Abstract
There is no evidence of the effect of exercise training on human brown-like adipose tissue vascular density (BAT-d). Here, we report whether whole-body strength training (ST) in a cold environment increased BAT-d. The participants were 18 men aged 20–31 years. They were randomly assigned to two groups: one that performed ST twice a week at 75% intensity of one-repetition maximum for 10 weeks during winter (EX; n = 9) and a control group that did not perform ST (CT; n = 9). The total hemoglobin concentration in the supraclavicular region determined by time-resolved near-infrared spectroscopy was used as a parameter of BAT-d. ST volume (Tvol) was defined as the mean of the weight × repetition × sets of seven training movements. The number of occasions where the room temperature was lower than the median (NRcold) was counted as an index of potential cold exposure during ST. There was no significant between-group difference in BAT-d. Multiple regression analysis using body mass index, body fat percentage, NRcold, and Tvol as independent variables revealed that NRcold and Tvol were determined as predictive of changes in BAT-d. An appropriate combination of ST with cold environments could be an effective strategy for modulating BAT.
1. Introduction
The number of patients with cardiometabolic diseases has increased dramatically worldwide over the past 50 years, partly due to daily energy imbalance [1]. Therefore, there is an urgent need to develop integrated strategies to prevent cardiometabolic disorders and their complications [2]. White adipose tissue (WAT) is used for energy storage. Contrarily, classical brown adipose tissue (cBAT) and beige adipose tissue, a brown-like adipose tissue (BAT) habitable in WAT, are “adaptive heaters” that use excess energy for responding to stimuli such as cold [3], certain drugs, and dietary components with certain modifications by circadian rhythms, exercise training, and aging [3,4,5,6,7]. Activation of BAT improves systemic energy expenditure and glucose and lipid metabolism [8,9,10]. A large cross-sectional study has recently reported that individuals with high BAT activity have a lower risk of developing various non-communicable diseases such as type 2 diabetes mellitus, dyslipidemia, and coronary artery disease [11].
Exercise training is well-known to improve metabolic status by decreasing fat mass [12], increasing skeletal muscle mass (SMM) [13], and improving insulin sensitivity [13]. In addition, previous animal studies have shown the potential of exercise training to increase cBAT and BAT by increasing sympathetic innervation of WAT and endocrine secretion of catecholamines, irisin, and others [14,15,16,17,18,19]. Although very few human studies have been conducted, cross-sectional studies in humans have reported that men and women with endurance exercise training have significantly lower BAT activities (determined by 18F-fluorodeoxyglucose (FDG) uptake in the supraclavicular region) than sedentary men and non-athlete women [20,21]. Lower BAT activation in endurance athletes is associated with an insufficient activation of sympathetic nerve activity (SNA) during exercise, which is the major drive to enhance cBAT and BAT activities [22,23]. Further, several previous studies have not provided relevant data on BAT or daily physical activity before and after exercise training [24,25] or data on environmental and seasonal fluctuations in temperature without setting a control group. In contrast to endurance exercise training, strength training is known to cause an explosive increase in SNA outflow, leading to BAT activation [26,27]; however, this has not been focused upon in any previous study and warrants further research investigation.
This study used time-resolved near-infrared spectroscopy (TR-NIRS) to estimate human BAT characteristics by measuring the total hemoglobin concentration ([Total-Hb]) in the supraclavicular region. RNA sequencing data analysis revealed that adult human BAT in the supraclavicular region exhibits a molecular signature similar to beige adipose tissue [28]. Taking advantage of the abundant capillaries in BAT morphology [29,30,31], TR-NIRS detects BAT vascular density (BAT-d) and can safely and non-invasively evaluate BAT-d without requiring cold or radiation exposure [32,33]. A previous study [Total-Hb] assessed by TR-NIRS and standardized uptake values (SUVmean) assessed by 18F-FDG- positron emission tomography/computed tomography (PET/CT) showed a positive correlation in the supraclavicular region, where BAT was potentially localized [33].
Here, we hypothesized that whole-body strength training in the winter would increase BAT-d. Thus, we aimed to study whether strength training in a cold environment would increase BAT-d and to identify factors related to its changes.
2. Materials and Methods
2.1. Study Design
On the participant’s first laboratory visit, baseline measurements were conducted to determine body composition, BAT-d, and muscular strength. Within 1 week after the first visit, the exercise group (EX) began exercise training for 10 weeks, twice per week on non-consecutive days for 60 min each at 75% intensity of one-repetition maximum of seven whole-body movements. Exercise training intensity was increased progressively and individually by modulating the weight to be lifted by all participants in the EX once every 2 weeks during the 10-week exercise training program. All exercise training programs were supervised by skilled instructors at all times to ensure that the appropriate weight was handled, adequate rest was taken, the correct form was used for lifting, and risks were managed to prevent injuries and accidents. In all exercise training sessions, the ratio of participants to instructors was 1:1. All measurements were repeated within a week from two days after the end of the exercise training program. While the temperature of the training room was controlled by an air conditioner set at 25.0 °C, the temperature fluctuated from 15.0 to 22.6 °C during training, as the room was relatively large. Then, the relationship between fluctuation in room temperature during training and changes in BAT-d was analyzed as below.
2.2. Participants and Interventions Inclusion/Exclusion Criteria
This study’s inclusion criteria were as follows: men aged 20–59 years with a body mass index (BMI) of less than 30 kg/m2. Women were not included in the study because the hormonal imbalance caused by the menstrual cycle may affect the whole-body metabolism, including BAT. People with one or more of the following conditions were excluded from the study: daily strength training, smoking, glucose intolerance, dyslipidemia, hypertension, hyperuricemia, gout, coronary artery disease, brain infarction, non-alcoholic fatty liver disease, menstrual disorder, sleep apnea syndrome, motor disorder, obesity related-glomerulopathy, and cardiovascular disease risk. As a result, out of the 19 people who applied for participation in the study, 18 healthy men aged 20–31 years who met the inclusion criteria described above were randomly assigned to the EX (n = 9) and control groups (CT; n = 9) (Figure 1). This study was conducted per the Declaration of Helsinki, and the study design was approved by the Tokyo Medical University Medical Ethics Review Board (approval number: T2019-0028). Written informed consent was obtained from the participants before conducting the study.
Figure 1.
Inclusion criteria.
2.3. Anthropometric Measurements
Body weight, fat mass, body fat percentage (%BF), and SMM were measured using bioelectric impedance (InBody 720 Body Composition Analyzer; InBody Japan, Tokyo, Japan). BMI was calculated as an individual’s weight (kg) divided by the square of their height (m). Visceral fat area was measured in a standing position using the impedance method (Bioelectrical impedance analysis EW-FA90; Panasonic, Osaka, Japan). The thickness of subcutaneous fat in the supraclavicular, deltoid, and abdominal regions was monitored using B-mode ultrasound (Vscan Dual Probe; GE Vingmed Ultrasound AS, Horten, Norway).
2.4. Brown-like Adipose Tissue Vascular Density
BAT-d was evaluated by TR-NIRS (Hamamatsu Photonics K.K., Hamamatsu, Japan) according to previous studies [33,34]. The abundance of capillaries in BAT helped distinguish it from other tissues based on the optical properties of the tissue as evaluated by TR-NIRS. The 3 cm probe used in this study allowed light to reach an average depth of 2 cm [35], which is the depth at which BAT is potentially present [36]. [Total-Hb] in the supraclavicular region, an indicator of BAT-d, was calculated as the sum of oxygenated and deoxygenated hemoglobin, i.e., blood volume. As control regions, [Total-Hb] in the deltoid muscle ([Total-Hb]delt) and [Total-Hb] in the abdominal subcutaneous fat ([Total-Hb]sub) were measured by using TR-NIRS and BAT-d measurements simultaneously. [Total-Hb] in the supraclavicular region was adjusted for the thickness of the subcutaneous adipose tissue layer [37]. TR-NIRS data were extracted every 10 s and averaged over 1 min. The coefficient of variation within an individual on repeated evaluation was 4.9% [33].
2.5. Exercise Training
Before the intervention and after 2, 4, 8, and 10 weeks of the intervention, the EX group underwent a muscular strength test on a strength training machine (AXT-225, TuffStuff Fitness International, Chino, CA, USA), which was evaluated by a health fitness programmer. Muscle strength was assessed for the seven training types: lower limb exercises: leg extension and leg curl; upper limb exercises: chest press, lat pulldown, triceps extension, and biceps curl; and a core exercise of crunches excluding the handgrip strength test. One-repetition maximal muscle strength (1 RM) was not measured directly because the subjects were untrained. For the estimation of the 1 RM value, the Wathen equation was used [38]. Strength training volume (Tvol) was defined as the mean of the weight × the repetition × sets.
Based on this equation, participants lifted the maximum weight they could lift 1–5 times [38]. All participants practiced with lighter weights and tested their muscle strength during the three trials. If they found that they could perform more than five repetitions with a certain weight, they stopped once, rested until their muscles recovered, and then tried again with a heavier weight. If fewer than five repetitions were performed at maximal muscle strength, the exercise was considered complete.
The handgrip strength test was assessed using a Takei 5401 digital Grip-D hand dynamometer (Takei, Niigata, Japan). Participants stood and extended their elbows carefully, without letting their arms touch their bodies. The participants gripped the dynamometer at maximum effort with a grip width almost perpendicular to the second joint of the index finger. The right–left alternation was performed twice, and the better record of each was averaged.
2.6. Questionnaires
Total physical activity was assessed using the International Physical Activity Questionnaire (IPAQ; long version) during a representative week. The IPAQ assesses physical activity lasting longer than 10 min. The total amount of physical activity was calculated as physical activity duration (hours) × physical activity intensity (metabolic equivalents).
The brief-type self-administered diet history questionnaire is a questionnaire designed to examine the amount of nutrients habitually consumed from the diet and to obtain information on individuals’ nutrient and food intakes. We assessed the intake of the three macronutrients: protein, fat, and carbohydrates.
2.7. Ambient Temperature
Data on average ambient temperatures were obtained from the Japan Meteorological Agency (Japan Meteorological Agency. “Historical Weather Data”, available at: https://www.jma.go.jp/jma/menu/menureport.html accessed 19 April 2022). The room temperature was contentiously measured during the strength training using a radio clock with a thermometer (8RZ140, CITIZEN, Tokyo, Japan). This thermometer could measure from −9.9 to 50 °C with a measurement error of ±1 °C. Then, the number of occasions with room temperature less than the median (NRcold) and the number of occasions with outside temperature less than the median (NOcold) were counted as an index of potential cold exposure during the strength training. Participants dressed in personal thin summer clothing during exercise training. Time spent outside (Tout) during the intervention period and area under the curve (AUCout; Tout × outside temperature of the training day) were calculated based on the IPAQ questionnaire.
2.8. Statistical Analysis
The sample size was calculated considering the type 1 and type 2 errors based on the statistical testing of BAT-d, which was one of the primary outcomes. No previous studies have examined the effect of exercise training on BAT-d. Therefore, we calculated the sample size using the G*Power software (Version 3.1; Bonn University, Bonn, Germany), based on our previous work evaluating the changes in BAT-d in healthy adults who ingested capsinoids or placebo capsules for 8 weeks [39]. In this setting, the sample size was calculated at 80% power and 5% significance level. This resulted in a net sample size of eight subjects in each group. Therefore, we decided to enroll nine subjects in each group in case of dropouts.
Interactions in a two-way repeated-measures analysis of variance (ANOVA) were used to evaluate the pre- and post-intervention changes between the two groups. The difference before and after the intervention was calculated as the amount of change (Δ). Pearson’s correlation coefficient (r) was used for the correlation between the two variables; however, only for the correlation coefficient (rxy·z) between ΔBAT and Tvol, a partial correlation analysis was used to account for the effect of SMM. Stepwise multiple linear regression analysis (MLR) was used to evaluate the independent relationships between parameters (p < 0.20) with ΔBAT-d. Data are represented as the mean (standard deviation). All statistical analyses were performed using SPSS Statistics 27 or 28 (IBM SPSS Japan, Tokyo, Japan), and the significance level was set at p < 0.05.
3. Results
3.1. Participant Characteristics and BAT-d
There was no significant difference in the mean values of all pre- and post-intervention indices in the two groups, except for a significant increase in SMM and muscle strength (Table 1 and Table 2). The change in BAT-d appears to be greater in the control group than in the exercise group, probably due to seasonal variation [34]; however, no significant change was observed (Figure 2).

Table 1.
Participant body composition.

Table 2.
Participants’ exercise training and dietary status.
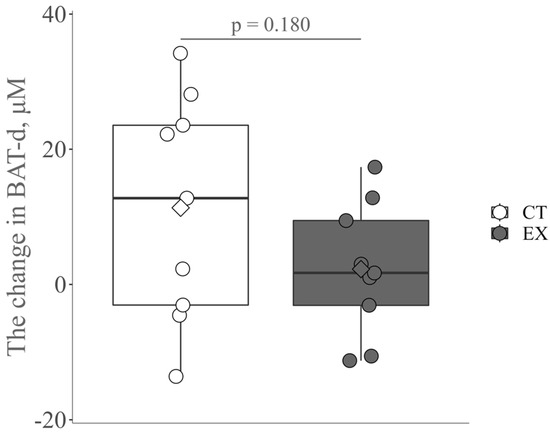
Figure 2.
The change in brown-like adipose tissue vascular density (BAT-d) pre- and post-intervention in the control group (CT) and the exercise group (Ex). The circle and diamond plots indicate individual values and the mean values, respectively.
3.2. Ambient Temperature
Tout and AUCout had no significant between-group differences (Table 3). The median values of outside temperature on the training day and room temperature during strength training were 10.5 and 18.8 °C, respectively (Table 3). In studies where human BAT is activated by cold stimuli, the room temperature is set below 19 °C, where shivering does not occur.

Table 3.
Ambient temperature.
3.3. Relationship between BAT-d and the Number of Occasions Lower Than the Median Room Temperature, the Number of Occasions Lower Than the Median outside Temperature, Skeletal Muscle Mass, and Training Volume
ΔBAT-d is positively correlated with NRcold (Table 4). However, no significant correlation between ΔBAT and NOcold was observed (r = 0.312, p = 0.414; Table 4). ΔBAT-d was not associated with pre-SMM (r = 0.461, p = 0.212). A partial correlation analysis used to account for the effect of pre-SMM revealed that BAT-d was marginally associated with Tvol (rxy·z = 0.690, p = 0.058). MLR, using BMI, %BF, NRcold, and Tvol as independent variables, showed that NRcold and Tvol were predictors of ΔBAT-d (Y = −53.59 + 2.49x1 + 0.03x2 (x1: NRcold, x2: Tvol,), F = 11.23, p = 0.009). The contribution was 78.9% (r = 0.888) and the standard error of the estimate was 5.22 µM (Table 4). On the other hand, Δ[Total-Hbdelt] and Δ[Total-Hbsub] as parameters of the control region were not correlated with NRcold (r = 0.258, p = 0.504 and r = −0.111, p = 0.776, respectively) and Tvol (r = 0.478, p = 0.193 and r = 0.139, p = 0.721, respectively).

Table 4.
Multiple linear regression analysis for ΔBAT-d.
4. Discussion
Contrary to our hypothesis, ΔBAT-d did not significantly differ between the EX and CT groups. MLR using a stepwise method with BMI, %BF, NRcold, and Tvol as independent variables revealed that NRcold and Tvol were factors predicting ΔBAT-d, suggesting that some stimuli such as elevated SNA occurring due to a lower room temperature and strength training volume may be associated with the increase in BAT-d.
We have observed the large interindividual variability in the change in BAT-d following strength training and the same for the control group. The interindividual heterogeneity in BAT-d dynamics pre- and post-intervention may be due to the susceptibility to seasonal ambient temperature fluctuation in CT and/or the stimuli elicited by the strength training in EX. Further research is needed to clarify the interindividual variability and the physiological mechanisms behind these findings. Similar to the present results, one previous study reported that 18F-FDG uptake in the supraclavicular region did not change after 2 weeks of high-intensity cycling, which would have led to adequate activation of SNA [40]. One previous study in an animal model reported that high-intensity resistance training, consisting of 50–100% of maximal load, five times a week for 8 consecutive weeks, induced the browning of inguinal and retroperitoneal white adipose tissue or increased BAT [16]. In one animal model study, BAT weights were comparable between the cold acclimation group (CG) and cold acclimation–exercise training group (CTG). However, BAT lipid content was 9.3% lower in the CTG than in the CG [41], which would be a marginal difference in lipid density resulting in a small increase in BAT-d when adopted in our study. In this study, the most important reason for comparable results of BAT-d in the EX and CT was the increase in body temperature due to exercise training that may have offset the increase in BAT-d due to the increased SNA.
In other words, the rapid increase in body temperature associated with increased thermogenesis due to exercise training is similar to a state of intermittent mild heat exposure. As heat exposure has been reported to have an inhibitory effect on norepinephrine (NE)-dependent non-shivering thermogenesis (NST) [42], it is possible that the increase in body temperature due to strength training also inhibited the increase in BAT-d in this study. As BAT is essentially an adaptive thermogenic tissue in cold environments, the attenuated increase in BAT-d as a whole in this study might be a reasonable outcome. The association of strength training volume with ΔBAT-d indicates that a larger amount of muscle work elicits a greater efferent increase in SNA through larger stimuli. In fact, subjects with greater muscle mass or strength easily induce higher intramuscular pressure resulting in spontaneous blood flow restriction, which, in turn, creates enhanced SNA [43,44,45].
Another possible reason for the comparable BAT-d between EX and CT in this study could be that the increase in SNA during strength training was lower than that during cold exposure. In an animal study, the exercise training group (EXG; 90.6 g (SD 11.9)) had a lower BAT weight than the chronic cold exposure group (CCE; 283.7 g (SD 25.2)). Furthermore, the total amount of urinary catecholamine metabolites such as 3-methoxy-4-hydroxymandelic acid (VMA) and homovanillic acid (HVA) was significantly lower in the EXG than in the CCE. On the other hand, urinary catecholamine metabolites per unit time were higher in the EXG (VMA: 15.6 µg/h (SD 0.57), HVA: 9.94 µg/h (SD 0.27)) than in the CCE (VMA: 7.80 µg/h (SD 1.96), HVA: 1.94 µg/h (SD 0.67)). In short, it is suggested that prolonged, low-dose cold exposure may enhance NE-dependent NST better than that with short-duration, high-dose SNA stimulation derived from exercise training [46].
Cold exposure is the primary factor that activates BAT [5]. The present strength training program was selected according to the American College of Sports Medicine exercise guidelines that allow the major muscle groups to be trained twice a week [47]. However, SNA created by the strength training in the present study may be lower than that of a two-hour cold exposure for 6 weeks obtained in a previous study reporting an increase in BAT activity and a decrease in body fat mass in healthy young men [48]. A lower amount of SNA may be one of the reasons for no increase in BAT by the current strength training. However, increasing exercise volume to enhance exercise-induced SNA may suppress BAT because it causes a greater increase in body temperature in humans. Quantitative assessment of SNA during a combination of cold exposure with exercise training should be investigated to determine measures to effectively increase BAT, which would be a more comfortable method than a high-stress cold-exposure regimen itself.
Limitations
This study has four limitations. Firstly, while TR-NIRS allows noninvasive measurements, it may be less valid than invasive tissue biopsies. BAT-d measurement using TR-NIRS has been validated over and over. In this study, only [Total-Hb] in the supraclavicular region ([Total-Hb]sup), not [Total-Hb]delt or [Total-Hb]sub, was significantly correlated with Tvol and NRcold. Furthermore, previous studies have reported that [Total-Hb]sup can be characterized distinctly from [Total-Hb]sub [49]. To further enhance the validity of this study, it is necessary to link observations to tissue biopsy. However, we realize that obtaining this in a human subject study is challenging. Instead, we can examine our hypotheses in part through the use of animal models. Secondly, because blood hemoglobin levels were not measured in this study, it was not possible to determine changes in blood hemoglobin levels before and after exercise and in the control group. Among the limited longitudinal investigations, Schobersberger et al. [50] reported that 6 weeks of strength training slightly increased erythropoiesis. Others have shown that 12 weeks of strength training either has no effect on erythrocyte volume and hemoglobin concentration [51] or decreases hemoglobin concentration with no change in hematocrit [52]. One strength training study showed no significant changes in hematocrit or red blood cell count at 10 weeks, but an increase in hematocrit and red blood cell count was observed at 20 weeks. Further research is needed to clarify the effects of strength training on blood hemoglobin levels and the physiological mechanisms behind these findings. Thirdly, the room temperature during strength training was not controlled due to the relatively large room size and the lack of an air conditioner to effectively control the temperature. However, coincidently occurring temperature variations allowed us to examine the relationship between the occurrence of cold exposure during strength training and changes in BAT-d. Finally, only young male participants were included. As this is a pilot study investigating the effects of exercise on human BAT, women were not included due to the wide fluctuations in hormones during the menstrual cycle. In the future, it will be necessary to monitor hormonal imbalances during the menstrual cycle and then study sex differences in exercise-related changes in BAT. It is also important to study the effect of exercise on BAT among different sexes by monitoring the hormone balance of the menstrual cycle.
5. Conclusions
There were individual differences in ΔBAT-d and no overall increase in BAT-d, possibly due to negative factors such as increased body temperature elicited from strength training. However, when the analysis focused on individual data, the fact that both NRcold and Tvol were independently extracted as factors explaining ΔBAT-d in the MLR suggests that an appropriate combination of strength training in a mildly cold environment could be a strategy for effective and comfortable modulation of BAT.
Author Contributions
Conceptualization, R.T., M.K., T.E., R.K., Y.K. and T.H.; methodology, R.T., M.K. and S.F.-H.; validation, M.K. and S.F.-H.; formal analysis, R.T., S.F.-H., T.Y. and T.H.; investigation, R.T., S.F.-H., M.K. and T.H.; data curation, R.T. and S.F.-H.; writing—original draft preparation, R.T.; writing—review and editing, R.T., S.F.-H. and T.H.; visualization, R.T. and S.F.-H.; supervision, S.F.-H. and T.H.; project administration, T.H.; funding acquisition, R.T. and T.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by JSPS KAKENHI (19H04061) and Japan Health Promotion & Fitness Foundation (no funding number).
Institutional Review Board Statement
The study was conducted per the Declaration of Helsinki and approved by the Tokyo Medical University Medical Ethics Review Board (approval number: T2019-0028, date of approval: 13 June 2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
Derived data supporting the findings of this study are available from the corresponding author T.H. on request.
Acknowledgments
This work was supported by JSPS KAKENHI (19H04061) and Japan Health Promotion & Fitness Foundation (no funding number). The authors wish to acknowledge the volunteers who participated in this study.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
References
- Hruby, A.; Hu, F.B. The Epidemiology of Obesity: A Big Picture. Pharmacoeconomics 2015, 33, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Poher, A.L.; Altirriba, J.; Veyrat-Durebex, C.; Rohner-Jeanrenaud, F. Brown Adipose Tissue Activity as a Target for the Treatment of Obesity/Insulin Resistance. Front. Physiol. 2015, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Yoneshiro, T.; Aita, S.; Matsushita, M.; Okamatsu-Ogura, Y.; Kameya, T.; Kawai, Y.; Miyagawa, M.; Tsujisaki, M.; Saito, M. Age-Related Decrease in Cold-Activated Brown Adipose Tissue and Accumulation of Body Fat in Healthy Humans. Obesity 2011, 19, 1755–1760. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.R.; Martinez-Tellez, B.; Sanchez-Delgado, G.; Osuna-Prieto, F.J.; Rensen, P.C.N.; Boon, M.R. Role of Human Brown Fat in Obesity, Metabolism and Cardiovascular Disease: Strategies to Turn Up the Heat. Prog. Cardiovasc. Dis. 2018, 61, 232–245. [Google Scholar] [CrossRef]
- Van Marken Lichtenbelt, W.D.; Vanhommerig, J.W.; Smulders, N.M.; Drossaerts, J.M.A.F.L.; Kemerink, G.J.; Bouvy, N.D.; Schrauwen, P.; Teule, G.J.J. Cold-Activated Brown Adipose Tissue in Healthy Men. N. Engl. J. Med. 2009, 360, 1500–1508. [Google Scholar] [CrossRef]
- Scheele, C.; Nielsen, S. Metabolic Regulation and the Anti-Obesity Perspectives of Human Brown Fat. Redox Biol. 2017, 12, 770–775. [Google Scholar] [CrossRef]
- Froy, O.; Garaulet, M. The Circadian Clock in White and Brown Adipose Tissue: Mechanistic, Endocrine, and Clinical Aspects. Endocr. Rev. 2018, 39, 261–273. [Google Scholar] [CrossRef]
- Stanford, K.I.; Middelbeek, R.J.W.; Townsend, K.L.; An, D.; Nygaard, E.B.; Hitchcox, K.M.; Markan, K.R.; Nakano, K.; Hirshman, M.F.; Tseng, Y.H.; et al. Brown Adipose Tissue Regulates Glucose Homeostasis and Insulin Sensitivity. J. Clin. Investig. 2013, 123, 215–223. [Google Scholar] [CrossRef]
- Ouellet, V.; Labbé, S.M.; Blondin, D.P.; Phoenix, S.; Guérin, B.; Haman, F.; Turcotte, E.E.; Richard, D.; Carpentier, A.C. Brown Adipose Tissue Oxidative Metabolism Contributes to Energy Expenditure during Acute Cold Exposure in Humans. J. Clin. Investig. 2012, 122, 545–552. [Google Scholar] [CrossRef]
- Chen, K.Y.; Brychta, R.J.; Linderman, J.D.; Smith, S.; Courville, A.; Dieckmann, W.; Herscovitch, P.; Millo, C.M.; Remaley, A.; Lee, P.; et al. Brown Fat Activation Mediates Cold-Induced Thermogenesis in Adult Humans in Response to a Mild Decrease in Ambient Temperature. J. Clin. Endocrinol. Metab. 2013, 98, 1218–1223. [Google Scholar] [CrossRef]
- Becher, T.; Palanisamy, S.; Kramer, D.J.; Eljalby, M.; Marx, S.J.; Wibmer, A.G.; Butler, S.D.; Jiang, C.S.; Vaughan, R.; Schöder, H.; et al. Brown Adipose Tissue Is Associated with Cardiometabolic Health. Nat. Med. 2021, 27, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Després, J.P.; Bouchard, C.; Savard, R.; Tremblay, A.; Allard, C. Lack of Relationship between Changes in Adiposity and Plasma Lipids Following Endurance Training. Atherosclerosis 1985, 54, 135–143. [Google Scholar] [CrossRef]
- Pearson, A.M. Muscle Growth and Exercise. Crit. Rev. Food Sci. Nutr. 1990, 29, 167–196. [Google Scholar] [CrossRef] [PubMed]
- Aldiss, P.; Betts, J.; Sale, C.; Pope, M.; Budge, H.; Symonds, M.E. Exercise-Induced ‘Browning’ of Adipose Tissues. Metabolism 2018, 81, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Peppler, W.T.; Anderson, Z.G.; MacRae, L.M.; MacPherson, R.E.K.; Wright, D.C. Habitual Physical Activity Protects against Lipopolysaccharide-Induced Inflammation in Mouse Adipose Tissue. Adipocyte 2017, 6, 1–11. [Google Scholar] [CrossRef]
- Picoli, C.D.C.; Gilio, G.R.; Henriques, F.; Leal, L.G.; Besson, J.C.; Lopes, M.A.; Franzói de Moraes, S.M.; Hernandes, L.; Batista Junior, M.L.; Peres, S.B. Resistance Exercise Training Induces Subcutaneous and Visceral Adipose Tissue Browning in Swiss Mice. J. Appl. Physiol. 2020, 129, 66–74. [Google Scholar] [CrossRef]
- Peppler, W.T.; Townsend, L.K.; Knuth, C.M.; Foster, M.T.; Wright, D.C. Subcutaneous Inguinal White Adipose Tissue Is Responsive to, but Dispensable for, the Metabolic Health Benefits of Exercise. Am. J. Physiol. Endocrinol. Metab. 2018, 314, E66–E77. [Google Scholar] [CrossRef]
- Stallknecht, B.; Vinten, J.; Ploug, T.; Galbo, H. Increased Activities of Mitochondrial Enzymes in White Adipose Tissue in Trained Rats. Am. J. Physiol. Endocrinol. Metab. 1991, 261, E410–E414. [Google Scholar] [CrossRef]
- De Matteis, R.; Lucertini, F.; Guescini, M.; Polidori, E.; Zeppa, S.; Stocchi, V.; Cinti, S.; Cuppini, R. Exercise as a New Physiological Stimulus for Brown Adipose Tissue Activity. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 582–590. [Google Scholar] [CrossRef]
- Vosselman, M.J.; Hoeks, J.; Brans, B.; Pallubinsky, H.; Nascimento, E.B.M.; Van Der Lans, A.A.J.J.; Broeders, E.P.M.; Mottaghy, F.M.; Schrauwen, P.; Van Marken Lichtenbelt, W.D. Low Brown Adipose Tissue Activity in Endurance-Trained Compared with Lean Sedentary Men. Int. J. Obes. 2015, 39, 1696–1702. [Google Scholar] [CrossRef]
- Singhal, V.; Maffazioli, G.D.; Ackerman, K.E.; Lee, H.; Elia, E.F.; Woolley, R.; Kolodny, G.; Cypess, A.M.; Misra, M. Effect of Chronic Athletic Activity on Brown Fat in Young Women. PLoS ONE 2016, 11, e0156353. [Google Scholar] [CrossRef]
- Bachman, E.S.; Dhillon, H.; Zhang, C.Y.; Cinti, S.; Bianco, A.C.; Kobilka, B.K.; Lowell, B.B. BAR Signaling Required for Diet-Induced Thermogenesis and Obesity Resistance. Science 2002, 297, 843–845. [Google Scholar] [CrossRef] [PubMed]
- Bartness, T.J.; Vaughan, C.H.; Song, C.K. Sympathetic and Sensory Innervation of Brown Adipose Tissue. Int. J. Obes. 2010, 34, S36–S42. [Google Scholar] [CrossRef] [PubMed]
- Norheim, F.; Langleite, T.M.; Hjorth, M.; Holen, T.; Kielland, A.; Stadheim, H.K.; Gulseth, H.L.; Birkeland, K.I.; Jensen, J.; Drevon, C.A. The Effects of Acute and Chronic Exercise on PGC-1α, Irisin and Browning of Subcutaneous Adipose Tissue in Humans. FEBS J. 2014, 281, 739–749. [Google Scholar] [CrossRef]
- Tsiloulis, T.; Carey, A.L.; Bayliss, J.; Canny, B.; Meex, R.C.R.; Watt, M.J. No Evidence of White Adipocyte Browning after Endurance Exercise Training in Obese Men. Int. J. Obes. 2018, 42, 721–727. [Google Scholar] [CrossRef] [PubMed]
- MacDougall, J.D.; Tuxen, D.; Sale, D.G.; Moroz, J.R.; Sutton, J.R. Arterial Blood Pressure Response to Heavy Resistance Exercise. J. Appl. Physiol. 1985, 58, 785–790. [Google Scholar] [CrossRef]
- Ruivo, J.A.; Alcântara, P. Hypertension and exercise. Rev. Port. Cardiol. 2012, 31, 151–158. [Google Scholar] [CrossRef]
- Shinoda, K.; Luijten, I.H.N.; Hasegawa, Y.; Hong, H.; Sonne, S.B.; Kim, M.; Xue, R.; Chondronikola, M.; Cypess, A.M.; Tseng, Y.-H.; et al. Genetic and Functional Characterization of Clonally Derived Adult Human Brown Adipocytes. Nat. Med. 2015, 21, 389–394. [Google Scholar] [CrossRef]
- Wang, W.; Seale, P. Control of Brown and Beige Fat Development. Nat. Rev. Mol. Cell Biol. 2016, 17, 691–702. [Google Scholar] [CrossRef]
- Lee, P.; Swarbrick, M.M.; Ho, K.K.Y. Brown Adipose Tissue in Adult Humans: A Metabolic Renaissance. Endocr. Rev. 2013, 34, 413–438. [Google Scholar] [CrossRef]
- Cinti, S. Symposium on “New Perspectives on Adipose Tissue Function”: The Adipose Organ.: Morphological Perspectives of Adipose Tissues. Proc. Nutr. Soc. 2001, 60, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Hamaoka, T.; Nirengi, S.; Fuse, S.; Amagasa, S.; Kime, R.; Kuroiwa, M.; Endo, T.; Sakane, N.; Matsushita, M.; Saito, M.; et al. Near-Infrared Time-Resolved Spectroscopy for Assessing Brown Adipose Tissue Density in Humans: A Review. Front. Endocrinol. 2020, 11, 261. [Google Scholar] [CrossRef] [PubMed]
- Nirengi, S.; Yoneshiro, T.; Sugie, H.; Saito, M.; Hamaoka, T. Human Brown Adipose Tissue Assessed by Simple, Noninvasive near-Infrared Time-Resolved Spectroscopy. Obesity 2015, 23, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Fuse, S.; Nirengi, S.; Amagasa, S.; Homma, T.; Kime, R.; Endo, T.; Sakane, N.; Matsushita, M.; Saito, M.; Yoneshiro, T.; et al. Brown Adipose Tissue Density Measured by Near-Infrared Time-Resolved Spectroscopy in Japanese, across a Wide Age Range. J. Biomed. Opt. 2018, 23, 1. [Google Scholar] [CrossRef]
- Gunadi, S.; Leung, T.S.; Elwell, C.E.; Tachtsidis, I. Spatial Sensitivity and Penetration Depth of Three Cerebral Oxygenation Monitors. Biomed. Opt. Express 2014, 5, 2896. [Google Scholar] [CrossRef]
- Flynn, A.; Li, Q.; Panagia, M.; Abdelbaky, A.; Macnabb, M.; Samir, A.; Cypess, A.M.; Weyman, A.E.; Tawakol, A.; Scherrer-Crosbie, M. Contrast-Enhanced Ultrasound: A Novel Noninvasive, Nonionizing Method for the Detection of Brown Adipose Tissue in Humans. J. Am. Soc. Echocardiogr. 2015, 28, 1247–1254. [Google Scholar] [CrossRef]
- Niwayama, M.; Suzuki, H.; Yamashita, T.; Yasuda, Y. Error Factors in Oxygenation Measurement Using Continuous Wave and Spatially Resolved Near-Infrared Spectroscopy. J. Jpn. Coll. Angiol. 2012, 52, 211–215. [Google Scholar] [CrossRef][Green Version]
- Wood, T.M.; Maddalozzo, G.F.; Harter, R.A. Accuracy of Seven Equations for Predicting 1-RM Performance of Apparently Healthy, Sedentary Older Adults. Meas. Phys. Educ. Exerc. Sci. 2002, 6, 67–94. [Google Scholar] [CrossRef]
- Nirengi, S.; Homma, T.; Inoue, N.; Sato, H.; Yoneshiro, T.; Matsushita, M.; Kameya, T.; Sugie, H.; Tsuzaki, K.; Saito, M.; et al. Assessment of Human Brown Adipose Tissue Density during Daily Ingestion of Thermogenic Capsinoids Using Near-Infrared Time-Resolved Spectroscopy. J. Biomed. Opt. 2016, 21, 091305. [Google Scholar] [CrossRef]
- Motiani, P.; Virtanen, K.A.; Motiani, K.K.; Eskelinen, J.J.; Middelbeek, R.J.; Goodyear, L.J.; Savolainen, A.M.; Kemppainen, J.; Jensen, J.; Din, M.U.; et al. Decreased Insulin-Stimulated Brown Adipose Tissue Glucose Uptake after Short-Term Exercise Training in Healthy Middle-Aged Men. Diabetes Obes. Metab. 2017, 19, 1379–1388. [Google Scholar] [CrossRef]
- Kashimura, O.; Yanagidaira, Y.; Ueda, G. Effect of Physical Activity Training on Enhanced Nonshivering Thermogenesis in Cold Acclimated Rats. Ann. Environ. Sci. Shinshu Univ. 1989, 11, 79–84. [Google Scholar]
- Kuroshima, A.; Habara, Y.; Ohno, T. Noradrenaline-Induced Secretions of Pancreatic Hormones in Cold- and Heat-Acclimated Rats. Jpn. J. Physiol. 1983, 33, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Joyner, M.J.; Wieling, W. Increased Muscle Perfusion Reduces Muscle Sympathetic Nerve Activity during Handgripping. J. Appl. Physiol. 1993, 75, 2450–2455. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Kagaya, A.; Ogita, F.; Shinohara, M. Changes in Muscle Sympathetic Nerve Activity and Calf Blood Flow during Combined Leg and Forearm Exercise. Acta Physiol. Scand. 1992, 146, 449–456. [Google Scholar] [CrossRef]
- Seals, D.R. Sympathetic Neural Discharge and Vascular Resistance during Exercise in Humans. J. Appl. Physiol. 1989, 66, 2472–2478. [Google Scholar] [CrossRef]
- Kashimura, O.; Yanagidaira, Y.; Sakai, A.; Ueda, G. Comparative Study of Urinary Catecholamine Metabolites by Chronic Cold Exposure and Exercise Training. Jpn.J.Biometeor. 1991, 28, 85–93. [Google Scholar] [CrossRef]
- American College of Sports Medicine. Progression Models in Resistance Training for Healthy Adults. Med. Sci. Sports Exerc. 2009, 41, 687–708. [Google Scholar] [CrossRef]
- Yoneshiro, T.; Aita, S.; Matsushita, M.; Kayahara, T.; Kameya, T.; Kawai, Y.; Iwanaga, T.; Saito, M. Recruited Brown Adipose Tissue as an Antiobesity Agent in Humans. J. Clin. Investig. 2013, 123, 3404–3408. [Google Scholar] [CrossRef]
- Kuroiwa, M.; Fuse, S.; Amagasa, S.; Kime, R.; Endo, T.; Kurosawa, Y.; Hamaoka, T. Relationship of Total Hemoglobin in Subcutaneous Adipose Tissue with Whole-Body and Visceral Adiposity in Humans. Appl. Sci. 2019, 9, 2442. [Google Scholar] [CrossRef]
- Schobersberger, W.; Tschann, M.; Hasibeder, W.; Steidl, M.; Herold, M.; Nachbauer, W.; Koller, A. Consequences of 6 Weeks of Strength Training on Red Cell O2 Transport and Iron Status. Eur. J. Appl. Physiol. Occup. Physiol. 1990, 60, 163–168. [Google Scholar] [CrossRef]
- Frontera, W.R.; Meredith, C.N.; O’Reilly, K.P.; Evans, W.J. Strength Training and Determinants of VO2max in Older Men. J. Appl. Physiol. 1990, 68, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Deruisseau, K.C.; Roberts, L.M.; Kushnick, M.R.; Evans, A.M.; Austin, K.; Haymes, E.M. Iron Status of Young Males and Females Performing Weight-Training Exercise. Med. Sci. Sports Exerc. 2004, 36, 241–248. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).