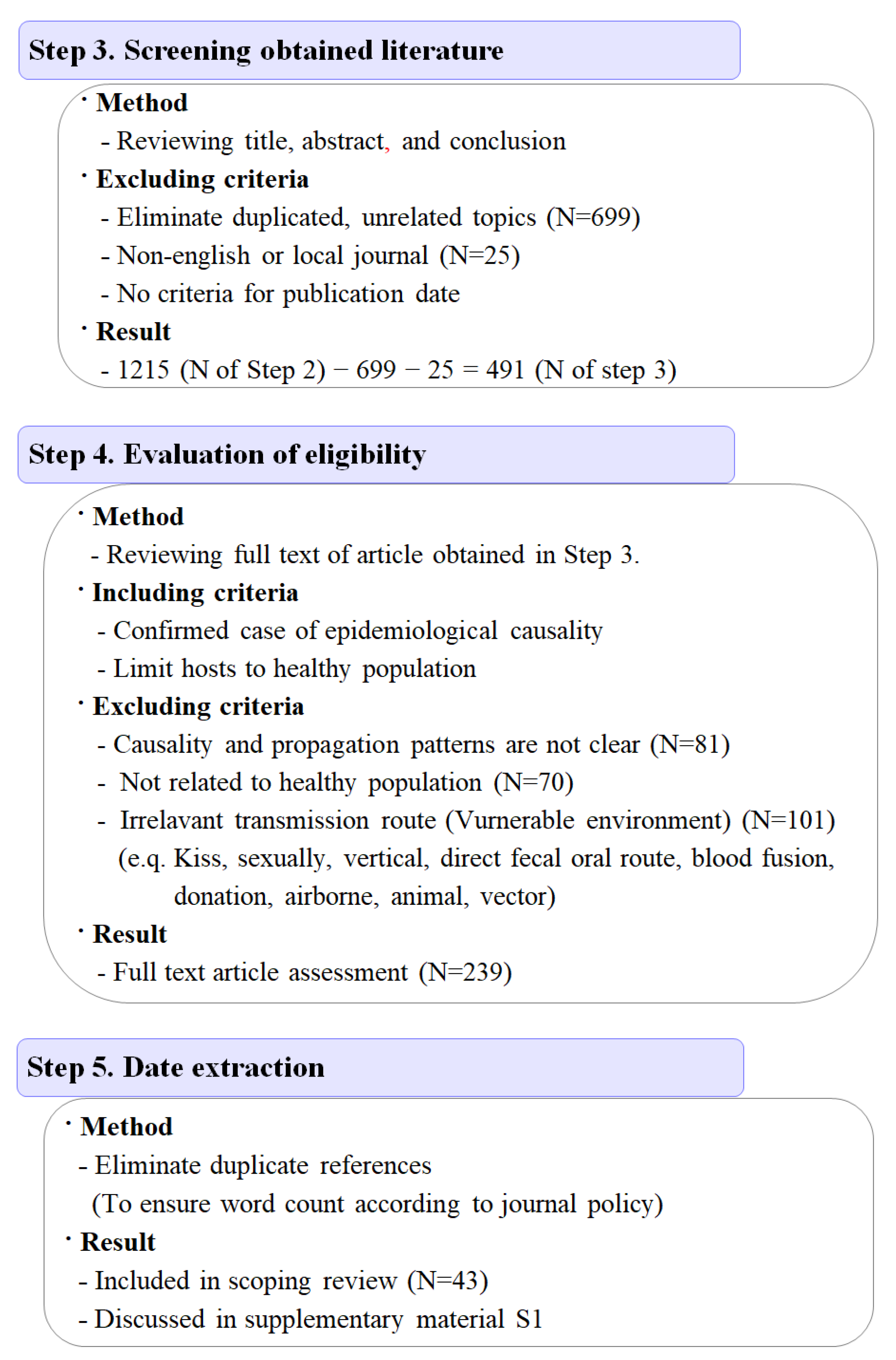
2.5. Scoping Review
3. Results
3.1. Environmental Risk Sources
3.2. Transmission Routes as Risk Sources
3.3. Human Reservoir as Risk Sources
3.4. Risk Analysis
3.5. Risk Treatment Option: Direction, Goal, Scenarios, and SOP
3.6. Scenarios
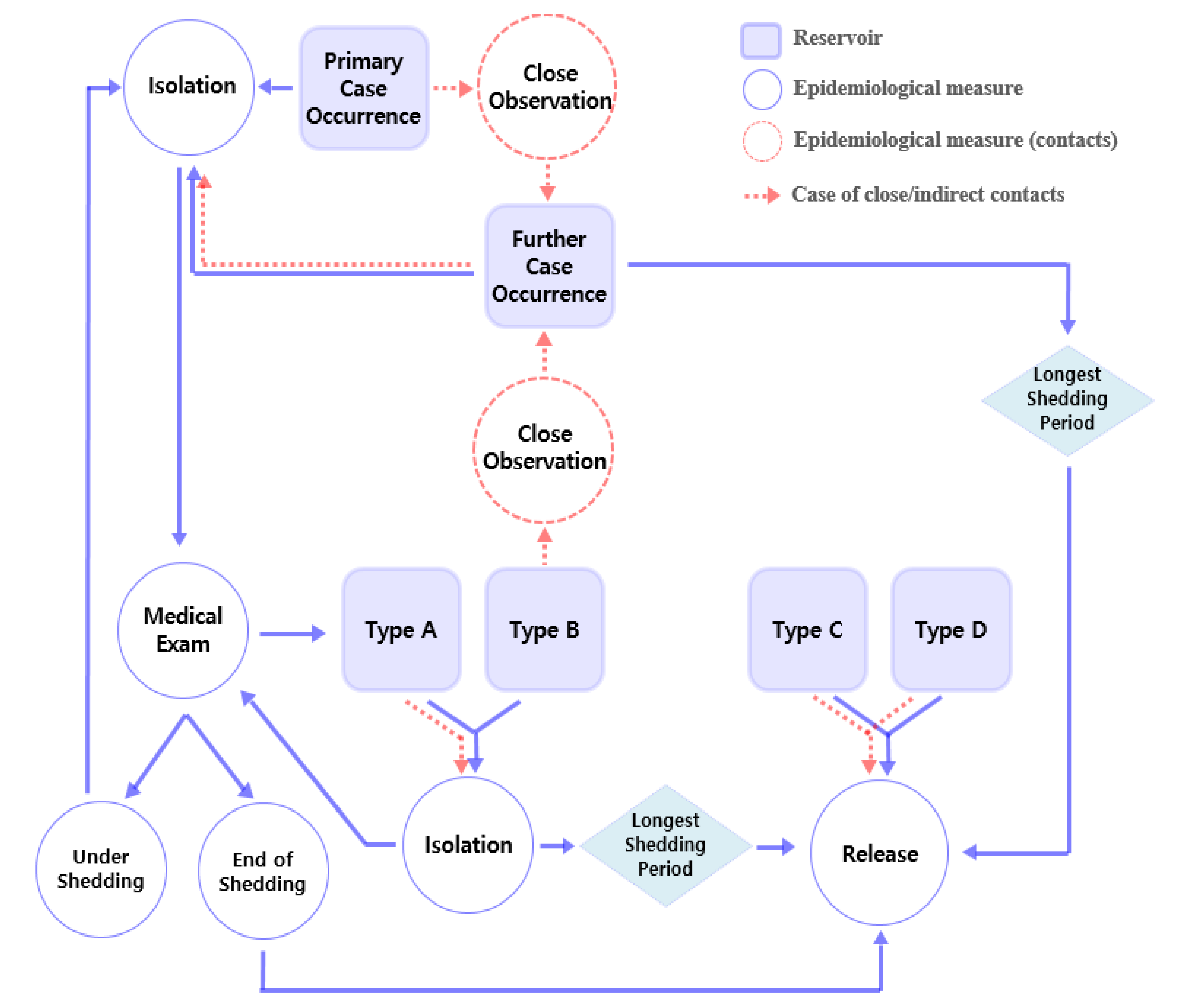
3.6.1. First Step of Configuration of Procedures over Time: Primary Case Occurrence
| Part 3. Further Case Occurrences | |
|---|---|
| 3.1 | Health quarantine, close observation, and isolation |
| (1) | Further case occurrence indicates Type A propagation, even before the medical evaluation of primary case deduction. Close and indirect contacts should be isolated from occupation. The reason for attempting indirect contact isolation is that the risk of indirect transmission increases in the case of direct transmission. |
| (2) | Medical evaluation and treatment must be performed in consideration of asymptomatic case that discharge causative agents in both close and indirect contacts. |
| (3) | If someone does not correspond to close or indirect contacts, close observation is required because of a shared general domestic environment. |
| 3.2 | Record management |
| (1) | Symptoms of further cases and medical evaluation results should be recorded and managed. |
| Part 4. With Deduction of Medical Evaluation | |
| 4.1 | Type A |
| (1) | Even if there is no further case occurrence, all close and indirect contacts must maintain isolation to block or slow down propagation and obey the procedures of (2.3) |
| 4.2 | Type B |
| (1) | Close and indirect contacts maintain close observation because of the possibility of indirect transmission through close and indirect contact. |
| (2) | Isolation should be performed whenever additional symptoms occur during close observation. Rapid propagation among close and indirect contacts can be blocked by maintaining close observation. |
| 4.3 | Type C |
| (1) | Primary case can be released from isolation. |
| (2) | Close and indirect contacts can be released from health quarantine or close observation. |
| 4.4 | Type D |
| (1) | Primary case might be isolated until symptom extinguished. |
| (2) | Close and indirect contacts might be released from health quarantine and close observation. |
| Part 5. Release | |
| 5.1 | Confirmed case |
| (1) | This is limited to Types A and B. Types C and D comply with the regulations of 4.3 or 4.4 |
| (2) | Since shedding period varies by etiology and host factor, it is necessary to return to occupation after confirming that there is no discharge through medical evaluation (2.3). |
| (3) | If medical evaluation is not possible due to financial conditions, the case should be excluded from occupation until the longest known period of each agent’s discharging period. |
| 5.2 | Health quarantine |
| (1) | This is limited to Types A and B. Types C and D comply with the regulations of 4.3 or 4.4. |
| (2) | Close and indirect contacts without any symptoms can return to occupation after the maximum shedding periods has elapsed from the last case isolation date, and other close observations can be released. |
3.6.2. Second Step of Configuration of Procedures over Time: Medical Evaluation and Further Case Occurrence
3.6.3. Third Step of Configuration of Procedures over Time: Categorized Scenario
3.6.4. Fourth Step of Configuration of Procedures over Time: Release
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burgos, D.; Ivanov, D. Food retail supply chain resilience and the COVID-19 pandemic: A digital twin-based impact analysis and improvement directions. Transp. Res. E Logist. Transp. Rev. 2021, 152, 102412. [Google Scholar] [CrossRef] [PubMed]
- Correia, S.; Luck, S.; Verner, E. Pandemics Depress the Economy, Public Health Interventions Do Not: Evidence from the 1918 Flu 2020. 2021. Available online: https://www.ssrn.com/abstract=3561560 (accessed on 22 June 2022).
- Huang, F.; Qu, M.; Liu, Y.; Yan, H.Q.; Gao, Z.Y.; Dou, X.F.; Zhang, H.Y.; Zhang, Z.; Ma, J.X.; Guo, J. Etiological analysis of enteric infectious diseases during Beijing Olympic Games. Zhonghua Yu Fang Yi Xue Za Zhi 2009, 43, 789–792. [Google Scholar] [PubMed]
- Kim, K.; Jang, J.Y.; Moon, G.; Shim, H.; Jung, P.Y.; Kim, S.; Choi, Y.U.; Bae, K.S. Experiences of the emergency department at the Pyeongchang polyclinic during the 2018 PyeongChang winter olympic games. Yonsei Med. J. 2019, 60, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; You, Y.H.; Cho, H.M.; Hong, J.W.; Ghim, S.Y. Foodborne infectious diseases mediated by inappropriate infection control in food service businesses and relevant countermeasures in Korea. Osong Public Health Res. Perspect. 2017, 8, 159–168. [Google Scholar] [CrossRef]
- Carbone, A. Food supply chains: Coordination governance and other shaping forces. Agric. Food Econ. 2017, 5, 3. [Google Scholar] [CrossRef]
- Khan, S.Z.; Ashfaq, M.A.; Awan, M.U.; URehman, H.; Kamal, S.A.; Hoa, N.T.X.; Shafiq, M. Investigating supply chain issues in the food processing industry. IOP Conf. Ser. Mater. Sci. Eng. 2020, 847, 012071. [Google Scholar] [CrossRef]
- De Brito, A.C.; Kauffmann, C.; Pelkmans, J. The Contribution of Mutual Recognition to International Regulatory Co-Operation; OECD Regulatory Policy Working Papers, No. 2; OECD Publishing: Paris, France, 2016. [Google Scholar]
- Kim, E.A. Social Distancing and Public Health Guidelines at Workplaces in Korea: Responses to Coronavirus Disease-19. Saf. Health Work 2020, 11, 275–283. [Google Scholar] [CrossRef]
- Aven, T. The flaws of the ISO 31000 conceptualisation of risk. Proceedings of the Institution of mechanical engineers. Proc. Inst. Mech. Eng. 2017, 231, 467–468. [Google Scholar]
- ISO 31000:2018(en); International Standard for Risk Management. ISO: Geneva, Switzerland, 2018.
- Lalonde, C.; Boiral, O. Managing risks through ISO 31000: A critical analysis. Risk Manag. 2012, 14, 272–300. [Google Scholar] [CrossRef]
- Leitch, M. ISO 31000:2009—The new international standard on risk management. Risk Anal. 2010, 30, 887–892. [Google Scholar] [CrossRef]
- Kaya, G.K.; Ward, J.R.; Clarkson, P.J. A framework to support risk assessment in hospitals. Int. J. Qual. Health Care 2019, 31, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.I.; Han, S.W.; Shin, S.; Byeon, S.H. Comparison of the qualitative and the quantitative risk assessment of hazardous substances requiring management under the occupational safety and health act in South Korea. Int. J. Environ. Res. Public Health 2021, 2, 1354. [Google Scholar] [CrossRef] [PubMed]
- Pascarella, G.; Rossi, M.; Montella, E.; Capasso, A.; De Feo, G.; Botti, G.; Nardone, A.; Montuori, P.; Triassi, M.; D’Auria, S.; et al. Risk analysis in healthcare organizations: Methodological framework and critical variables. Risk Manag. Healthc. Policy 2021, 14, 2897–2911. [Google Scholar] [CrossRef]
- Wilbanks, D.W.; Byrd, T. The Relevance & Benefit of ISO 31000 to OSH Practice. Prof. Saf. 2020, 65, 32–38. [Google Scholar]
- Zhou, L.J.; Cao, Q.G.; Yu, K.; Wang, L.L.; Wang, H.B. Research on occupational safety, health management and risk control technology in coal mines. Int. J. Environ. Res. Public Health 2018, 15, 868. [Google Scholar] [CrossRef]
- Dicker, R.C.; Coronado, F.; Koo, D.; Parrish, R.G. Principles of Epidemiology in Public Health Practice: An Introduction to Applied Epidemiology and Biostatistics, 3rd ed.; Department of Health and Human Services, CDC: Atlanta, GA, USA, 2011. [Google Scholar]
- Scholthof, K.B. The disease triangle: Pathogens, the environment and society. Nat. Rev. Microbiol. 2007, 5, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Munn, Z.; Peters, M.D.J.; Stern, C.; Tufanaru, C.; McArthur, A.; Aromataris, E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med. Res. Methodol. 2018, 18, 143. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.; Colquhoun, H.; Kastner, M.; Levac, D.; Ng, C.; Sharpe, J.P.; Wilson, K.; et al. A scoping review on the conduct and reporting of scoping reviews. BMC Med. Res. Methodol. 2016, 9, 15. [Google Scholar] [CrossRef]
- ISO (International Standardization Organization). ISO/IEC 17025 International Standard(E), General Requirements for the Competence of Testing and Calibration Laboratories, 3rd ed.; ISO: Geneva, Switzerland, 2017. [Google Scholar]
- FDA (Food and Drug Administration of United States) and OSHA (Occupational Safety and Health Administration). Employee Health and Food Safety Checklist for Human and Animal Food Operations during the COVID-19 Pandemic; FDA: Silver Spring, MD, USA, 2020. [Google Scholar]
- FAO (Food and Agricultural Administration of United Nation) and WHO (World Health Organization). COVID-19 and Food Safety: Guidance for Food Businesses; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Burns, C.J.; LaKind, J.S. Using the Matrix to bridge the epidemiology/risk assessment gap: A case study of 2,4-D. Crit. Rev. Toxicol. 2021, 51, 591–599. [Google Scholar] [CrossRef]
- Chaiklieng, S.; Suggaravetsiri, P.; Autrup, H. Biomatrix of health risk assessment of benzene-exposed workers at Thai gasoline stations. J. Occup. Health 2021, 63, e12307. [Google Scholar] [CrossRef]
- Gaffar, B.; Alhumaid, J.; Alhareky, M.; Alonaizan, F.; Almas, K. Dental facilities during the new corona outbreak: A SWOT analysis. Risk Manag. Healthc. Policy 2020, 13, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Ledwaba, S.E.; Becker, P.; Traore-Hoffman, A.; Potgieter, N. Bacterial contamination of children’s toys in rural Day care centres and households in South Africa. Int. J. Environ. Res. Public Health 2019, 16, 2900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ota, M.; Kamigaki, T.; Mimura, S.; Nakashima, K.; Ogami, T. An enterohaemorrhagic Escherichia coli outbreak spread through the environment at an institute for people with intellectual disabilities in Japan in 2005. West. Pac. Surveill. Response J. 2019, 10, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Strachan, N.J.; Dunn, G.M.; Locking, M.E.; Reid, T.M.; Ogden, I.D. Escherichia coli O157: Burger bug or environmental pathogen? Int. J. Food Microbiol. 2006, 112, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Weil, A.A.; Begum, Y.; Chowdhury, F.; Khan, A.I.; Leung, D.T.; LaRocque, R.C. Harris JB. Bacterial shedding in household contacts of cholera patients in Dhaka, Bangladesh. Am. J. Trop. Med. Hyg. 2014, 91, 738–742. [Google Scholar] [CrossRef]
- Baker-Austin, C.; Oliver, J.D.; Alam, M.; Ali, A.; Waldor, M.K.; Qadri, F.; Martinez-Urtaza, J. Vibrio spp. infections. Nat. Rev. Dis. Primers 2018, 4, 1–19. [Google Scholar]
- Gopinath, S.; Carden, S.; Monack, D. Shedding light on Salmonella carriers. Trends Microbiol. 2012, 20, 320–327. [Google Scholar] [CrossRef]
- Beatty, M.E.; Shevick, G.; Shupe-Ricksecker, K.; Bannister, E.; Tulu, A.; Lancaster, K.; Braden, C.R. Large Salmonella enteritidis outbreak with prolonged transmission attributed to an infected food handler, Texas, 2002. Epidemiol. Infect. 2009, 137, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Loewenstein, M.S. An outbreak of salmonellosis propagated by person-to-person transmission on an Indian reservation. Am. J. Epidemiol. 1975, 102, 257–262. [Google Scholar] [CrossRef]
- Palmer, S.R.; Jephcott, A.E.; Rowlands, A.J.; Sylvester, D.G.H. Person-to-person spread of Salmonella typhimurium phage type 10 after a common source outbreak. Lancet 1981, 317, 881–884. [Google Scholar] [CrossRef]
- Majee, S.; Chowdhury, A.R.; Pinto, R.; Chattopadhyay, A.; Agharkar, A.N.; Chakravortty, D.; Basu, S. Spatiotemporal evaporating droplet dynamics on fomites enhances long term bacterial pathogenesis. Commun. Biol. 2021, 4, 1173. [Google Scholar] [CrossRef] [PubMed]
- Korpela, J.; Kärpänoja, P.; Taipalinen, R.; Siitonen, A. Subtyping of Shigella sonnei for tracing nosocomial transmission. J. Hosp. Infect. 1995, 30, 261–266. [Google Scholar] [CrossRef]
- Islam, M.S.; Hossain, M.A.; Khan, S.I.; Khan, M.N.; Sack, R.B.; Albert, M.J.; Huq, A.; Colwell, R.R. Survival of Shigella dysenteriae type 1 on fomites. J. Health Popul. Nutr. 2001, 19, 177–182. [Google Scholar] [PubMed]
- Kotloff, K.L.; Riddle, M.S.; Platts-Mills, J.A.; Pavlinac, P.; Zaidi, A.K.M. Shigellosis. Lancet 2018, 391, 801–812. [Google Scholar] [CrossRef]
- Butz, A.M.; Fosareli, P.; Dick, J.; Cusack, T.; Yolken, R. Prevalence of Rotavirus on high-risk fomites in day care facilities. Pediatrics 1993, 92, 202–205. [Google Scholar] [CrossRef]
- Sattar, S.A.; Lloyd-Evans, N.; Springthorpe, V.S. Institutional outbreaks of Rotavirus diarrhea: Potential role of fomites and environmental surfaces as vehicles for virus transmission. J. Hyg. 1986, 96, 277–289. [Google Scholar] [CrossRef]
- Alidjinou, E.K.; Sane, F.; Firquet, S.; Lobert, P.E.; Hober, D. Resistance of enteric viruses on fomites. Intervirology 2018, 61, 205–213. [Google Scholar] [CrossRef]
- Kraay, A.N.M.; Brouwer, A.F.; Lin, N.; Collender, P.A.; Remais, J.V.; Eisenberg, J.N.S. Modeling environmentally mediated rotavirus transmission: The role of temperature and hydrologic factors. Proc. Natl. Acad. Sci. USA 2018, 115, E2782–E2790. [Google Scholar] [CrossRef]
- Abad, F.X.; Villena, C.; Guix, S.; Caballero, S.; Pintó, R.M.; Bosch, A. Potential role of fomites in the vehicular transmission of human astroviruses. Appl. Environ. Microbiol. 2001, 67, 3904–3907. [Google Scholar] [CrossRef]
- Chimonas, M.A.; Vaughan, G.H.; Andre, Z.; Ames, J.T.; Tarling, G.A.; Beard, S.; Widdowson, M.A.; Cramer, E. Passenger behaviors associated with norovirus infection on board a cruise ship—Alaska, May to June 2004. J. Travel Med. 2008, 15, 177–183. [Google Scholar] [CrossRef]
- Ho, M.S.; Glass, R.I.; Monroe, S.S.; Madore, H.P.; Stine, S.; Pinsky, P.F.; Cubitt, D.; Ashley, C.; Caul, E.O. Viral gastroenteritis aboard a cruise ship. Lancet 1989, 334, 961–965. [Google Scholar] [CrossRef]
- Hutson, A.M.; Atmar, R.L.; Graham, D.Y.; Estes, M.K. Norwalk virus infection and disease is associated with ABO histo-blood group type. J. Infect. Dis. 2002, 185, 1335–1337. [Google Scholar] [CrossRef] [PubMed]
- Kebisek, J.; Richards, E.E.; Buckelew, V.; Hourihan, M.K.; Finder, S.; Ambrose, J.F. Norovirus outbreak in army service members, Camp Arifjan, Kuwait, May 2018. MSMR 2019, 26, 8–13. [Google Scholar] [PubMed]
- La Rosa, G.; Fratini, M.; Della Libera, S.; Iaconelli, M.; Muscillo, M. Viral infections acquired indoors through airborne, droplet or contact transmission. Ann. Ist. Super. Sanita 2013, 49, 124–132. [Google Scholar]
- Widdowson, M.A.; Glass, R.; Monroe, S.; Beard, R.S.; Bateman, J.W.; Lurie, P.; Johnson, C. Probable transmission of norovirus on an airplane. JAMA 2005, 293, 1859–1860. [Google Scholar]
- Yap, J.; Qadir, A.; Liu, I.; Loh, J.; Tan, B.H.; Lee, V.J. Outbreak of acute norovirus gastroenteritis in a military facility in Singapore: A public health perspective. Singap. Med. J. 2012, 53, 249–254. [Google Scholar]
- Leoni, E.; Bevini, C.; Esposti, S.D.; Graziano, A. An outbreak of intrafamiliar hepatitis A associated with clam consumption: Epidemic transmission to a school community. Eur. J. Epidemiol. 1998, 14, 187–192. [Google Scholar] [CrossRef]
- Yu, P.; Huang, L.; Li, H.; Liu, M.; Zong, J.; Li, C.; Chen, F. Epidemiological investigation of an outbreak of hepatitis A in rural China. Int. J. Infect. Dis. 2015, 33, 191–195. [Google Scholar] [CrossRef]
- Abad, F.X.; Pintó, R.M.; Bosch, A. Survival of enteric viruses on environmental fomites. Appl. Environ. Microbiol. 1994, 60, 3704–3710. [Google Scholar] [CrossRef]
- Mbithi, J.N.; Springthorpe, V.S.; Sattar, S.A. Effect of relative humidity and air temperature on survival of hepatitis: A virus on environmental surfaces. Appl. Environ. Microbiol. 1991, 157, 1394–1399. [Google Scholar] [CrossRef]
- Leung, A.K.C.; Leung, A.A.M.; Wong, A.H.C.; Sergi, C.M.; Kam, J.K.M. Giardiasis: An Overview. Recent Pat. Inflamm. Allergy Drug Discov. 2019, 13, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Hajipour, N.; Moosavy, M.H.; Rostamzadeh, B.; Hajibemani, A. Contamination of coins and banknotes as sources of transmission of parasitic pathogens: A pilot study from Iran. Public Health 2020, 186, 116–118. [Google Scholar] [CrossRef]
- Hasan, H.; Nasirudeen, N.A.; Ruzlan, M.A.F.; Mohd Jamil, M.A.; Ismail, N.A.S.; Wahab, A.A.; Ali, A. Acute infectious gastroenteritis: The causative agents, omics-based detection of antigens and novel biomarkers. Children 2021, 8, 1112. [Google Scholar] [CrossRef] [PubMed]
- Salit, I.E.; Khairnar, K.; Gough, K.; Pillai, D.R. A possible cluster of sexually transmitted Entamoeba histolytica: Genetic analysis of a highly virulent strain. Clin. Infect. Dis. 2009, 49, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Battersby, T.; Whyte, P.; Bolton, D.J. Protecting broilers against Campylobacter infection by preventing direct contact between farm staff and broilers. Food Control 2016, 69, 346–351. [Google Scholar] [CrossRef]
- WHO. Cholera. In Global Defense against the Infected Disease Threat; WHO: Geneva, Switzerland, 2003; pp. 74–79. [Google Scholar]
- Mori, M.; Roest, H.J. Farming, Q fever and public health: Agricultural practices and beyond. Arch. Public Health 2018, 76, 2. [Google Scholar] [CrossRef] [Green Version]
- Mesner, O.; Riesenberg, K.; Biliar, N.; Borstein, E.; Bouhnik, L.; Peled, N.; Yagupsky, P. The many faces of human-to-human transmission of brucellosis: Congenital infection and outbreak of nosocomial disease related to an unrecognized clinical case. Clin. Infect. Dis. 2007, 45, e135–e140. [Google Scholar] [CrossRef]
- Eriksen, J.; Zenner, D.; Anderson, S.R.; Grant, K.; Kumar, D. Clostridium perfringens in London, July 2009: Two weddings and an outbreak. Eurosurveillance 2010, 15, 19598. [Google Scholar] [CrossRef]
- Adams, N.L.; Rose, T.C.; Hawker, J.; Violato, M.; O’Brien, S.J.; Barr, B.; Howard, V.J.K.; Whitehead, M.; Harris, R.; Taylor-Robinson, D.C. Relationship between socioeconomic status and gastrointestinal infections in developed countries: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0191633. [Google Scholar] [CrossRef]
- Armstrong-Esther, C.A. Carriage patterns of Staphylococcus aureus in a healthy non-hospital population of adults and children. Ann. Hum. Biol. 1976, 3, 221–227. [Google Scholar] [CrossRef]
- Dalman, M.; Bhatta, S.; Nagajothi, N.; Thapaliya, D.; Olson, H.; Naimi, H.M.; Smith, T.C. Characterizing the molecular epidemiology of Staphylococcus aureus across and within fitness facility types. BMC Infect. 2019, 18, 69. [Google Scholar] [CrossRef]
- Amieva, M.R. Important bacterial gastrointestinal pathogens in children: A pathogenesis perspective. Pediat. Clin. N. 2005, 52, 749–777. [Google Scholar] [CrossRef]
- Jones, R.M.; Brosseau, L.M. Aerosol transmission of infectious disease. J. Occup. Environ. Med. 2015, 57, 501–508. [Google Scholar] [CrossRef]
- Wikswo, M.E.; Kambhampati, A.; Shioda, K.; Walsh, K.A.; Bowen, A.; Hall, A.J. Centers for Disease Control and Prevention (CDC). Outbreaks of acute gastroenteritis transmitted by person-to-person contact, environmental contamination, and unknown modes of transmission—United States, 2009–2013. MMWR Surveill. Summ. 2015, 64, 1–16. [Google Scholar] [CrossRef]
- Bhandari, J.; Thada, P.K.; DeVos, E. Typhoid Fever; StatPearls Publishing: StatPearls Treasure Island, FL, USA, 2022. [Google Scholar]
- Felsenfeld, O. Notes on food, beverages and fomites contaminated with Vibrio cholerae. Bull. World Health Organ. 1965, 33, 725–734. [Google Scholar]
- Murray, P.R.; Rosenthal, K.S.; Pfaller, M.A. Medical Microbiology, 6th ed.; Mosby Elsevier: St. Louis, MO, USA, 2009. [Google Scholar]
- Ghazani, M.; FitzGerald, G.; Hu, W.; Toloo, G.S.; Xu, Z. Temperature variability and gastrointestinal infections: A review of impacts and future perspectives. Int. J. Environ. Res. Public Health 2018, 15, 766. [Google Scholar] [CrossRef]
- EFSA. Opinion of the scientific panel on biological hazards on Bacillus cereus and other Bacillus spp. in foodstuffs. EFSA J. 2005, 3, 175. [Google Scholar]
- Winterscheid, A. Application of scenario technique in flood risk management. Water Sci. Technol. 2007, 56, 87–95. [Google Scholar] [CrossRef] [Green Version]
- Chaifetz, A.; Chapman, B. Evaluating North Carolina food pantry food safety-related operating procedures. J. Food Prot. 2015, 78, 2033–2042. [Google Scholar] [CrossRef]
- Freeman, K.P.; Cook, J.R.; Hooijberg, E.H. Standard operating procedures. J. Am. Vet. Med. Assoc. 2021, 258, 477–481. [Google Scholar] [CrossRef]
- Koo, W.H.; Baek, M.H. A Study on the development of the standard operation procedures for disaster response at occurring the urban forest fire. Korean Soc. Hazard Mitig. 2014, 14, 169–176. [Google Scholar] [CrossRef]
- Tannock, G.W. Normal Microflora: An Introduction to Microbes Inhabiting the Human Body; Kluwer Academic Publishers: Alphen aan den Rijn, Netherlands, 1995. [Google Scholar]
| Stratification of Environment | Environmental Risk Sources Potentially Causing Direct or Indirect Transmission |
|---|---|
| A. General daily space | Direct contact (talking, meal, coughing) |
| Indirect contact (toilet or meal facility) | |
| B. General office space | Direct contact (meeting, hand-shaking, talking, coughing) |
| Indirect contact (joint phone, handle, meeting room and door) | |
| C. Space unique to analytical and testing facility | Direct contact (co-working, collaboration) |
| Indirect contact (joint space, facility, equipment, tools) |
| Transmission Route | Risk Source | |
|---|---|---|
| Direct transmission (1) | Collaboration | Sampling (4 days, 9 people) |
| Pretreatment of experimental sample (3 days, 10 people) | ||
| Co-working | Use of communal experimental room at the same time (12 days, 11 people) | |
| Use of experimental desks at the same time (10 days, 11 people) | ||
| Use of clean bench or home hood at the same time (11 days, 5 people) | ||
| Fomite or surface transmission (2) | Joint space | Communal experimental room (11 count/day) |
| Joint facility | Clean bench or home hood (6.5 count/day) | |
| Chair (8.8 count/day) | ||
| Deionizer (9 count/day) | ||
| Gas chromatograph (1.5 count/day) | ||
| Inductively coupled plasma (0 count/day) | ||
| Liquid chromatograph (1.5 count/day) | ||
| Experimental desk (11.5 count/day) | ||
| Joint equipment, utensils | Pipette (6.5 count/day) | |
| Syringe (2.8 count/day) | ||
| Types of handles (8.5 count/day) | ||
| Sprayer (8.8 count/day) | ||
| Sterilizer (3.3 count/day) | ||
| Categorization Type | Risk Sources |
|---|---|
| Type A. Direct, fomite- and surface-mediated transmission | Enterohemorrhagic Escherichia coli [29,30,31] |
| Vibrio cholerae [32,33] | |
| Salmonella typhi [34]; S. enteritidis [35]; S. typhimurium [36,37,38] | |
| Shigella sp. [39,40,41] | |
| Rotavirus [42,43,44,45] | |
| Astrovirus [46] | |
| Norovirus [44,47,48,49,50,51,52,53] | |
| Hepatitis A virus [44,54,55,56,57] | |
| Giardia intestinalis [7,58] | |
| Type B. Fomites or surface transmission | Entamoeba histolytica, coli [59,60,61] |
| Type C. Water or foodborne | Vibro vulnificus, V. parahemolyticus [33] |
| Campylobacter sp. [62] | |
| Entero-invasive, -aggresive, -pathogenic, and -toxigenic E. coli [63] | |
| Coxienella burnetii [64] | |
| Brucella sp. [65] | |
| Type D. Intoxication or atypical carrier | Clostridium perfringens [66] |
| Bacillus cereus [67] | |
| Staphylococcus aureus [68,69] |
| Part 1. Potential Reservoirs | ||
|---|---|---|
| 1.1 | Primary case | First symptomatic workers with visible symptoms |
| 1.2 | Further case | Additional case under similar symptoms as the primary case |
| 1.3 | Confirmed case | Cases in which an intestinal infection or causative agent was identified in a medical evaluation |
| 1.4 | Close contacts | In cases of sharing both time and space through co-work (collaboration) with cases, e.g., sampling, pretreatment procedures, using a shared laboratory room, facility, utensils at the same time |
| 1.5 | Indirect contacts | In cases involving sharing of space and property with a time difference (if joint facilities, equipment, and utensil are shared) |
| Part 2. Measures for Potential Reservoirs | ||
| 2.1 | Isolation | Exclusion from occupation of all cases or contact, according to a response procedure scenario |
| 2.2 | Close observation | All close or indirect contacts should be observed by manager with the onset of symptoms in mind, to slow down or block propagation |
| 2.3 | Health quarantine | Even if there are no symptoms, isolation is performed if there is a risk of infection after exposure to the primary or confirmed cases, to slow down or block propagation |
| 2.4 | Release | Return to occupation from isolation, close observation, health quarantine |
| 2.5 | Medical evaluation | Clinical estimation diagnosis or laboratory diagnosis by medical staff to estimate or determine the cause of symptoms. |
| Internal Factor | |||
|---|---|---|---|
| Strengths | Weakness | ||
| S1 | Proceduralization and standardization of work | W1 | Increased possibility of close contact |
| S2 | Acceptability of regulations and procedures to workers | W2 | Increased possibility of indirect contact |
| S3 | Acceptability of documentation to workers | ||
| S4 | Familiarity with documentation | ||
| External Factor | |||
| Opportunities | Threats | ||
| O1 | Traceability of infection through working procedures | T1 | Increasing infectious disease risk and likelihood |
| O2 | Accumulation of prior study cases via infectious disease epidemiology | T2 | Depend on group capabilities for infectious disease management |
| Decision Making Strategies | |||
| Active response | Step-by-step implementation | ||
| SO1 | Standardizing scenario | WO1 | Continual revision of scenarios |
| SO2 | Stipulation of scenario | ||
| SO3 | On-site application of stipulated scenario | ||
| Defensive response | Differentiation strategy | ||
| WT1 | Minimize close/indirect contact through scenario | ||
| WT2 | Ensuring continuity of industrial roles of institution through scenario | ||
| Part 1. General Requirement for Prevention of Infection and Transmission | |
|---|---|
| 1.1 | Monitoring and record management |
| (1) | Employees’ health status should always be monitored, and visible symptoms should be recorded and managed. |
| (2) | The issue of infectious diseases outside the organization is always monitored and considered. |
| 1.2 | Risk source management |
| (1) | Since the laboratory facilities have relatively high chance of indirect transmission, cross-contamination behavior and opportunities in workplaces should be avoided as much as possible. |
| (2) | Sufficient experimental utensils, tools, or equipment should be prepared as much as possible to control the possibility of fomite- or surface-mediated transmission. |
| 1.3 | Exposure traceability |
| (1) | To determine whether employees have direct or indirect contact with the primary case, procedures for reviewing an analytical testing manual, experiment or working diary, access record, etc. should be organized. |
| (2) | Infrastructure should be established to enable the implementation of the procedures proposed in this scenario. |
| Part 2. Primary Case Occurrence | |
| 2.1 | Health quarantine, close observation, isolation |
| (1) | In the case of primary case occurrence, it should be isolated in the workplace, assuming type of direct transmission (Type A), even before medical evaluation. |
| (2) | Close and indirect contacts should be closely observed until medical evaluation and (2.3) deduction. Since the discharge of agent takes place after the onset of symptoms, analyzing test activities is possible only if there are no similar symptoms. |
| 2.2 | Record management |
| (1) | If the primary case occurs, record symptoms and signs for further case occurrence situation. Propagation can be determined through record comparison even before the medical evaluation of primary case deduction. |
| (2) | Record management includes all symptoms of the body (fever, pain, vomit, food consumed, travel history) and backgrounds that can cause it. |
| 2.3 | Medical evaluation and treatment |
| (1) | The type of disease must be specified through medical evaluation and isolated from work until the results of the medical examination are derived. |
| (2) | Primary case should receive appropriate medical treatment. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.-M.; Cho, J.-H.; Jun, N.-S.; Bang, K.-I.; Hong, J.-W. Worker Protection Scenarios for General Analytical Testing Facility under Several Infection Propagation Risks: Scoping Review, Epidemiological Model and ISO 31000. Int. J. Environ. Res. Public Health 2022, 19, 12001. https://doi.org/10.3390/ijerph191912001
Park J-M, Cho J-H, Jun N-S, Bang K-I, Hong J-W. Worker Protection Scenarios for General Analytical Testing Facility under Several Infection Propagation Risks: Scoping Review, Epidemiological Model and ISO 31000. International Journal of Environmental Research and Public Health. 2022; 19(19):12001. https://doi.org/10.3390/ijerph191912001
Chicago/Turabian StylePark, Jong-Myong, Joong-Hee Cho, Nam-Soo Jun, Ki-In Bang, and Ji-Won Hong. 2022. "Worker Protection Scenarios for General Analytical Testing Facility under Several Infection Propagation Risks: Scoping Review, Epidemiological Model and ISO 31000" International Journal of Environmental Research and Public Health 19, no. 19: 12001. https://doi.org/10.3390/ijerph191912001
APA StylePark, J.-M., Cho, J.-H., Jun, N.-S., Bang, K.-I., & Hong, J.-W. (2022). Worker Protection Scenarios for General Analytical Testing Facility under Several Infection Propagation Risks: Scoping Review, Epidemiological Model and ISO 31000. International Journal of Environmental Research and Public Health, 19(19), 12001. https://doi.org/10.3390/ijerph191912001







