Abstract
In this study, a newly synthesized sepiolite-supported nanoscale zero-valent iron (S-nZVI) adsorbent was tested for the efficient removal of As(III) and As(V) in aqueous solution. Compared with ZVI nanoparticles, the As(III) and As(V) adsorption abilities of S-nZVI were substantially enhanced to 165.86 mg/g and 95.76 mg/g, respectively, owing to the good dispersion of nZVI on sepiolite. The results showed that the adsorption kinetics were well fitted with the pseudo-second-order model, and the adsorption isotherms were fitted with the Freundlich model, denoting a multilayer chemical adsorption process. The increase in the initial solution pH of the solution inhibited As(III) and As(V) adsorption, but a weaker influence on As(III) than As(V) adsorption was observed with increasing pH. Additionally, the presence of SO42− and NO3− ions had no pronounced effect on As(III) and As(V) removal, while PO43− and humic acid (HA) significantly restrained the As(III) and As(V) adsorption ability, and Mg2+/Ca2+ promoted the As(V) adsorption efficiency. Spectral analysis showed that As(III) and As(V) formed inner-sphere complexes on S-nZVI. As(III) oxidation and As(V) reduction occurred with the adsorption process on S-nZVI. Overall, the study demonstrated a potential adsorbent, S-nZVI, for the efficient removal of As(III) and As(V) from contaminated water.
1. Introduction
Arsenic (As) is a toxic metalloid that is widely distributed in the environment and is known to be a serious threat to food safety and human health [1,2,3,4]. Numerous health effects are associated with As exposure and As can cause severe health problems, such as liver, lung, kidney, and skin cancers [5,6,7,8]. Arsenic exposure pathways from the environment normally include consumption of contaminated food and water, inhalation of dust, and incidental ingestion of soil. In some well-documented regions (e.g., Bangladesh; West Bengal, India; and Shimen County, China), consumption of As-contaminated drinking water and crops represents the major exposure sources and pathways [9,10,11]. Hence, arsenic has been categorized as the first priority issue among toxic substances, and a maximum contamination level of 10 μg·L−1 for As in drinking water was established by the World Health Organization (WHO) [8]. Arsenic is most commonly found in two inorganic forms in the environment: arsenate (As(V)) and arsenite (As(III)). This normally depends on the environmental conditions, e.g., pH and redox potential (Eh). In groundwater with a pH range of 6–8.5, As(III) dominates as the H3AsO3 species under reducing conditions (low Eh values), while As(V) appears as a mixture of the H2AsO4− and HAsO42− species under oxidizing conditions (high Eh values) [12]. However, their toxicity and adsorption affinity are significantly different: As(III) is more mobile, soluble, and toxic than As(V) and is more difficult to remove by adsorbents than As(V) [13,14]. It is noteworthy that nanoparticle size adsorbent displayed a ~10% greater removal efficiency (RE) for As(III) than for As(V) under certain experimental conditions [15].
Arsenic removal by adsorbents largely depends on solution pH, increasing the difficulty of wastewater disposal considering the wide pH range of contaminated water. However, it is relatively difficult to remove arsenite (As(III)), which can remain in the form of H3AsO3 when the pH is lower than 9.2 [16,17]. Previous research indicated that uncharged H3AsO3 is more difficult to remove using traditional physical-chemical treatment methods [18]. Therefore, it is necessary to seek integrated materials that exhibit highly efficient As(III) oxidation and adsorption for the removal of As(III) from arsenic-polluted water, and additional oxidation is often required for conversion from As(III) to As(V).
There are many methods for removing heavy metals, such as coagulation, ion exchange, adsorption, membrane filtration, and electrochemistry; among them, adsorption is widely used because of its simple operation and economic and feasible characteristics [19,20,21]. Many studies have focused on iron oxides and their precursors, such as nano zero-valent iron (nZVI). Gil-Diaz et al. [22] found that when nZVI was employed for in situ remediation of arsenate in soil, both oxidation and adsorption of As(III) happened at the same time. The attractive properties of nanoadsorbents used for arsenic removal from water include a high surface area and high reactivity [23]. However, as an adsorbent for arsenic removal in wastewater, nZVI suffers from the drawback of easy aggregation. Therefore, supporting substances such as zeolite, biochar, and sepiolite are usually employed to improve the dispersion of nZVI nanoparticles [24,25,26]. Sepiolite (SEP) is an abundant natural clay mineral with a stable structure and low cost, and it also exhibits remarkable effectiveness in removing As from waste water [27]. Its polyporous structure is a good choice for dispersing the nZVI homogeneously in the aqueous solution, which can maximize the effectiveness of nZVI. Contaminants including lead, chromium, and metoprolol were removed using sepiolite-supported nZVI (S-nZVI) from aqueous solution in previous studies [28,29]. However, less attention has been devoted to the removal ability of As(III) and As(V) and the underlying mechanism of arsenic adsorption by S-nZVI.
The objective of the present study was to prepare different S-nZVI adsorbents with the highest removal capacity for As(III) and As(V). The factors influencing the arsenic adsorption efficiency were well explored and included pH, dosage, and coexisting ions. In addition, the adsorption kinetics and isotherms of As(III) and As(V) were investigated. Finally, the mechanisms of As(III)/As(V) removal were interpreted with the help of X-ray photoelectron spectroscopy (XPS) and X-ray diffraction (XRD) analysis. This study will contribute to the knowledge on the application of S-nZVI for the remediation of As(III) and As(V) in contaminated water.
2. Materials and Methods
2.1. Chemicals
All chemical reagents used in this study were of analytical grade. The sepiolite was obtained from Neixiang, Henan Province, China. The sepiolite was ground and passed through a 0.15 mm pore size sieve. Stock solutions of As(III) and As(V) were prepared by dissolving appropriate amounts of NaAsO3 and Na2HAsO4.12H2O in deionized water (Millipore Milli-Q 18 MX).
2.2. Synthesis of Materials
Acid-activated sepiolite (ASEP) was prepared according to Zhang et al. [30]: natural sepiolite (SEP) was immersed in HCl solution (2 mol/L) at 80 °C for 8 h under magnetic stirring [30]. The synthesis process of the S-nZVI composite was simplified and improved based on the method mentioned in a previous study [24]. Briefly, 8 g of ASEP and 200 mL of FeCl3·6H2O solution were mixed in a 1000 mL three-necked flask. The mass ratios of Fe:sepiolite were set to 1:3, 1:9, and 1:15. All operations were conducted under room temperature and pressure conditions. After 15 min of ultrasound treatment, the mixture was stirred for another three hours. Subsequently, 200 mL of 0.98 mol/L NaBH4 was added dropwise with stirring to perform the reduction reaction, and a black mixture of S-nZVI was formed. The reduction reaction is as follows (1):
4Fe3+ + 3BH4− + 9H2O → 4Fe0 + 3H2BO3− + 6H2 +12H+
Then, the S-nZVI was separated from the mixture by a magnetic field, washed with deionized water and absolute ethanol, freeze-dried, and stored under dry conditions. The nZVI particles were prepared as described above, except that absolute ethanol was added during stirring to control the sizes of the nanoparticles.
2.3. Characterization of Samples
The solid phases were characterized through XRD using a Rigaku Ultima IV (Japan) device with Cu Kα radiation (λ = 0.1541 nm) at a voltage and current of 40 kV and 40 mA, respectively. A scan range between 2° and 70° and a step size of 0.02° were used, with a change rate of 5 s/step. The infrared spectra of each sample were collected using a Nicolet 6700 FTIR spectrometer (Thermo Fisher, Waltham, MA, USA) within a range of 4000–400 cm−1. The specific surface area (SSA) and pore volume were estimated by Brunner–Emmet–Teller (BET) analysis (Belsorp-Mini II, Microtracbel Inc., Osaka, Japan). The valence states of the composites were determined by performing XPS with monochromatic Al Kα radiation (1486.8 eV) by using an Amicus XPS instrument (Shimazu Corp., Kyoto, Japan). The morphology and elemental composition of the selected samples were examined using scanning electron microscopy (SEM) (SU8100, HITACHI Corp., Tokyo, Japan).
2.4. Batch Sorption Experiments
2.4.1. Optimization of the Adsorbent Preparation Conditions
To obtain S-nZVI with the highest RE for arsenic, S-nZVI was prepared with different mass ratios of Fe:sepiolite as the starting material. Then, the arsenic RE of S-nZVI with different Fe:sepiolite ratios was evaluated by adding 30 mg adsorbent to 30 mL arsenic solutions with 0.01 mol/L NaCl (background electrolyte) in a 50 mL centrifuge tube. The mixture was shaken at 180 rpm at 25 °C for 24 h. The initial pH of the solution was adjusted to 7.0 using diluted HCl and NaOH (0.1 mol/L). The adsorption capacity and RE at equilibrium were calculated according to Equations (2) and (3):
where qe is the equilibrium adsorption capacity (mg/g), V is the volume of solution (mL), Ce and C0 represent the concentrations at equilibrium and the initial time (mg/L), respectively, and m is the weight of the adsorbent (g).
The suspensions were filtered through 0.22 μm filters before being tested. The As concentration was determined by atomic fluorescence spectrometry (AFS-8220, JiTian instrument Co., Ltd, Beijing, China).
2.4.2. Adsorption Kinetics
The adsorption kinetics of As(III) and As (V) by S-nZVI (sepiolite: Fe at a ratio of 3:1) were evaluated by batch adsorption experiments. In general, 1 g/L S-nZVI was dosed into flasks containing 50 mg/L As(III) and As(V) solutions at an initial pH of 7.0. At predetermined time intervals until 24 h, the aliquots of the solution were collected and centrifuged. The mixture was shaken in a thermostatic shaker at 180 rpm and 25 °C. The As(III) and As(V) concentrations in the solutions were tested with the AFS system as mentioned in Section 2.4.1. The pseudo-first-order and pseudo-second-order models can be expressed as Equations (4) and (5):
where qt and qe (mg/g) are the adsorption quantity at adsorption time (t) and equilibrium, respectively. k1 and k2 represent the adsorption rate constants.
2.4.3. Adsorption Isotherm
The adsorption isotherms of S-nZVI were tested by dosing 30 mg adsorbent into flasks containing 30 mL As(III) and As(V) at different concentrations (varying from 5 to 600 mg/L). The mixture of adsorbent and arsenic solution was shaken in a thermostatic shaker at 180 rpm and 25 °C with an initial pH of 7.0 for 14 h. The adsorption isotherms were fitted by the Langmuir (assuming a monolayer adsorption process) and Freundlich models (assuming that multilayer adsorption occurred) [31]. The Langmuir and Freundlich models can be expressed by Equations (6) and (7), respectively:
where Ce and qe are the equilibrium concentration (mg/L) and adsorption amount (mg/g) of heavy metal, respectively; qm (mg/g) is the maximum adsorption capacity; KL (L/mg) is the Langmuir constant; and Kf and 1/n are the Freundlich constants.
To evaluate the feasibility of the adsorption process, RL was calculated according to Equation (8) [32]:
The adsorption isotherms were also fitted with the Dubinin–Astakhov (D-A) model and Dubinin–Radushkevich (D-R) model. The Dubinin–Astakhov (D-A) model was expressed as Equations (9) and (10) [33,34]
where qmD-A (mg/g) and qmD-R (mg/g) are the maximum adsorption amount, ε (kJ/mol) is the adsorption potential, Cs (mg/L) is the solubility of arsenate and arsenite, which was 2.46 × 105 mg/L and 9.01 × 105 mg/L for As(V) and As(III) at 25 °C, R (kJ/K/mol) is the gas constant equal to 8.314 × 10−3, Ce (mg/L) is the equilibrium concentration, qe (mg/g) is the adsorption amount, T = 273.15 K at the experimental condition, EDA (kJ/mol) is the characteristic energy, nDA is a constant related to the percent of pore filling, and KDR (mol2/kJ2) is the model constant of the D-R model [35].
2.4.4. Effect of Initial pH, Adsorbent Dosage and Coexisting Ions
The influence of the initial pH, adsorbent dosage, and coexisting ions was assessed by examining the equilibrium adsorption amounts of As(III) and As(V) on S-nZVI. For the study of pH influence, the initial pH of the sorption solution was adjusted from 3.0 to 9.0 by adding HCl (0.1 mol/L) or NaOH (0.1 mol/L). The S-nZVI adsorbent was dosed into an arsenic solution of 50 mg/L at a dosage ratio of 1 g/L. For the study of the adsorbent dosage effect, the adsorbent was dosed into arsenic solution at concentrations ranging from 0.2 to 8 g/L. To study the influence of coexisting ions, the S-nZVI adsorbent (1 g/L) was dosed into a mixed solution with 50 mg/L arsenic and each coexisting ion (0.01 mol/L Ca2+, Mg2+, NO3−, SO42− or PO43−) or 200 mg/L humic acid (HA).
3. Results
3.1. Adsorption Kinetics of As(III)/As(V) by S-nZVI
The composites were prepared under a graded series of theoretical mass ratios of sepiolite to Fe (3:1, 9:1, and 15:1), and RE was examined based on the adsorption kinetics at pH = 7.0 (Figure 1). Figure 1a shows the As(III) and As(V) adsorption amounts of S-nZVI when the Fe:sepiolite ratio varied from 1:3 to 1:9 and 1:15. When the mass ratio of sepiolite:Fe increased from 3:1 to 15:1, the RE of As(III) decreased from 97.89% to 41.87%, while that of As(V) decreased from 79.33% to 22.00%. The decrease in arsenic removal capacity with the increase in the relative sepiolite content in S-nZVI illustrated that nZVI was the key functional component in removing arsenic. Therefore, in the following experiments, S-nZVI with a sepiolite:Fe ratio of 3:1 was adopted as the adsorbent.
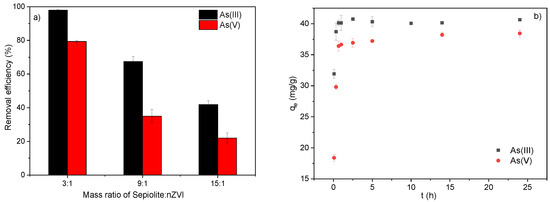
Figure 1.
(a) Comparison of the As(III)/As(V) adsorption capacity of S-nZVI prepared with different sepiolite:nZVI mass ratios; (b) adsorption kinetic curves of As(III) and As(V) by S-nZVI.
Figure 1b shows the As(III) and As(V) adsorption kinetics by S-nZVI. The adsorption process of both As(III) and As(V) exhibited a tendency of rapid adsorption in the first 2 h and then gradually reached equilibrium by 24 h. The equilibrium adsorption amounts were 38.44 ± 0.59 mg/g and 40.66 ± 0.21 mg/g for As(V) and As(III), respectively, consistent with the result (Figure 1a) that As(III) was more advantageous than As(V) for removal by the S-nZVI adsorbent.
The pseudo-first-order and pseudo-second-order kinetic parameters for adsorption are given in Table 1. For both adsorbates, the equilibrium adsorption quantity calculated from the pseudo-first-order and pseudo-second-order models was very close to that from the experimental data. The adsorption process of both As(III) and As(V) better suited the pseudo-second-order model (R2 > 0.99). Better fitting with the pseudo-second-order model indicated that the adsorption of As(III) and As(V) was a chemical adsorption process [36]. In addition, the adsorption rates (k2) of As(III) and As(V) were 1.12 g·mg−1·h−1 and 0.28 g·mg−1·h−1, respectively, indicating that the removal process of As(III) by S-nZVI was more rapid than that of As(V).

Table 1.
Fitted parameters of the pseudo-first-order and pseudo-second-order models for As(III) and As(V) adsorption by S-nZVI.
3.2. Isotherm Adsorption Study of As(III)/As(V) by S-nZVI
The parameters of the As(III) and As(V) adsorption isotherms fitted with the Langmuir and Freundlich models are shown in Figure S1 and Table 2. By comparing the R2 values of both models, it was found that the Freundlich model can better describe the absorption process of S-nZVI for As(V) (R2 = 0.974) while the Langmuir model can better describe the As(III) adsorption by S-nZVI (R2 = 0.967). The better fitting results of the Freundlich model suggest that ion sorption on S-nZVI is a multilayer adsorption process. The maximum adsorption capacities of S-nZVI towards As(III) and As(V) was 165.86 mg/g and 95.76 mg/g, respectively, which were much higher than those of other nZVI-based materials reported in previous studies (Table S1). For example, the As(III) and As(V) adsorption capacity by Mn-nZVI was reported to be 59.90 mg/g and 45.50 mg/g, respectively. This excellent removal ability of As(III) and As(V) indicated that S-nZVI is an effective sorbent for arsenic treatment of contaminated water. Table 2 shows that 1/n was 0.38 and 0.41 for As(III) and As(V), respectively, and RL ranged between 0 and 1, indicating that As(III) and As(V) sorption on S-nZVI is a favorable process [31]. The difference between the adsorption capacities of As(III) and As(V) was most likely associated with the chemical behavior of As(III) and As(V) anions, which might be controlled by diverse adsorption mechanisms [37]. The adsorption isotherms were also fitted with the Dubinin–Astakhov (D-A) model and Dubinin–Radushkevich (D-R) model (Figure S2 and Table S2). The D-A and D-R models can be applied to describe the adsorption process on adsorbents with pores following Gaussian energy distribution [34]. From the fitting results (Table S2), it can be seen that As(III)/As(V) adsorption isotherms can be interpreted by both models, indicating that adsorption of As(III)/As(V) on S-nZVI happened in homogeneous microporous systems [38].

Table 2.
Isothermal parameters of As(III) and As(V) adsorption on nZVI and S-nZVI.
3.3. Influence of Initial pH on As(III)/As(V) Adsorption
The speciation of heavy metal ion species at different pH values can influence the reaction as well. To elucidate the effect of pH on the sorption process, adsorption edge experiments were conducted in the pH range from 3.0 to 9.0 for both As(III) and As(V). Figure 2a,b shows that the As(III) and As(V) sorption capacities of SEP were relatively stable with pH variation but were significantly lower than those of nZVI and S-nZVI, ranging between 5 and 7 mg/g. The adsorption capacities of As(V) and As(III) decreased with increasing initial pH for both nZVI and S-nZVI, which is in accordance with previous studies [39,40]. However, the As(III) adsorption capacity of S-nZVI decreased slowly from 48.71 to 44.74 mg/g when the pH changed from 3 to 9, while the As(V) sorption capacity decreased from 47.72 to 33.33 mg/g. From the chemical species calculation results (Figure 2c,d), it can be seen that As(V) existed in anion form (H2AsO4− or HAsO42−) between pH 3 and 9, while As(III) was mostly an uncharged ion (H3AsO3) with a small part as a charged anion (H2AsO3−) when the pH was higher than 8. The pH increase led to the deprotonation of the S-nZVI adsorbent, which may lower the electrostatic attraction between the adsorbent and anionic pollutants (H2AsO4−, HAsO42−, H2AsO3−). This explained why pH variation had a larger influence on the sorption of As(V) than that of As(III). The dashed lines in Figure 2a,b demonstrate the pH variation before and after As(III) or As(V) adsorption, which reflects the pH buffering capacity of each adsorbent. The deviation of the dashed lines from the line with a slope of 1 and an intercept of 0 (orange dot line) show how much the pH of aqueous solution can be retained after adsorption. Figure 2a,b shows that nZVI and S-nZVI had a higher pH buffering capacity compared with sepiolite adsorbent, possibly due to the nZVI modification.
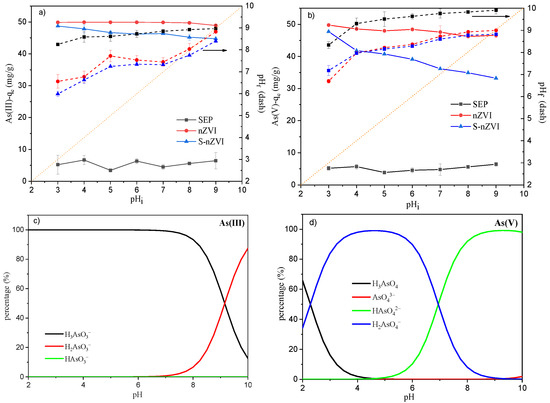
Figure 2.
Influence of initial pH on (a) As(III) and (b) As(V) adsorption capacity (pHi and pHf indicate initial pH before S-nZVI dosing and final pH after adsorption, respectively); the distribution of different (c) As(III) and (d) As(V) species in aqueous solution calculated with Visual MINTEQ.
3.4. Influence of Adsorbent Dosage
With the objective of decreasing the arsenic concentration as much as possible in an economical way, it is necessary to optimize the sorbent dosage in practical applications. The effect of the adsorbent dosage was studied for As(III) and As(V) adsorption with the concentration of S-nZVI varying from 0.2 to 8.0 g/L (Figure S3). The results showed that RE increased from 45.14% to 99.76% for As(III) and from 35.39% to 97.51% for As(V) when the S-nZVI dosage increased from 0.2 to 8.0 g/L. When the S-nZVI dose was 1 g/L, the As(III) and As(V) RE were above 80%, while the heavy metal RE almost reached 100% when the dosage was 4 g/L. Comparing As(III) and As(V) adsorption, it can be found that the As(III) RE was higher than that of As(V) at each S-nZVI dosage, reflecting the excellent affinity between the adsorption sites of S-nZVI and As(III). The reason for this phenomenon was that the increased material content led to an increased surface area and number of adsorption sites.
3.5. Influence of Coexisting Ions/Substances on As(III)/As(V) Adsorption
Normally, there are various coexisting anions in wastewater, and thus, determining the influence of these ions on arsenic RE is vital for adsorbent development. Figure 3 shows the influence of coexisting ions (0.01 mol/L Ca2+, Mg2+, NO3−, SO42−, and PO43− and 200 mg/L HA) on the adsorption of As(III) and As(V) by S-nZVI. From Figure 3, it is clear that the ions have different effects on sorption capacity. It can be concluded that the presence of SO42− and NO3− had little influence on the adsorption of either As(V) or As(III). The interaction between sulfate/nitrate and nZVI was reported to be due to the formation of outer-sphere complexes, which are not as tightly bound as inner-sphere complexes [41,42]. Therefore, it is difficult for SO42− and NO3− to compete for binding sites with arsenic due to their weaker affinity with S-nZVI. On the other hand, the presence of PO43− and HA significantly inhibited the adsorption of As(III) and As(V). The great loss of As(III)/As(V) adsorption capacity by competition with PO43−/HA could be due to the similarity of their molecular structures [43,44]. Coexisting HA introduced not only A− but also protons, thus increasing the positive charges on the adsorbent. The decreased arsenic RE due to HA competition illustrated that the increased electrostatic attraction due to introduced protons could not overcome the competition effect of A−. Ca2+ and Mg2+ considerably enhanced the RE of As(V) from 76.88% to 98.36% and 97.73%, while no such promoting effect was found for As(III) adsorption. It is assumed that Ca2+/Mg2+ can increase the positive charges on the S-nZVI adsorbent, which is beneficial for anion adsorption through the bridging effect. As presented in Figure 2c,d, at the experimental pH value of 7, As(III) was electrically neutral, while As(V) was in anion form. Therefore, the coexistence of Ca2+/Mg2+ and the change in surface charge showed a greater influence on As(V) adsorption than on As(III) adsorption. This observation was consistent with some previous studies [45,46].
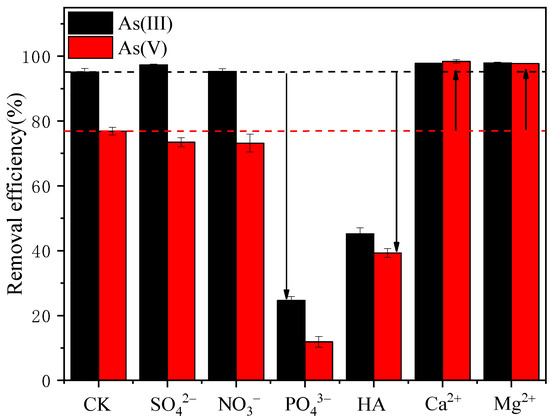
Figure 3.
Influence of coexisting ions (SO42−, PO42−, Ca2+, Mg2+, and NO3−) and HA on As(III) and As(V) adsorption (experimental conditions: pH = 7). ‘CK’ stands for ‘control check’, which means no competing ions existed in the aqueous solution.
3.6. S-nZVI Characterization and Sorption Mechanisms
It was demonstrated that spherical nZVI was well dispersed on the sepiolite surface without agglomeration (Figure S4). The specific surface areas of SEP, ASEP, and S-nZVI were 23.21, 110.00, and 59.01 m2/g, respectively. The above results confirmed that nZVI was well loaded on the surface of SEP and that sepiolite could inhibit nZVI agglomeration.
The broad XPS scan indicated that S-nZVI was primarily composed of Fe, O, and Si. Small amounts of C, Ca, and Mg were also detected, which might be contributed by SEP (Figure 4a). The curve fitting of the As3d peaks involved four subpeaks because the As3d peak for As(III) and As(V) has two spin-orbit splittings, denoted as As3d3/2 and As3d5/2, with a constant binding energy gap of 0.7 eV. By comparing the areas of the subpeaks corresponding to As(III) and As(V), the relative abundance of each valence state can be calculated. From Figure 4b, it can be seen that after As(V) was adsorbed on S-nZVI, 53.55% of the total arsenic was detected as As(III), indicating that some As(V) was reduced to As(III). Figure 4c demonstrates that after As(III) was adsorbed on S-nZVI, 39.64% of the total arsenic was oxidized to As(V), while 60.36% of arsenic remained as As(III). The results suggest that As(V) can be reduced by S-nZVI and that As(III) oxidation can happen by reacting with S-nZVI. In other words, the S-nZVI adsorbent served as both a reductant and an oxidant for arsenic [40,47]. The same results were reported in a previous study, in which the arsenic reduction and oxidation mechanism was interpreted to be due to the Fe oxide shell outside the Fe0 core of nZVI particles [48]. Electrons from Fe0 can be transferred via the oxide layer and lead to the reduction of As(V). On the other hand, the iron oxides on the outer layer account for As(III) oxidation. It was also explained in previous studies that the corrosion of Fe0 in solution generated intermediate compounds known as reactive oxygen species (ROS), leading to the oxidation of As(III) [49,50]. This phenomenon is consistent with the Fe2p spectrum. Figure 4d–f represents the valence state change of Fe on S-nZVI before and after As(V) or As(III) adsorption. Before adsorption, iron on the adsorbent surface was largely oxidized to Fe2+ and Fe3+, with only 10.82% of the total iron remaining as Fe0. After As(V) or As(III) adsorption, the ratio of Fe0 was further decreased to 3.40% and 4.12%, respectively. Furthermore, the binding energy of Fe0, Fe2+, and Fe3+ was shifted to higher positions after As(III) and As(V) adsorption, suggesting that As(III) and As(V) were adsorbed onto Fe composites and formed inner-sphere complexes [51,52,53]. The Fe0 loss of nZVI confirmed the hypothesis that Fe0 was oxidized and that an Fe oxide shell was formed on the adsorbent surface during arsenic adsorption. After As(V) was adsorbed on S-nZVI, the ratios of Fe0 and Fe2+ were determined to be 3.40% and 43.80%, respectively, which are lower than the values of 4.12% and 46.53% for As(III) adsorption. The higher degree of iron oxidation caused by As(V) adsorption indicated that As(V) reduction happened at the same time as adsorption, in agreement with the As3d spectrum.
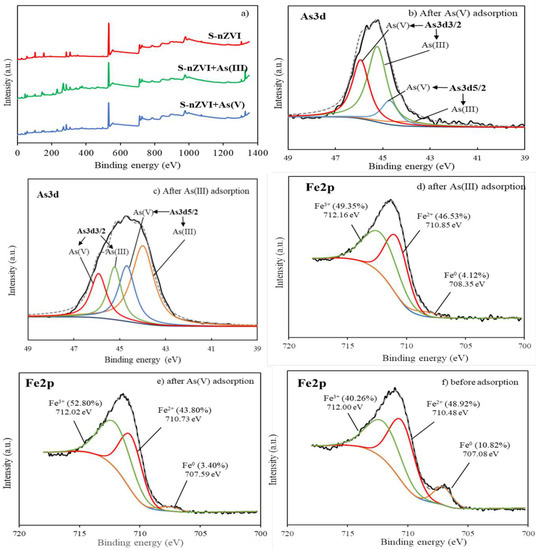
Figure 4.
XPS wide-scan spectra (a), As3d spectra after As(V) adsorption (b) and As(III) adsorption (c); and Fe2p spectra after As(III) adsorption (d), after As(V) adsorption (e), and before adsorption (f).
The spectra of O1s consisted of peaks at ~530 eV, ~531 eV, and ~532.5 eV, which represent the binding energies of O2−(M-O), OH-, and physically or chemically adsorbed water (H2O), respectively [30]. For the adsorption of As(III) and As(V), the ratio of the peak area for O2− increased from 19.32% to 40.51% and 30.54%, respectively. The variation in O2− may be due to the adsorption of arsenic and the formation of As-O bonds on S-nZVI. The higher peak area ratio of O2− for As(III) also proved that the As(III) adsorption capacity was higher than that of As(V). Furthermore, the percentage of -OH groups on S-nZVI increased from 22.64% to 42.09% and 44.89% after As(III) and As(V) adsorption, respectively. Thus, it is reasonable to conclude that the increase in the intensity of the -OH peak was due to surface adsorption reactions on the surface of S-nZVI [1].
Figure S5 shows the XRD patterns of S-nZVI before and after adsorption of As(III) and As(V). After arsenic adsorption, the peak for Fe0 at 44.68° was largely eliminated, indicating the oxidation of Fe0 on S-nZVI during the adsorption process. The presence of the characteristic Fe2O3 peak (35.5°) on S-nZVI before and after arsenic adsorption corresponded to the oxidation of Fe0. From the patterns after As(V) adsorption, FeAsO4 (59.05°) precipitation was shown to be formed on S-nZVI [31]. In addition to FeAsO4 precipitation, Fe2As4O12 (21.05°) was observed to be formed on S-nZVI after As(III) adsorption, proving that As(III) oxidation simultaneously happened with adsorption. In accordance with the XPS Fe2p spectrum, the formation of Fe2As4O12 indicates the existence of Fe(II) during As(III) adsorption and oxidation. The spectral analysis provided evidence that the mechanism of As(III) and As(V) removal by S-nZVI involves surface precipitation and inner-sphere complexation. Furthermore, the adsorption of As(III) and As(V) was accompanied by the oxidation/reduction process of iron and arsenic [1,52,54,55].
4. Conclusions
In this study, a sepiolite-supported nanoscale zero-valence iron adsorbent (S-nZVI) was successfully prepared and utilized in As(III) and As(V) adsorption from aqueous solution. An adsorption isothermal study was carried out. Removal of both As(III) and As(V) followed pseudo-second-order kinetics with a higher rate constant for As(III) than As(V), revealing a faster adsorption process for As(III). As(III) and As(V) adsorption isotherms were well fitted by the Freundlich isotherm model, and the maximum adsorption capacity of S-nZVI for As(III) and As(V) was 165.86 mg/g and 95.76 mg/g, respectively. In addition, increases in solution pH substantially inhibited the adsorption capacity of As(III) and As(V) by S-nZVI, with a stronger influence on As(V) being observed. Coexisting PO43− and humic acid (HA) competed strongly with the adsorption of As(III) and As(V) on S-nZVI, while the influence of SO42− and NO3− was negligible. The presence of Ca2+ and Mg2+ in solution enhanced only the As(V) adsorption ability, with little effect on As(III) adsorption. XPS As3d, Fe2p, and O1s analysis confirmed that the mechanisms of As(III) and As(V) adsorption include precipitation and surface complexation, which means that Fe-O bonds were formed between As(III)/As(V) and S-nZVI. Furthermore, the oxidation and reduction of arsenic happened simultaneously with the adsorption process. The present study provides an alternative adsorbent for the highly efficient removal of arsenic from wastewater, which could reduce the human exposure risk to arsenic.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijerph191811401/s1, Figure S1: Adsorption isotherms of (a) As(III) and (b) As(V) on nZVI and S-nZVI; Figure S2: The Dubinin–Astakhov model (a–d) and Dubinin–Radushkevich model (e–f) fitting results of As(III)/As(V) adsorption isotherms on nZVI and S-nZVI.; Figure S3: Effect of S-nZVI dosage on the As(III) and As(V) removal; Figure S4: SEM images of S-nZVI; Figure S5: XRD patters of S-nZVI before and after adsorption of As(III) and As(V); Table S1: Comparison of the maximum As(III)/As(V) adsorption capacity by adsorbents reported in literatures; Table S2: Fitting results of As(III) and As(V) adsorption on nZVI and S-nZVI by Dubinin–Astakhov model (a–d) and Dubinin–Radushkevich model.
Author Contributions
M.A.: investigation, writing; T.Z. and X.Z.: conceptualization, funding acquisition; X.Y.: funding acquisition; J.W.: investigation; S.S., Y.W. and Y.Z.: software and data analysis; N.Z. and T.Z.: supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (NSFC) (Project No. U19A2048), the Natural Science Foundation of Sichuan Province (NSFSC) (Project No. 2022NSFSC1059), the Agricultural Science and Technology Innovation Program (ASTIP) and the Central Public-interest Scientific Institution Basal Research Fund (Project No. BSRF202213).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Zhongkebaice Technology Co., Ltd. for helping us with material characterization.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, B.; Zhu, Q.H.; Zhang, Q.; Zhu, H.H.; Huang, D.Y.; Su, S.M.; Wang, Y.N.; Zeng, X.B. Cadmium and arsenic availability in soil under submerged incubation: The influence of humic substances on iron speciation. Ecotoxicol. Environ. Saf. 2021, 225, 112773. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-M.; Tang, D.-D.; Yuan, X.-Y.; Uchimiya, M.; Li, J.-Z.; Li, Z.-Y.; Luo, Z.-C.; Xu, Z.-W.; Sun, S.-G. Effect of amendments on soil Cd sorption and trophic transfer of Cd and mineral nutrition along the food chain. Ecotoxicol. Environ. Saf. 2020, 189, 110045. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.; Li, B.; Li, J.; Chen, W.; Xu, L. Heavy metals in paddy soil-rice systems of industrial and township areas from subtropical China: Levels, transfer and health risks. J. Geochem. Explor. 2018, 194, 210–217. [Google Scholar] [CrossRef]
- Jing, M.; Chen, W.; Zheng, T.; Liao, Y.; Ellis Burnet, J.; Xu, M.; Yang, C.; Shen, L.; Liang, M. Water geochemical characteristic variations in and around a karst-dominated natural reserve area, southwestern China. Environ. Earth Sci. 2011, 64, 1051–1058. [Google Scholar] [CrossRef]
- Mäki-Paakkanen, J.; Kurttio, P.; Paldy, A.; Pekkanen, J. Association between the clastogenic effect in peripheral lymphocytes and human exposure to arsenic through drinking water. Environ. Mol. Mutagen. 1998, 32, 301–313. [Google Scholar] [CrossRef]
- Rahman, M.A.; Hasegawa, H.; Rahman, M.M.; Miah, M.M.; Tasmin, A. Arsenic accumulation in rice (Oryza sativa L.): Human exposure through food chain. Ecotoxicol. Environ. Saf. 2008, 69, 317–324. [Google Scholar] [CrossRef]
- Cubadda, F.; Jackson, B.P.; Cottingham, K.L.; Van Horne, Y.O.; Kurzius-Spencer, M. Human exposure to dietary inorganic arsenic and other arsenic species: State of knowledge, gaps and uncertainties. Sci. Total Environ. 2017, 579, 1228–1239. [Google Scholar] [CrossRef]
- Abdul, K.S.M.; Jayasinghe, S.S.; Chandana, E.P.; Jayasumana, C.; De Silva, P.M.C. Arsenic and human health effects: A review. Environ. Toxicol. Pharmacol. 2015, 40, 828–846. [Google Scholar] [CrossRef]
- Rahman, M.M.; Dong, Z.; Naidu, R. Concentrations of arsenic and other elements in groundwater of Bangladesh and West Bengal, India: Potential cancer risk. Chemosphere 2015, 139, 54–64. [Google Scholar] [CrossRef]
- Tang, J.; Liao, Y.; Yang, Z.; Chai, L.; Yang, W. Characterization of arsenic serious-contaminated soils from Shimen realgar mine area, the Asian largest realgar deposit in China. J. Soils Sediments 2016, 16, 1519–1528. [Google Scholar] [CrossRef]
- Adlassnig, W.; Schmidt, B.; Jirsa, F.; Gradwohl, A.; Ivesic, C.; Koller-Peroutka, M. The Arsenic–Antimony Creek at Sauerbrunn/Burgenland, Austria: A Toxic Habitat for Amphibians. Int. J. Environ. Res. Public Health 2022, 19, 6010. [Google Scholar] [CrossRef] [PubMed]
- Cuong, D.V.; Wu, P.C.; Chen, L.I.; Hou, C.H. Active MnO2/biochar composite for efficient As (III) removal: Insight into the mechanisms of redox transformation and adsorption. Water Res. 2021, 188, 116495. [Google Scholar] [CrossRef] [PubMed]
- Jain, C.K.; Ali, I. Arsenic: Occurrence, toxicity and speciation techniques. Water Res. 2000, 34, 4304–4312. [Google Scholar] [CrossRef]
- Petrick, J.S.; Ayala-Fierro, F.; Cullen, W.R.; Carter, D.E.; Vasken Aposhian, H. Monomethylarsonous acid (MMA(III)) is more toxic than arsenite in Chang human hepatocytes. Toxicol. Appl. Pharmacol. 2000, 163, 203–207. [Google Scholar] [CrossRef] [PubMed]
- An, B.; Zhao, D. Immobilization of As(III) in soil and groundwater using a new class of polysaccharide stabilized Fe-Mn oxide nanoparticles. J. Hazard. Mater. 2012, 211–212, 332–341. [Google Scholar] [CrossRef]
- Ding, Z.; Fu, F.; Cheng, Z.; Lu, J.; Tang, B. Novel mesoporous Fe[sbnd]Al bimetal oxides for As(III) removal: Performance and mechanism. Chemosphere 2017, 169, 297–307. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U. Arsenic removal from water/wastewater using adsorbents—A critical review. J. Hazard. Mater. 2007, 142, 1–53. [Google Scholar] [CrossRef]
- Fu, F.; Dionysiou, D.D.; Liu, H. The use of zero-valent iron for groundwater remediation and wastewater treatment: A review. J. Hazard. Mater. 2014, 267, 194–205. [Google Scholar] [CrossRef]
- Shrestha, R.; Ban, S.; Devkota, S.; Sharma, S.; Joshi, R.; Tiwari, A.P.; Kim, H.Y.; Joshi, M.K. Technological trends in heavy metals removal from industrial wastewater: A review. J. Environ. Chem. Eng. 2021, 9, 105688. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhou, S.; Zhou, X.; Mo, S.; Zhu, Y.; Zhang, L.; Tang, S.; Fang, Z.; Fan, Y. Effective Remediation of Arsenic-Contaminated Soils by EK-PRB of Fe/Mn/C-LDH: Performance, Characteristics, and Mechanism. Int. J. Environ. Res. Public Health 2022, 19, 4389. [Google Scholar] [CrossRef]
- Ali, A.; Siddique, M.; Chen, W.; Han, Z.; Khan, R.; Bilal, M.; Waheed, U.; Shahzadi, I. Promising Low-Cost Adsorbent from Waste Green Tea Leaves for Phenol Removal in Aqueous Solution. Int. J. Environ. Res. Public Health 2022, 19, 6396. [Google Scholar] [CrossRef]
- Gil-Díaz, M.; Diez-Pascual, S.; González, A.; Alonso, J.; Rodríguez-Valdés, E.; Gallego, J.R.; Lobo, M.C. A nanoremediation strategy for the recovery of an As-polluted soil. Chemosphere 2016, 149, 137–145. [Google Scholar] [CrossRef]
- Wadhawan, S.; Jain, A.; Nayyar, J.; Mehta, S.K. Role of nanomaterials as adsorbents in heavy metal ion removal from waste water: A review. J. Water Process Eng. 2020, 33, 101038. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Meng, J.; Liu, X.; Xu, J.; Wang, F.; Brookes, P. Zeolite-supported nanoscale zero-valent iron: New findings on simultaneous adsorption of Cd(II), Pb(II), and As(III) in aqueous solution and soil. J. Hazard. Mater. 2018, 344, 1–11. [Google Scholar] [CrossRef]
- Zhu, H.; Jia, Y.; Wu, X.; Wang, H. Removal of arsenic from water by supported nano zero-valent iron on activated carbon. J. Hazard. Mater. 2009, 172, 1591–1596. [Google Scholar] [CrossRef]
- Du, Y.; Zhen, S.; Wang, J.; Ma, Y.; Wu, J.; Dai, H. FeOOH-MnO2/Sepiolite and Fe2O3-MnO2/Diatomite: Highly efficient adsorbents for the removal of as (V). Appl. Clay Sci. 2022, 222, 106491. [Google Scholar] [CrossRef]
- Zhou, F.; Ye, G.; Gao, Y.; Wang, H.; Zhou, S.; Liu, Y.; Yan, C. Cadmium adsorption by thermal-activated sepiolite: Application to in-situ remediation of artificially contaminated soil. J. Hazard. Mater. 2022, 423, 127104. [Google Scholar] [CrossRef]
- Daneshkhah, M.; Hossaini, H.; Malakootian, M. Removal of metoprolol from water by sepiolite-supported nanoscale zero-valent iron. J. Environ. Chem. Eng. 2017, 5, 3490–3499. [Google Scholar] [CrossRef]
- Fu, R.; Yang, Y.; Xu, Z.; Zhang, X.; Guo, X.; Bi, D. The removal of chromium (VI) and lead (II) from groundwater using sepiolite-supported nanoscale zero-valent iron (S-NZVI). Chemosphere 2015, 138, 726–734. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, Z.; Ouyang, J.; Yang, H.; Chen, D. Highly dispersed sepiolite-based organic modified nanofibers for enhanced adsorption of Congo red. Appl. Clay Sci. 2018, 157, 76–85. [Google Scholar] [CrossRef]
- Yang, D.; Wang, L.; Li, Z.; Tang, X.; He, M.; Yang, S.; Liu, X.; Xu, J. Simultaneous adsorption of Cd(II) and As(III) by a novel biochar-supported nanoscale zero-valent iron in aqueous systems. Sci. Total Environ. 2020, 708, 134823. [Google Scholar] [CrossRef] [PubMed]
- Malana, M.A.; Qureshi, R.B.; Ashiq, M.N. Adsorption studies of arsenic on nano aluminium doped manganese copper ferrite polymer (MA, VA, AA) composite: Kinetics and mechanism. Chem. Eng. J. 2011, 172, 721–727. [Google Scholar] [CrossRef]
- Dubinin, M.M.; Astakhov, V.A. Development of the concepts of volume filling of micropores in the adsorption of gases and vapors by microporous adsorbents Communication 2. General bases of the theory of adsorption of gases and vapors on zeolites. Bull. Acad. Sci. Ussr Div. Chem. Sci. 1971, 20, 8–12. [Google Scholar] [CrossRef]
- Dubinin, M.M.; Radushkevich, L.V. The equation of the characteristic curve of the activated charcoal. Proc. Acad. Sci. USSR Phys. Chem. Sect. 1947, 55, 331–337. [Google Scholar]
- Wang, J.; Guo, X. Adsorption isotherm models: Classification, physical meaning, application and solving method. Chemosphere 2020, 258, 127279. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef] [PubMed]
- Kanematsu, M.; Young, T.M.; Fukushi, K.; Green, P.G.; Darby, J.L. Arsenic(III, V) adsorption on a goethite-based adsorbent in the presence of major co-existing ions: Modeling competitive adsorption consistent with spectroscopic and molecular evidence. Geochim. Cosmochim. Acta 2013, 106, 404–428. [Google Scholar] [CrossRef]
- Chen, S.G.; Yang, R.T. Theoretical Basis for the Potential Theory Adsorption Isotherms. The Dubinin-Radushkevich and Dubinin-Astakhov Equations. Langmuir 1994, 10, 4244–4249. [Google Scholar] [CrossRef]
- Kanel, S.R.; Greneche, J.M.; Choi, H. Arsenic(V) removal from groundwater using nano scale zero-valent iron as a colloidal reactive barrier material. Environ. Sci. Technol. 2006, 40, 2045–2050. [Google Scholar] [CrossRef]
- Kanel, S.R.; Manning, B.; Charlet, L.; Choi, H. Removal of arsenic(III) from groundwater by nanoscale zero-valent iron. Environ. Sci. Technol. 2005, 39, 1291–1298. [Google Scholar] [CrossRef]
- Stachowicz, M.; Hiemstra, T.; van Riemsdijk, W.H. Multi-competitive interaction of As(III) and As(V) oxyanions with Ca2+, Mg2+, PO43−, and CO32− ions on goethite. J. Colloid Interface Sci. 2008, 320, 400–414. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Wei, S.; Liu, C.; Chen, T.; Tang, Y.; Ma, J.; Yin, K.; Luo, S. Efficient removal of arsenic from groundwater using iron oxide nanoneedle array-decorated biochar fibers with high Fe utilization and fast adsorption kinetics. Water Res. 2019, 167, 115107. [Google Scholar] [CrossRef] [PubMed]
- Arai, Y.; Sparks, D.L. ATR-FTIR spectroscopic investigation on phosphate adsorption mechanisms at the ferrihydrite-water interface. J. Colloid Interface Sci. 2001, 241, 317–326. [Google Scholar] [CrossRef]
- Khare, N.; Hesterberg, D.; Martin, J.D. XANES investigation of phosphate sorption in single and binary systems of iron and aluminum oxide minerals. Environ. Sci. Technol. 2005, 39, 2152–2160. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, B.; Liu, H.; Qu, J. Simultaneous removal of arsenite and fluoride via an integrated electro-oxidation and electrocoagulation process. Chemosphere 2011, 83, 726–729. [Google Scholar] [CrossRef]
- Jones, R.G.; Loeppert, R.H. Calcite Surface Adsorption of As(V), As(III), MMAs(V), and DMAs(V) and the Impact of Calcium and Phosphate. Soil Sci. Soc. Am. J. 2013, 77, 83–93. [Google Scholar] [CrossRef]
- Naushad, M.; Ahamad, T.; Al-Maswari, B.M.; Abdullah Alqadami, A.; Alshehri, S.M. Nickel ferrite bearing nitrogen-doped mesoporous carbon as efficient adsorbent for the removal of highly toxic metal ion from aqueous medium. Chem. Eng. J. 2017, 330, 1351–1360. [Google Scholar] [CrossRef]
- Martin, J.E.; Herzing, A.A.; Yan, W.; Li, X.Q.; Koel, B.E.; Kiely, C.J.; Zhang, W. Determination of the oxide layer thickness in core-shell zerovalent iron nanoparticles. Langmuir 2008, 24, 4329–4334. [Google Scholar] [CrossRef]
- Leupin, O.X.; Hug, S.J. Oxidation and removal of arsenic (III) from aerated groundwater by filtration through sand and zero-valent iron. Water Res. 2005, 39, 1729–1740. [Google Scholar] [CrossRef]
- Yan, W.; Ramos, M.A.V.; Koel, B.E.; Zhang, W.X. As(III) sequestration by iron nanoparticles: Study of solid-phase redox transformations with X-ray photoelectron spectroscopy. J. Phys. Chem. C 2012, 116, 5303–5311. [Google Scholar] [CrossRef]
- Bakshi, S.; Banik, C.; Rathke, S.J.; Laird, D.A. Arsenic sorption on zero-valent iron-biochar complexes. Water Res. 2018, 137, 153–163. [Google Scholar] [CrossRef]
- Liu, X.; Xu, H.; Wang, L.; Qu, Z.; Yan, N. Surface nano-traps of Fe0/COFs for arsenic(III) depth removal from wastewater in non-ferrous smelting industry. Chem. Eng. J. 2020, 381, 122559. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, M.; Dong, H.; Li, H.; Pan, B. Simultaneous Oxidation and Sequestration of As(III) from Water by Using Redox Polymer-Based Fe(III) Oxide Nanocomposite. Environ. Sci. Technol. 2017, 51, 6326–6334. [Google Scholar] [CrossRef]
- Bhowmick, S.; Chakraborty, S.; Mondal, P.; Van Renterghem, W.; Van den Berghe, S.; Roman-Ross, G.; Chatterjee, D.; Iglesias, M. Montmorillonite-supported nanoscale zero-valent iron for removal of arsenic from aqueous solution: Kinetics and mechanism. Chem. Eng. J. 2014, 243, 14–23. [Google Scholar] [CrossRef]
- Cui, J.; Jin, Q.; Li, Y.; Li, F. Oxidation and removal of As(iii) from soil using novel magnetic nanocomposite derived from biomass waste. Environ. Sci. Nano 2019, 6, 478–488. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).