Application and Comparison of Different Models for Quantifying the Aquatic Community in a Dam-Controlled River
Abstract
1. Introduction
2. Materials and Methods
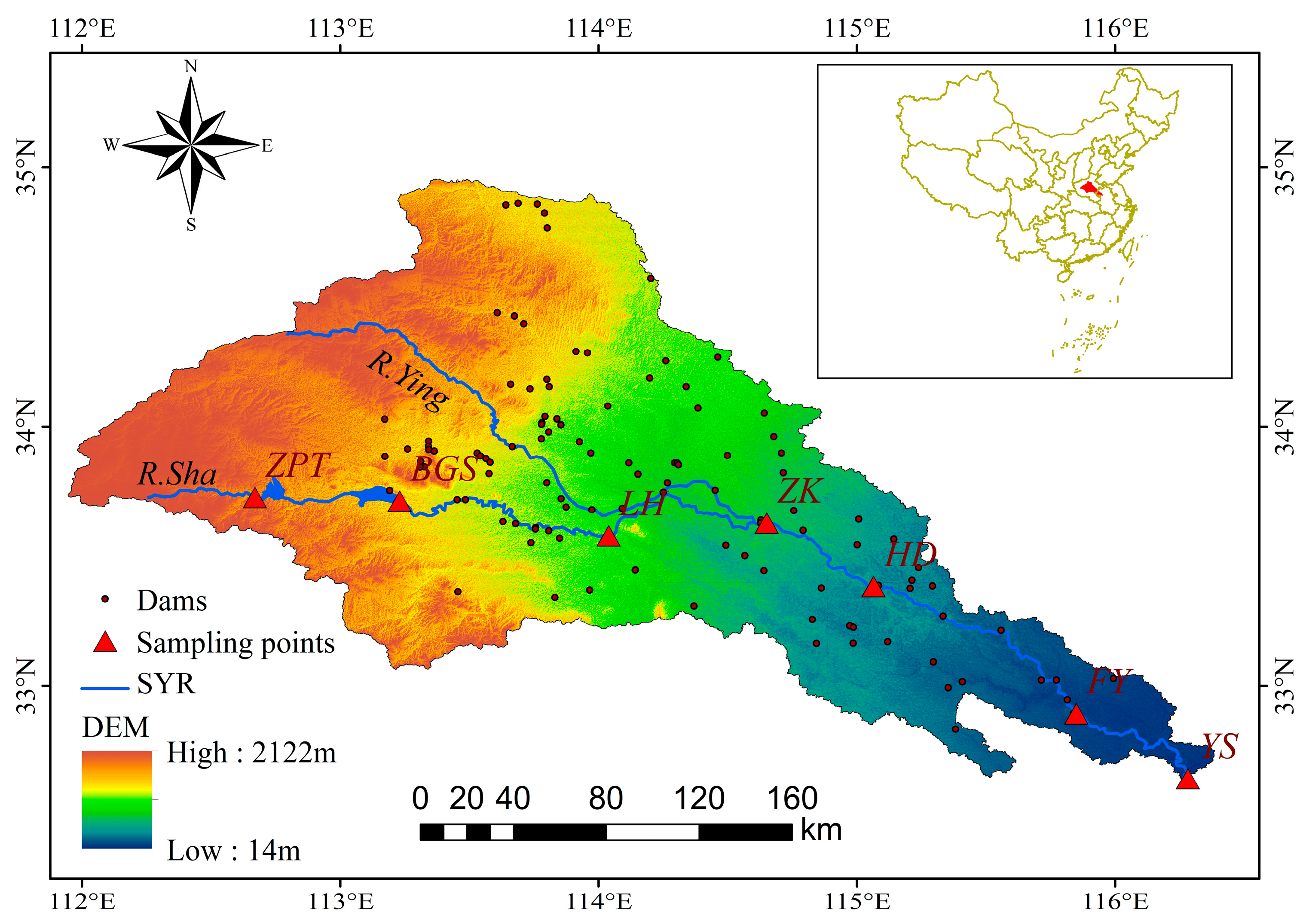
2.1. Study Area and Data Collection
2.2. Methodology
2.2.1. Output Variables
2.2.2. Input Variables
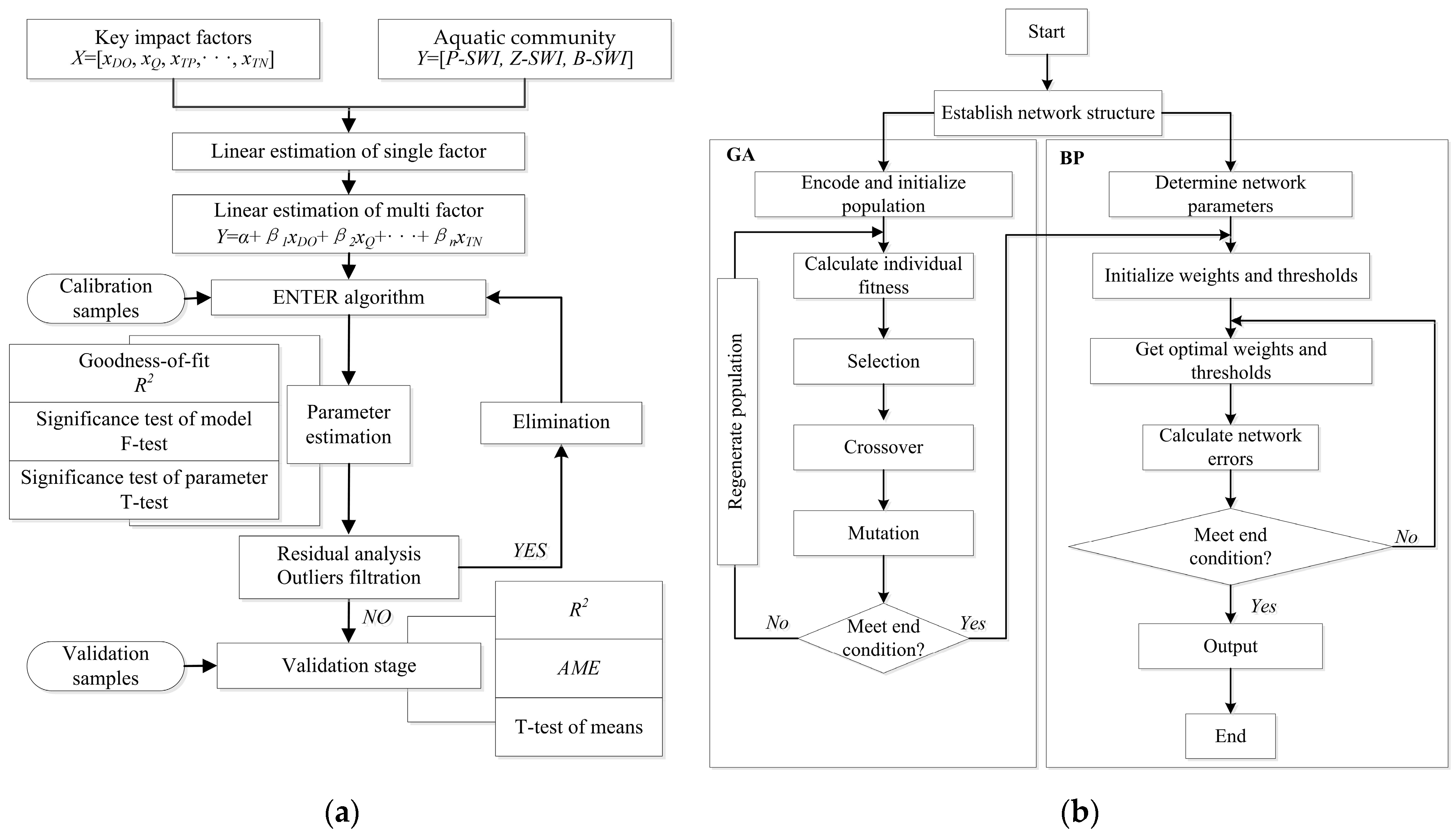
2.2.3. MLE Model Construction
2.2.4. GA-BP Model Construction
2.2.5. Statistical Analyses
3. Results
3.1. Model Structure and Parameter Setting
3.1.1. MLE Model
3.1.2. GA-BP Model
3.2. Model Calibration and Validation
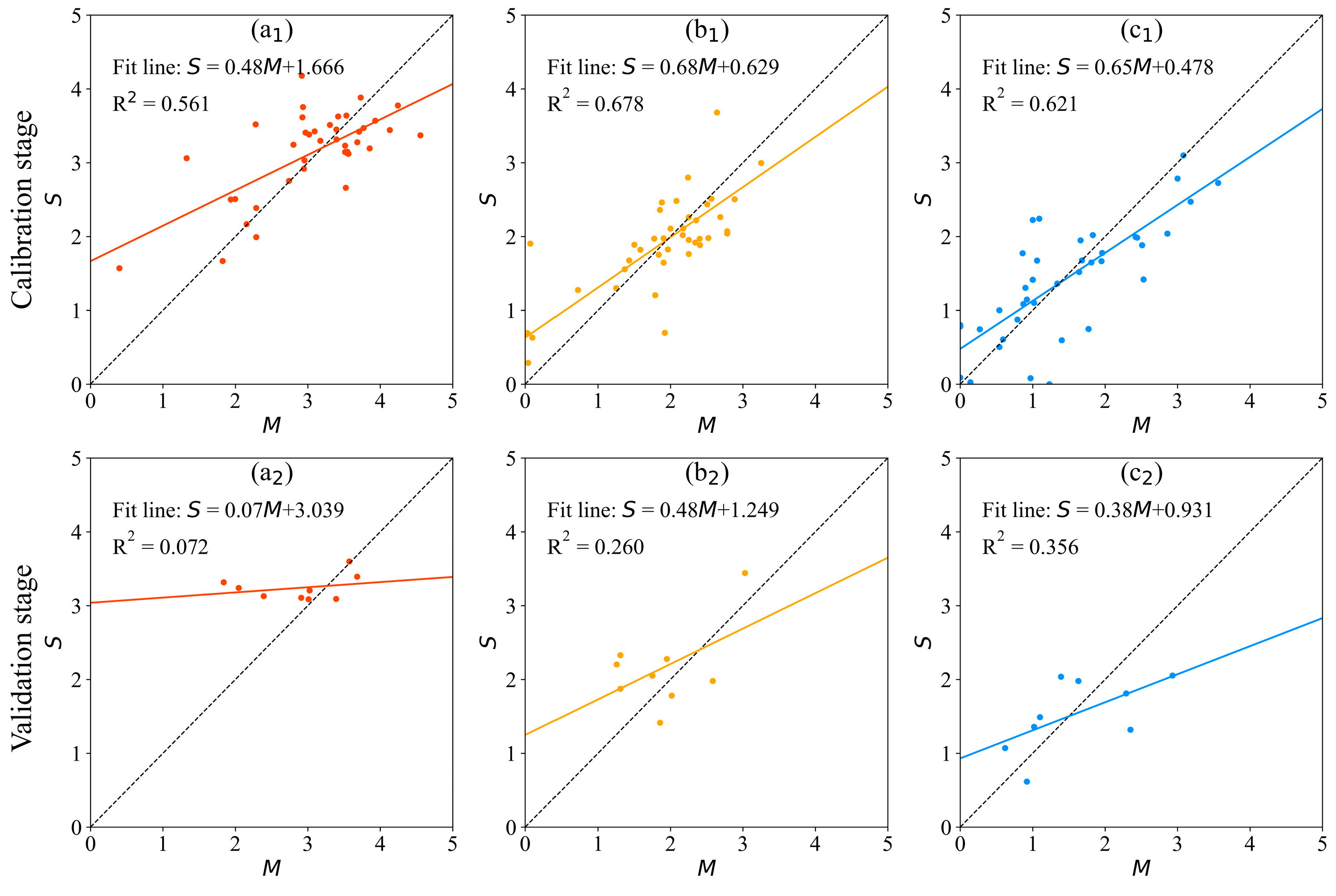
3.2.1. MLE Model
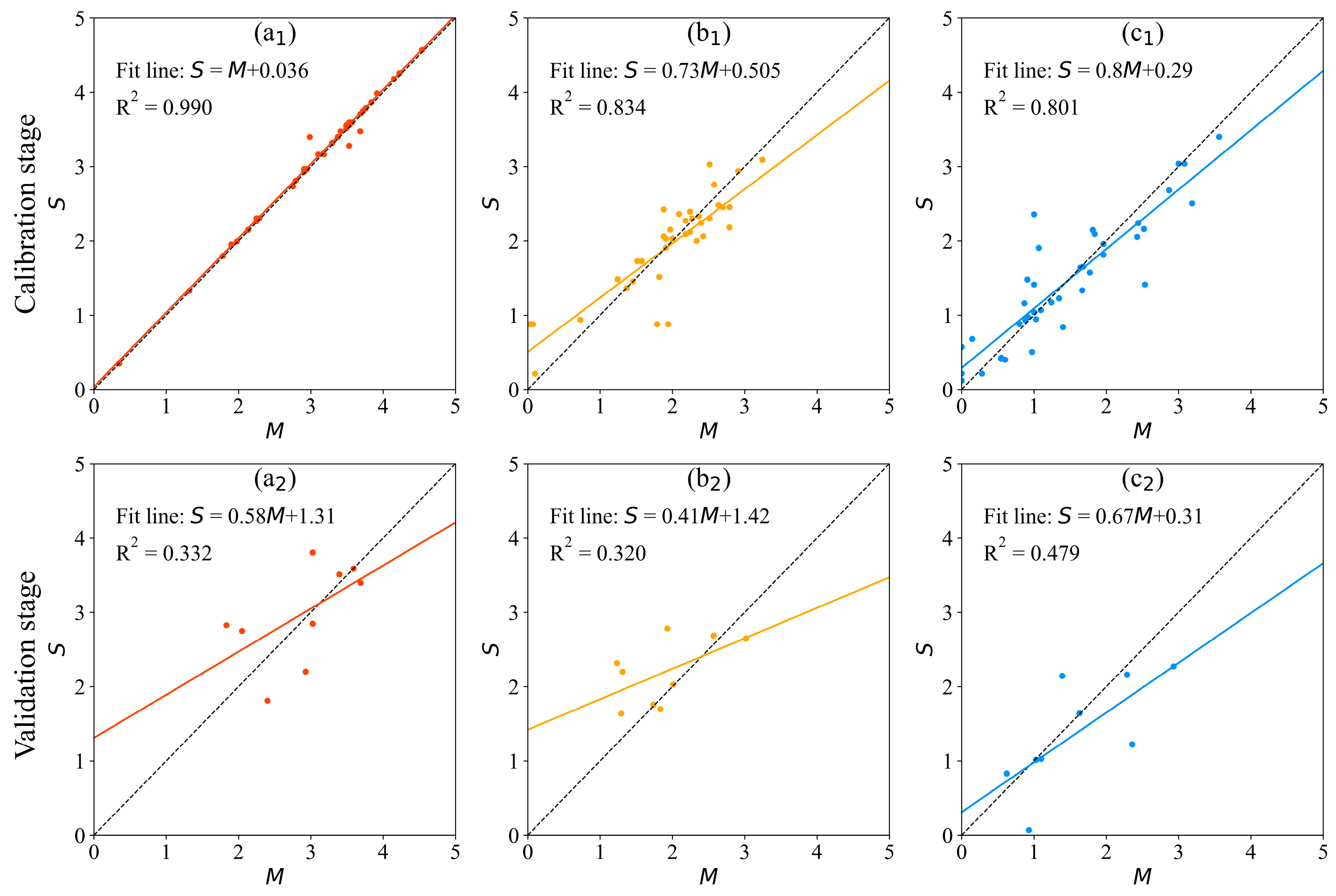
3.2.2. GA-BP Model
3.3. Model Comparison
4. Discussion
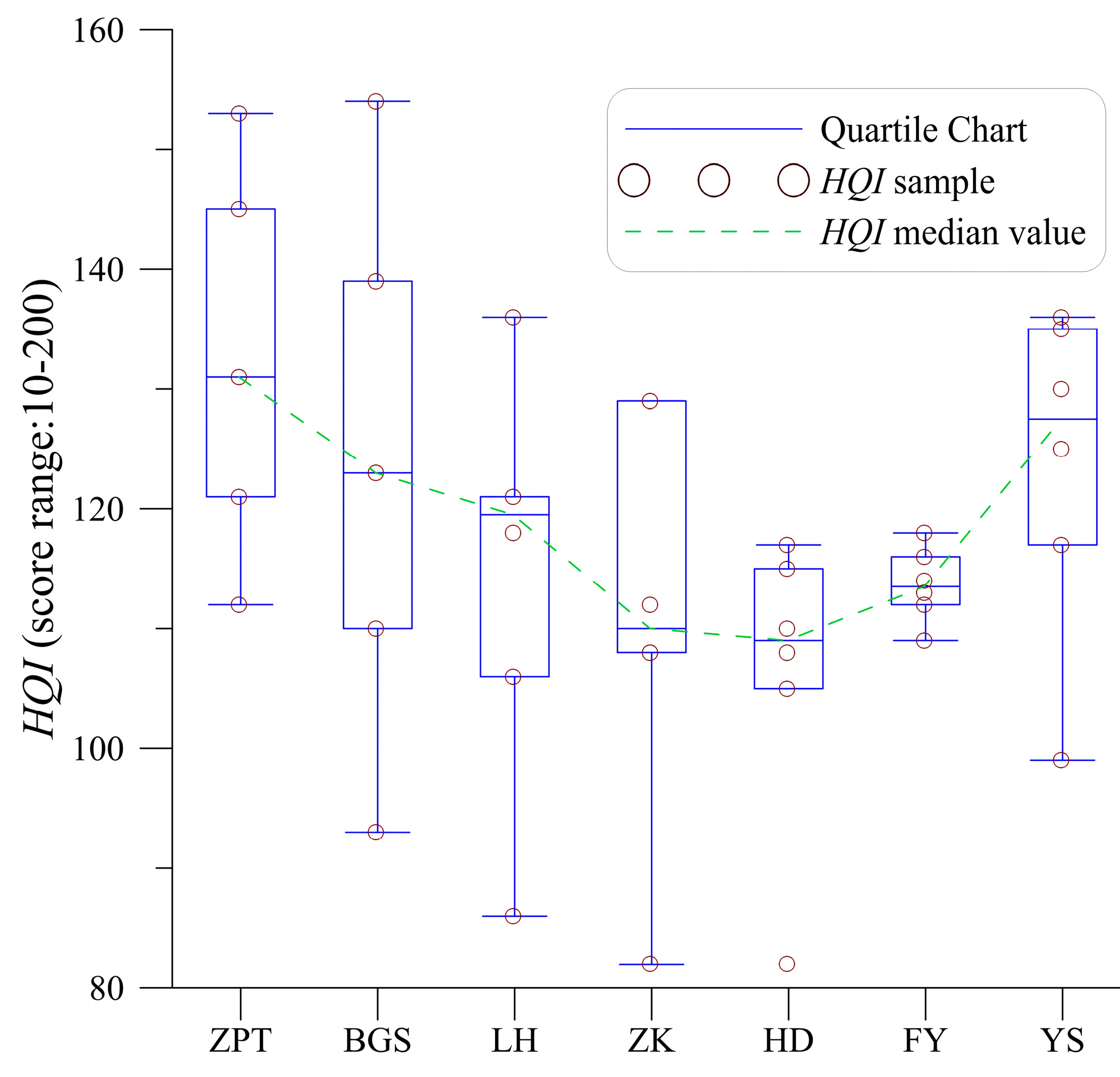
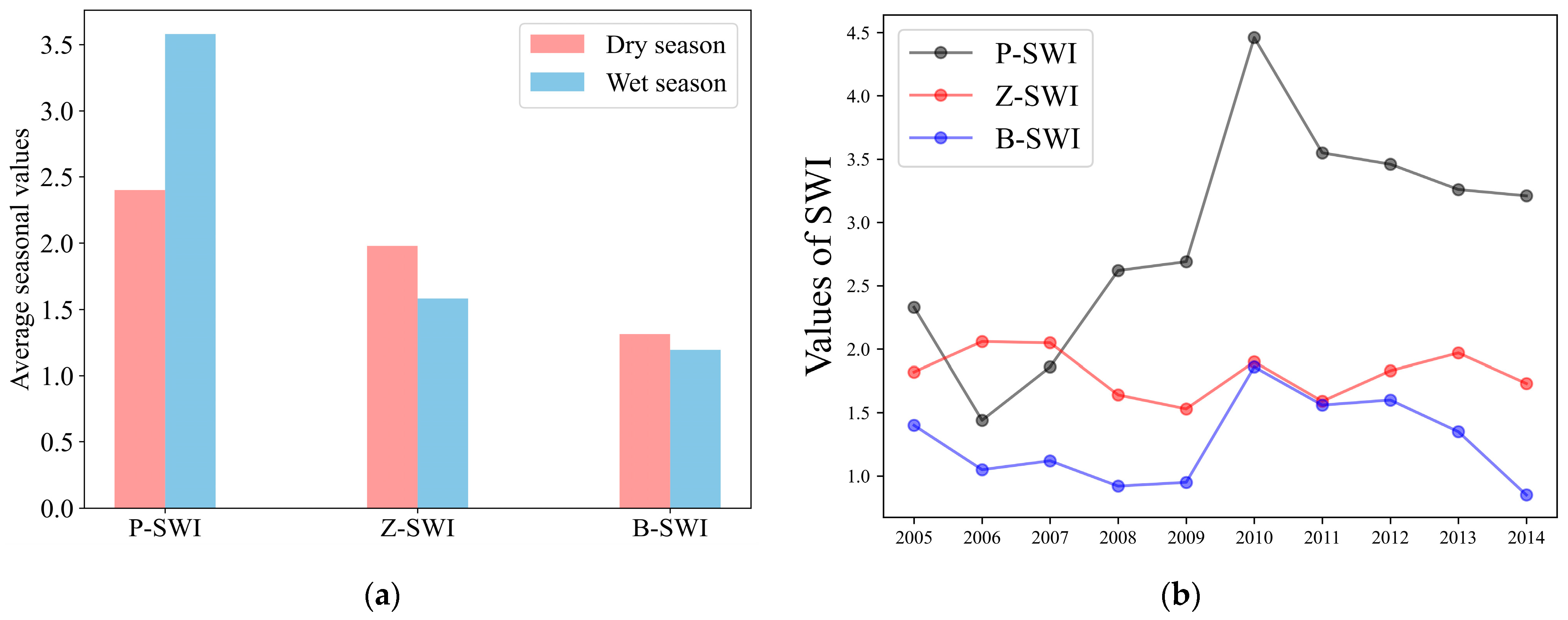
4.1. Variability of Habitat Quality
4.2. Aquatic Community Characteristics
4.3. Model Constraints
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reid, A.J.; Carlson, A.K.; Creed, I.F.; Eliason, E.J.; Gell, P.A.; Johnson, P.T.; Kidd, K.A.; MacCormack, T.J.; Olden, J.D.; Ormerod, S.J.; et al. Emerging threats and persistent conservation challenges for freshwater biodiversity. Biol. Rev. Camb. Phil. Soc. 2019, 94, 849–873. [Google Scholar] [CrossRef] [PubMed]
- Grizzetti, B.; Liquete, C.; Pistocchi, A.; Vigiak, O.; Zulian, G.; Bouraoui, F.; De Roo, A.; Cardoso, A.C. Relationship between ecological condition and ecosystem services in European rivers, lakes and coastal waters. Sci. Total Environ. 2019, 671, 452–465. [Google Scholar] [CrossRef] [PubMed]
- Bouska, K.L.; Houser, J.N.; De Jager, N.R.; Van Appledorn, M.; Rogala, J.T. Applying concepts of general resilience to large river ecosystems: A case study from the Upper Mississippi and Illinois rivers. Ecol. Indic. 2019, 101, 1094–1110. [Google Scholar] [CrossRef]
- Grill, G.; Lehner, B.; Thieme, M.; Geenen, B.; Tickner, D.; Antonelli, F.; Babu, S.; Borrelli, P.; Cheng, L.; Crochetiere, H.; et al. Mapping the world’s free-flowing rivers. Nature 2019, 569, 215–221. [Google Scholar] [CrossRef]
- Belletti, B.; Garcia de Leaniz, C.; Jones, J.; Bizzi, S.; Börger, L.; Segura, G.; Castelletti, A.; Van de Bund, W.; Aarestrup, K.; Barry, J.; et al. More than one million barriers fragment Europe’s rivers. Nature 2020, 588, 436–441. [Google Scholar] [CrossRef]
- Grigg, N.S. Global water infrastructure: State of the art review. Int. J. Water Resour. Dev. 2019, 35, 181–205. [Google Scholar] [CrossRef]
- Van Cappellen, P.; Maavara, T. Rivers in the Anthropocene: Global scale modifications of riverine nutrient fluxes by damming. Ecohydrol. Hydrobiol. 2016, 16, 106–111. [Google Scholar] [CrossRef]
- Winton, R.S.; Calamita, E.; Wehrli, B. Reviews and syntheses: Dams, water quality and tropical reservoir stratification. Biogeosciences 2019, 16, 1657–1671. [Google Scholar] [CrossRef]
- Poole, G.C.; Berman, C.H. An ecological perspective on in-stream temperature: Natural heat dynamics and mechanisms of human-caused thermal degradation. Environ. Manage. 2001, 27, 787–802. [Google Scholar] [CrossRef]
- McCartney, M. Living with dams: Managing the environmental impacts. Water Policy 2009, 11, 121–139. [Google Scholar] [CrossRef]
- Bizzi, S.; Dinh, Q.; Bernardi, D.; Denaro, S.; Schippa, L.; Soncini-Sessa, R. On the control of riverbed incision induced by run-of-river power plant. Resour. Res. 2015, 51, 5023–5040. [Google Scholar] [CrossRef]
- Poff, N.L.; Allen, J.D.; Bain, M.B.; Karr, J.R.; Prestegaard, K.L.; Richter, B.D.; Sparks, R.E.; Stromberg, J.C. The natural flow regime—A paradigm for river conservation and restoration. Bioscience 1997, 47, 769–784. [Google Scholar] [CrossRef]
- Maavara, T.; Chen, Q.; Van Meter, K.; Brown, L.E.; Zhang, J.; Ni, J.; Zarfl, C. River dam impacts on biogeochemical cycling. Nat. Rev. Earth Environ. 2020, 1, 103–116. [Google Scholar] [CrossRef]
- Carpenter-Bundhoo, L.; Butler, G.L.; Bond, N.R.; Bunn, S.E.; Reinfelds, I.V.; Kennard, M.J. Effects of a low-head weir on multi-scaled movement and behavior of three riverine fish species. Sci. Rep. 2020, 10, 6817. [Google Scholar] [CrossRef]
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.; Knowler, D.J.; Lévêque, C.; Naiman, R.J.; Prieur-Richard, A.H.; Soto, D.; Stiassny, M.L.; et al. Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol. Rev. Cambridge Philos. Soc. 2006, 81, 163–182. [Google Scholar] [CrossRef]
- Cardinale, B.J.; Duffy, J.E.; Gonzalez, A.; Hooper, D.U.; Perrings, C.; Venail, P.; Narwani, A.; Mace, G.M.; Tilman, D.; Wardle, D.A.; et al. Biodiversity loss and its impact on humanity. Nature 2012, 486, 59–67. [Google Scholar] [CrossRef]
- Liermann, C.R.; Nilsson, C.; Robertson, J.; Ng, R.Y. Implications of dam obstruction for global freshwater fish diversity. Bioscience 2012, 62, 539–548. [Google Scholar] [CrossRef]
- Poff, N.L.; Olden, J.D.; Merritt, D.M.; Pepin, D.M. Homogenization of regional river dynamics by dams and global biodiversity implications. Proc. Natl. Acad. Sci. USA 2007, 104, 5732–5737. [Google Scholar] [CrossRef]
- Poff, N.L.; Richter, B.D.; Arthington, A.H.; Bunn, S.E.; Naiman, R.J.; Kendy, E.; Acreman, M.; Apse, C.; Bledsoe, B.P.; Freeman, M.C.; et al. The ecological limits of hydrologic alteration (ELOHA): A new framework for developing regional environmental flow standards. Freshw. Biol. 2010, 55, 147–170. [Google Scholar] [CrossRef]
- Rameshkumar, S.; Radhakrishnan, K.; Aanand, S.; Rajaram, R. Influence of physicochemical water quality on aquatic macrophyte diversity in seasonal wetlands. Appl. Water Sci. 2019, 9, 12. [Google Scholar] [CrossRef]
- O’Hare, M.T.; Aguiar, F.C.; Asaeda, T.; Bakker, E.S.; Chambers, P.A.; Clayton, J.S.; Elger, A.; Ferreira, T.M.; Gross, E.M.; Gunn, I.D.; et al. Plants in aquatic ecosystems: Current trends and future directions. Hydrobiologia 2018, 812, 1–11. [Google Scholar] [CrossRef]
- Acreman, M. Environmental flows—Basics for novices. Wiley Interdiscip. Rev. Water 2016, 3, 622–628. [Google Scholar] [CrossRef]
- Trolle, D.; Hamilton, D.P.; Hipsey, M.R.; Bolding, K.; Bruggeman, J.; Mooij, W.M.; Janse, J.H.; Nielsen, A.; Jeppesen, E.; Elliott, J.A.; et al. A community-based framework for aquatic ecosystem models. Hydrobiologia 2012, 683, 25–34. [Google Scholar] [CrossRef]
- Even, S.; Poulin, M.; Garnier, J.; Billen, G.; Servais, P.; Chesterikoff, A.; Coste, M. River ecosystem modelling: Application of the PROSE model to the Seine river (France). Hydrobiologia 1998, 373, 27–45. [Google Scholar] [CrossRef]
- Gao, S.; Shen, A.; Jiang, J.; Wang, H.; Yuan, S. Kinetics of phosphate uptake in the dinoflagellate Karenia mikimotoi in response to phosphate stress and temperature. Ecol. Model. 2022, 468, 109909. [Google Scholar] [CrossRef]
- Lee, E.; Jalalizadeh, M.; Zhang, Q. Growth kinetic models for microalgae cultivation: A review. Algal. Res. 2015, 12, 497–512. [Google Scholar] [CrossRef]
- Van Poorten, B.T.; Walters, C.J.; Taylor, N.G. A field-based bioenergetics model for estimating time-varying food consumption and growth. Trans. Am. Fish. Soc. 2012, 141, 943–961. [Google Scholar] [CrossRef]
- Stokes, M.F.; Perron, J.T. Modeling the evolution of aquatic organisms in dynamic river basins. J. Geophys. Res. Earth Surf. 2020, 125, e2020JF005652. [Google Scholar] [CrossRef]
- Albert, J.S.; Schoolmaster, D.R., Jr.; Tagliacollo, V.; Duke-Sylvester, S.M. Barrier displacement on a neutral landscape: Toward a theory of continental biogeography. Syst. Biol. 2017, 66, 167–182. [Google Scholar] [CrossRef]
- Bicudo, T.C.; Sacek, V.; De Almeida, R.P.; Bates, J.M.; Ribas, C.C. Andean tectonics and mantle dynamics as a pervasive influence on Amazonian ecosystem. Sci. Rep. 2019, 9, 16879. [Google Scholar] [CrossRef]
- Priyadarshi, A.; Chandra, R.; Kishi, M.J.; Smith, S.L.; Yamazaki, H. Understanding plankton ecosystem dynamics under realistic micro-scale variability requires modeling at least three trophic levels. Ecol. Model. 2022, 467, 109936. [Google Scholar] [CrossRef]
- Sarker, S.; Al-Noman, M.; Basak, S.C.; Islam, M.M. Do biotic interactions explain zooplankton diversity differences in the Meghna River estuary ecosystems of Bangladesh? Estuar. Coast. Shelf Sci. 2018, 212, 146–152. [Google Scholar] [CrossRef]
- Larson, D.M.; DeJong, D.; Anteau, M.J.; Fitzpatrick, M.J.; Keith, B.; Schilling, E.G.; Thoele, B. High abundance of a single taxon (amphipods) predicts aquatic macrophyte biodiversity in prairie wetlands. Biodivers. Conserv. 2022, 31, 1073–1093. [Google Scholar] [CrossRef]
- De Moura, R.S.T.; Henry-Silva, G.G. Food web and ecological models used to assess aquatic ecosystems submitted to aqua-culture activities. Cienc. Rural. 2019, 49, e20180050. [Google Scholar] [CrossRef]
- Jardim, P.F.; Melo, M.M.M.; Ribeiro, L.D.C.; Collischonn, W.; Paz, A.R.D. A modeling assessment of large-scale hydrologic alteration in south American pantanal due to upstream dam operation. Front. Environ. Sci. 2020, 8, 567450. [Google Scholar] [CrossRef]
- Vishwakarma, D.K.; Ali, R.; Bhat, S.A.; Elbeltagi, A.; Kushwaha, N.L.; Kumar, R.; Rajput, J.; Heddam, S.; Kuriqi, A. Pre- and post-dam river water temperature alteration prediction using advanced machine learning models. Environ. Sci. Pollut. Res. 2022, 29, 83321–83346. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, J.; Chen, J.; Zhang, M. Water quantity and quality optimization modeling of dams operation based on SWAT in Wenyu River Catchment, China. Environ. Monit. Assess. 2011, 173, 409–430. [Google Scholar] [CrossRef]
- Campbell, D.E. Proposal for including what is valuable to ecosystems in environmental assessments. Environ. Sci. Technol. 2001, 35, 2867–2873. [Google Scholar] [CrossRef]
- Garcia, A.; Jorde, K.; Habit, E.; Caamano, D.; Parra, O. Downstream Environmental Effects of Dam Operations: Changes in Habitat Quality for Native Fish Species. River Res. Appl. 2011, 27, 312–327. [Google Scholar] [CrossRef]
- Marchant, R.; Hehir, G. The use of AUSRIVAS predictive models to assess the response of lotic macroinvertebrates to dams in south-east Australia. Freshw. Biol. 2002, 47, 1033–1050. [Google Scholar] [CrossRef]
- Chen, S.; Chen, B.; Fath, B.D. Assessing the cumulative environmental impact of hydropower construction on river systems based on energy network model. Renew. Sust. Energ. Rev. 2015, 42, 78–92. [Google Scholar] [CrossRef]
- Wu, N.; Tang, T.; Fu, X.; Jiang, W.; Li, F.; Zhou, S.; Cai, Q.; Fohrer, N. Impacts of cascade run-of-river dams on benthic diatoms in the Xiangxi River, China. Aquat. Sci. 2010, 72, 117–125. [Google Scholar] [CrossRef]
- Egger, G.; Politti, E.; Woo, H.; Cho, K.-H.; Park, M.; Cho, H.; Benjankar, R.; Lee, N.-J.; Lee, H. Dynamic vegetation model as a tool for ecological impact assessments of dam operation. J. Hydro-Environ. Res. 2012, 6, 151–161. [Google Scholar] [CrossRef]
- Zuo, Q.; Han, C.; Liu, J.; Li, J.; Li, W. Quantitative research on the water ecological environment of dam-controlled rivers: Case study of the Shaying River, China. Hydrol. Sci. J. 2019, 64, 2129–2140. [Google Scholar] [CrossRef]
- Zhao, Q.; Hastie, T. Causal interpretations of black-box models. J. Bus. Econ. Stat. 2021, 39, 272–281. [Google Scholar] [CrossRef]
- Zeng, Y.; Zeng, Y.; Choi, B.; Wang, L. Multifactor-influenced energy consumption forecasting using enhanced back-propagation neural network. Energy 2017, 127, 381–396. [Google Scholar] [CrossRef]
- Chau, K.W. Particle swarm optimization training algorithm for ANNs in stage prediction of Shing Mun River. J. Hydrol. 2006, 329, 363–367. [Google Scholar] [CrossRef]
- Meng, Z.; Long, X.; Gai, H.; Dang, Z.L.; Wang, S.; Xu, P. Identification of the shear parameters for lunar regolith based on a GA-BP neural network. J. Terramechanics 2020, 89, 22–29. [Google Scholar] [CrossRef]
- Chen, H.; Zuo, Q.T.; Zhang, Y.Y. Impact factor analysis of aquatic species diversity in the Huai River Basin, China. Water Supply 2019, 19, 2061–2071. [Google Scholar] [CrossRef]
- Luo, Z.; Zhang, S.; Liu, H.; Wang, L.; Wang, S.; Wang, L. Assessment of multiple dam- and sluice-induced alterations in hydrologic regime and ecological flow. J. Hydrol. 2023, 617, 128960. [Google Scholar] [CrossRef]
- Luo, Z.; Shao, Q.; Liu, H. Comparative evaluation of river water quality and ecological changes at upstream and downstream sites of dams/sluices in different regulation scenarios. J. Hydrol. 2021, 597, 126290. [Google Scholar] [CrossRef]
- Chen, H.; Li, W.; Zuo, Q.; Zhang, Y.; Liang, S. Evaluation of aquatic ecological health of sluice-controlled rivers in Huai River Basin (China) using evaluation index system. Environ. Sci. Pollut. Res. 2022, 29, 65128–65143. [Google Scholar] [CrossRef]
- Luo, Z.; Shao, Q.; Zuo, Q.; Cui, Y. Impact of land use and urbanization on river water quality and ecology in a dam dominated basin. J. Hydrol. 2020, 584, 124655. [Google Scholar] [CrossRef]
- Meng, W.; Zhang, N.; Zhang, Y. Integrated assessment of river health based on water quality, aquatic life and physical habitat. J. Environ. Sci. 2009, 21, 1017–1027. [Google Scholar] [CrossRef]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press, Urbana-Champaign: Champaign, IL, USA, 1964. [Google Scholar]
- McIntosh, R.P. An index of diversity and the relation of certain concepts to diversity. Ecology 1967, 48, 392–404. [Google Scholar] [CrossRef]
- Ibáñez, C.; Alcaraz, C.; Caiola, N.; Rovira, A.; Trobajo, R.; Alonso, M.; Duran, C.; Jiménez, P.J.; Munné, A.; Prat, N. Regime shift from phytoplankton to macrophyte dominance in a large river: Top-down versus bottom-up effects. Sci. Total Environ. 2012, 416, 314–322. [Google Scholar] [CrossRef]
- Chen, W.; Olden, J.D. Designing flows to resolve human and environmental water needs in a dam-regulated river. Nat. Commun. 2017, 8, 2158. [Google Scholar] [CrossRef]
- Alvarez-Garreton, C.; Boisier, J.P.; Billi, M.; Lefort, I.; Marinao, R.; Barría, P. Protecting environmental flows to achieve long-term water security. J. Environ. Manag. 2023, 328, 116914. [Google Scholar] [CrossRef]
| Input Variables | Output Variables |
|---|---|
| Q, DO, TP, and TN | fP-SWI |
| DO, Q, and TN | fZ-SWI |
| DO, Q, and CODMn | fB-SWI |
| Model | R2 | Significance Test | |
|---|---|---|---|
| F | p | ||
| fP-SWI | 0.561 | 10.538 | 0.000 * |
| fZ-SWI | 0.678 | 23.903 | 0.000 * |
| fB-SWI | 0.621 | 19.155 | 0.000 * |
| Model | Function Item | Parameter | Significance Test | ||
|---|---|---|---|---|---|
| Name | Value | t | p | ||
| fP-SWI | constant | αp | 2.1809 | 4.725 | 0.000 * |
| xDO | βp1 | 0.1228 | 3.317 | 0.002 * | |
| xQ | βp2 | 0.0043 | 1.421 | 0.165 | |
| xTP | βp3 | −0.7810 | −0.927 | 0.361 | |
| xTN | βp4 | −0.0598 | −1.293 | 0.205 | |
| fZ-SWI | constant | αz | −0.0553 | −0.1650 | 0.870 |
| xDO | βz1 | 0.1894 | 7.0842 | 0.000 * | |
| xQ | βz2 | 0.0062 | 2.6388 | 0.013 | |
| xTP | βz3 | −0.0048 | −0.1433 | 0.887 | |
| fB-SWI | constant | αb | 0.5772 | 1.0376 | 0.307 |
| xDO | βb1 | 0.1275 | 3.2575 | 0.003 * | |
| xQ | βb2 | 0.0138 | 4.3158 | 0.000 * | |
| xCODMn | βb3 | −0.2294 | −2.8501 | 0.007 * | |
| Model | GA-BP | |||
|---|---|---|---|---|
| P-SWI | Z-SWI | B-SWI | ||
| variables | input | DO, Q, TN, TP | Q, DO, TN | Q, DO, CODMn |
| output | fP-SWI | fZ-SWI | fB-SWI | |
| layer nodes | input layer neurons, m1 | 4 | 3 | 3 |
| hidden layer neurons, m2 | 9 | 3 | 4 | |
| output layer neurons, m3 | 1 | 1 | 1 | |
| parameters | transfer function | tansig and purelin | ||
| training function | trainlm | |||
| learning rate, v | 0.1 | 0.1 | 0.1 | |
| training times, epochs | 100 | 100 | 50 | |
| population, Mp | 30 | 20 | 20 | |
| iteration times, Ts | 80 | 100 | 50 | |
| crossover probability, pc | 0.3 | 0.3 | 0.3 | |
| mutation probability, pm | 0.1 | 0.1 | 0.1 | |
| Model | The Mean Value of Outputs | Calibration Stage | Validation Stage | |||||
|---|---|---|---|---|---|---|---|---|
| fP-SWI | fZ-SWI | fB-SWI | fP-SWI | fZ-SWI | fB-SWI | |||
| MLE | Simulation | 3.144 | 1.923 | 1.442 | 3.241 | 2.151 | 1.377 | |
| Observation | 3.055 | 1.916 | 1.440 | 2.880 | 1.881 | 1.586 | ||
| t-test (2-tailed) | H0 | mean simulation = mean observation | ||||||
| Z-score | 0.55 | 0.10 | −0.22 | 1.59 | 0.99 | −0.19 | ||
| results | no significant difference at the 0.05 level | |||||||
| GA-BP | Simulation | 3.082 | 1.908 | 1.442 | 2.971 | 2.195 | 1.377 | |
| Observation | 3.055 | 1.916 | 1.440 | 2.880 | 1.881 | 1.586 | ||
| t-test (2-tailed) | H0 | mean simulation = mean observation | ||||||
| Z-score | 0.14 | −0.05 | 0.01 | 0.29 | 1.26 | −0.58 | ||
| results | no significant difference at the 0.05 level | |||||||
| Model | Model Equation/Structure | Calibration | Validation | ||||
|---|---|---|---|---|---|---|---|
| MRE | MAE | R2 | MRE | MAE | R2 | ||
| GA-BP | 1.6% | 0.0338 | 0.990 | 20% | 0.4833 | 0.332 | |
| 13% | 0.3075 | 0.834 | 28.2% | 0.4217 | 0.320 | ||
| 18.5% | 0.2862 | 0.801 | 28.1% | 0.4523 | 0.479 | ||
| MLE | 20.7% | 0.4323 | 0.561 | 22.5% | 0.4979 | 0.072 | |
| 17% | 0.3551 | 0.678 | 33.7% | 0.5394 | 0.260 | ||
| 41.5% | 0.5246 | 0.621 | 37.5% | 0.5985 | 0.356 | ||
| MNLE | 9.1% | 0.2309 | 0.811 | 22% | 0.5136 | 0.357 | |
| 15.1% | 0.3443 | 0.723 | 34.5% | 0.4587 | 0.305 | ||
| 42% | 0.4262 | 0.657 | 29.5% | 0.5179 | 0.407 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Zang, C.; Zuo, Q.; Han, C.; Krause, S. Application and Comparison of Different Models for Quantifying the Aquatic Community in a Dam-Controlled River. Int. J. Environ. Res. Public Health 2023, 20, 4148. https://doi.org/10.3390/ijerph20054148
Liu J, Zang C, Zuo Q, Han C, Krause S. Application and Comparison of Different Models for Quantifying the Aquatic Community in a Dam-Controlled River. International Journal of Environmental Research and Public Health. 2023; 20(5):4148. https://doi.org/10.3390/ijerph20054148
Chicago/Turabian StyleLiu, Jing, Chao Zang, Qiting Zuo, Chunhui Han, and Stefan Krause. 2023. "Application and Comparison of Different Models for Quantifying the Aquatic Community in a Dam-Controlled River" International Journal of Environmental Research and Public Health 20, no. 5: 4148. https://doi.org/10.3390/ijerph20054148
APA StyleLiu, J., Zang, C., Zuo, Q., Han, C., & Krause, S. (2023). Application and Comparison of Different Models for Quantifying the Aquatic Community in a Dam-Controlled River. International Journal of Environmental Research and Public Health, 20(5), 4148. https://doi.org/10.3390/ijerph20054148












