Levels of Trace Elements in the Lens, Aqueous Humour, and Plasma of Cataractous Patients—A Narrative Review
Abstract
1. Introduction
2. Sodium and Potassium
2.1. The Role in Cataractogenesis
2.2. Studies In Vivo
3. Magnesium
3.1. The Role in Cataractogenesis
3.2. Studies In Vivo
4. Calcium
4.1. The Role in Cataractogenesis
4.2. Studies In Vivo
5. Iron
5.1. The Role in Cataractogenesis
5.2. Studies In Vivo
6. Selenium
6.1. The Role in Cataractogenesis
6.2. In Vitro and In Vivo Studies
7. Zinc
7.1. The Role in Cataractogenesis
7.2. Studies In Vivo
8. Copper
8.1. The Role in Cataractogenesis
8.2. Studies In Vivo
9. Toxic Elements
10. Cataracts and Special Diets
11. Discussion
12. Conclusions
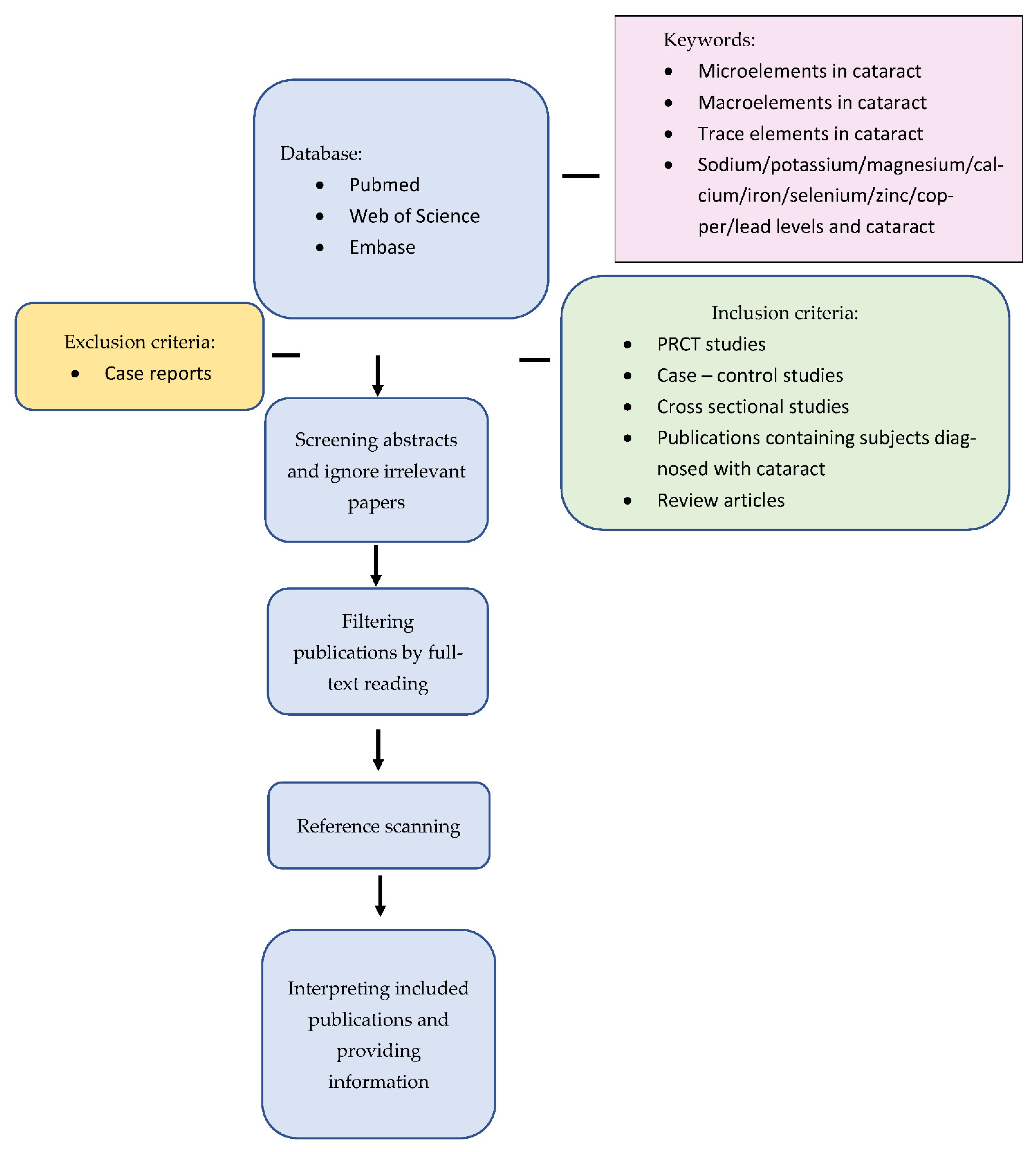
13. Methods of Literature Search
Exclusion Criteria
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Neuhann, I.; Neuhann, L.; Neuhann, T. Die senile Katarakt [Age-related Cataract]. Klin. Monbl. Augenheilkd. 2022, 239, 615–633. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, H.; Pakzad, R.; Yekta, A.; Aghamirsalim, M.; Pakbin, M.; Ramin, S.; Khabazkhoob, M. Global and regional prevalence of age-related cataract: A comprehensive systematic review and meta-analysis. Eye 2020, 34, 1357–1370. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; Wilkins, M.; Kim, T.; Malyugin, B.; Mehta, J.S. Cataracts. Lancet 2017, 390, 600–612. [Google Scholar] [CrossRef]
- GBD 2019 Blindness; Vision Impairment Collaborators; the Vision Loss Expert Group of the Global Burden of Disease Study. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: The Right to Sight: An analysis for the Global Burden of Disease Study. Lancet Glob. Health 2021, 9, e144–e160, Erratum in Lancet Glob Health 2021, 9, e408. [Google Scholar] [CrossRef]
- Ang, M.J.; Afshari, N.A. Cataract and systemic disease: A review. Clin. Exp. Ophthalmol. 2021, 49, 118–127. [Google Scholar] [CrossRef]
- James, E.R. The Etiology of Steroid Cataract. J. Ocul. Pharmacol. Ther. 2007, 23, 403–420. [Google Scholar] [CrossRef]
- Morris, M.S.; Jacques, P.F.; Hankinson, S.E.; Chylack, L.T.; Willett, W.C.; Taylor, A. Moderate alcoholic beverage intake and early nuclear and cortical lens opacities. Ophthalmic Epidemiol. 2004, 11, 53–65. [Google Scholar] [CrossRef]
- Evans, J.R.; Lawrenson, J.G. Antioxidant vitamin and mineral supplements for slowing the progression of age-related macular degeneration. Cochrane Database Syst Rev. 2017, 7, CD000254. [Google Scholar] [CrossRef]
- Młynarczyk, M.; Falkowska, M.; Micun, Z.; Obuchowska, I.; Kochanowicz, J.; Socha, K.; Konopińska, J. Diet, Oxidative Stress, and Blood Serum Nutrients in Various Types of Glaucoma: A Systematic Review. Nutrients 2022, 14, 1421. [Google Scholar] [CrossRef]
- Bunce, G.E. Nutrition and Cataract. Nutr. Rev. 2009, 37, 337–343. [Google Scholar] [CrossRef]
- Brown, G.C.; Brown, M.M.; Menezes, A.; Busbee, B.G.; Lieske, H.B.; Lieske, P.A. Cataract Surgery Cost Utility Revisited in 2012. Ophthalmology 2013, 120, 2367–2376. [Google Scholar] [CrossRef] [PubMed]
- Delamere, N.A.; Tamiya, S. Expression, regulation and function of Na,K-ATPase in the lens. Prog. Retin. Eye Res. 2004, 23, 593–615. [Google Scholar] [CrossRef] [PubMed]
- Mathur, G.; Pai, V. Comparison of serum sodium and potassium levels in patients with senile cataract and age-matched individuals without cataract. Indian J. Ophthalmol. 2016, 64, 446–447. [Google Scholar] [CrossRef]
- Garner, M.H.; Spector, A. ATP hydrolysis kinetics by Na,K-ATPase in cataract. Exp. Eye Res. 1986, 42, 339–348. [Google Scholar] [CrossRef]
- Gupta, J.D.; Harley, J.D. Decreased adenosine triphosphatase activity in human senile cataractous lenses. Exp. Eye Res. 1975, 20, 207–209. [Google Scholar] [CrossRef]
- Nordmann, J.; Klethi, J. Na-K-ATPase activity in the normal aging crystalline lens and in senile cataract. Arch. D’ophtalmol. 1976, 36, 523–528. [Google Scholar]
- Auricchio, G.; Rinaldi, E.; Savastano, S.; Albini, L.; Curto, A.; Landolfo, V. The Na-K-ATPase in relation to the Na, K and taurine levels in the senile cataract. Metab. Pediatr. Ophthalmol. 1980, 4, 15–17. [Google Scholar] [PubMed]
- Kobatashi, S.; Roy, D.; Spector, A. Sodium/potassium ATPase in normal and cataractous human lenses. Curr. Eye Res. 1982, 2, 327–334. [Google Scholar] [CrossRef]
- Laursen, A.B.; Klauber, A.; Jensen, O.A. Human senile cataract and Na–K-ATPase activity in the anterior lens struc-tures with special reference to anterior capsular/subcapsular opacity. Acta. Ophthalmol. 1980, 58, 496–506. [Google Scholar] [CrossRef]
- Pasino, M.; Maraini, G. Cation pump activity and membrane permeability in human senile cataractous lenses. Exp. Eye Res. 1982, 34, 887–893. [Google Scholar] [CrossRef]
- Paterson, C.A.; Delamere, N.; Mawhorter, L.; Cuizon, J.V. Na,K-ATPase in simulated eye bank and cryoextracted rabbit lenses, and human eye bank lenses and cataracts. Investig. Ophthalmol. Vis. Sci. 1983, 24, 1534–1538. [Google Scholar]
- Friedburg, D. Enzyme activity patterns in clear human lenses and in different types of human senile cataracts. In The Human Lens in Relation to Cataract, CIBA Foundation Symposium; El-liott, K., Fitzsimmons, D.W., Eds.; Elsevier: Amsterdam, The Netherlands, 1973; Volume 19, pp. 117–133. [Google Scholar]
- Gandolfi, S.A.; Tomba, M.C.; Maraini, G. 86-Rb Efflux in normal and cataractous human lenses. Curr. Eye Res. 1985, 4, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Cumming, R.; Mitchell, P.; Smith, W. Dietary Sodium Intake and Cataract: The Blue Mountains Eye Study. Am. J. Epidemiol. 2000, 151, 624–626. [Google Scholar] [CrossRef] [PubMed]
- Duncan, G.; Bushell, A. Ion analyses of human cataractous lenses. Exp. Eye Res. 1975, 20, 223–230. [Google Scholar] [CrossRef]
- Davies, P.D.; Duncan, G.; Pynsent, P.B.; Arber, D.L.; Lucas, V.A. Aqueous humor glucose concentration in cataract patients and its effect on the lens. Exp. Eye Res. 1984, 39, 605–609. [Google Scholar] [CrossRef]
- Stanojević-Paović, A.; Hristić, V.; Čuperlović, M.; Jovanović, S.; Krsmanović, J. Macro- and Microelements in the Cataractous Eye Lens. Ophthalmic Res. 1987, 19, 230–234. [Google Scholar] [CrossRef]
- Dilsiz, N.; Olcucu, A.; Atas, M. Determination of calcium, sodium, potassium and magnesium concentrations in human senile cataractous lenses. Cell Biochem. Funct. 2000, 18, 259–262. [Google Scholar] [CrossRef]
- Shukla, N.; Moitra, J.; Trivedi, R. Determination of lead, zinc, potassium, calcium, copper and sodium in human cataract lenses. Sci. Total Environ. 1996, 181, 161–165. [Google Scholar] [CrossRef]
- Mirsamadi, M.; Nourmohammadi, I.; Imamian, M. Comparative study of serum Na+ and K+ levels in senile cataract patients and normal individuals. Int. J. Med. Sci. 2004, 1, 165–169. [Google Scholar] [CrossRef]
- Tavani, A. Food and nutrient intake and risk of cataract. Ann. Epidemiol. 1996, 6, 41–46. [Google Scholar] [CrossRef]
- Miglior, S.; Marighi, P.E.; Musicco, M.; Balestreri, C.; Nicolosi, A.; Orzalesi, N. Risk factors for cortical, nuclear, posterior subcapsular and mixed cataract: A case-control study. Ophthalmic Epidemiol. 1994, 1, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Shoenfeld, E.R.; Leske, M.C.; Wu, S.Y. Recent epidemiologic studies on nutrition and cataract in India, Italy and the United States. J. Am. College Nutr. 1993, 12, 521–526. [Google Scholar] [CrossRef]
- Barber, C.W. Free amino acids in senile cataractous lenses: Possible osmotic etiology. Investig. Ophthalmol. 1963, 7, 564–567. [Google Scholar]
- Phillips, C.I.; Bartholomew, R.S.; Clayton, R.; Duffy, J. Cataract: A search for associated or causative factors. Excerpta Med. 1980, 34, 19–25. [Google Scholar]
- Donnelly, C.; Seth, J.; Clayton, R.; Phillips, C.; Cuthbert, J. Some Plasma Constituents Correlate with Human Cataract Location and Nuclear Colour. Ophthalmic Res. 1997, 29, 207–217. [Google Scholar] [CrossRef]
- Agarwal, R.; Iezhitsa, I.; Agarwal, P.; Spasov, A. Magnesium deficiency: Does it have a role to play in cataractogenesis? Exp. Eye Res. 2012, 101, 82–89. [Google Scholar] [CrossRef]
- Agarwal, R.; Iezhitsa, I.; Agarwal, P.; Spasov, A.A. Mechanisms of cataractogenesis in the presence of magnesium deficiency. Magnes. Res. 2013, 26, 2–8. [Google Scholar] [CrossRef]
- Nagai, N.; Fukuhata, T.; Ito, Y. Effect of Magnesium Deficiency on Intracellular ATP Levels in Human Lens Epithelial Cells. Biol. Pharm. Bull. 2007, 30, 6–10. [Google Scholar] [CrossRef][Green Version]
- Bara, M.; Guiet-Bara, A.; Durlach, J. Regulation of sodium and potassium pathways by magnesium in cell mem-branes. Magnes. Res. 1993, 6, 167–177. [Google Scholar]
- Kao, C.L.; Chou, C.K.; Tsai, D.C.; Hsu, W.M.; Liu, J.H.; Wang, C.S.; Lin, J.C.; Wu, C.C.; Peng, C.H.; Chang, C.J.; et al. Nitric Oxide Levels Aqueous Humor Cataract. Patients. J. Cataract. Refract. Surg. 2002, 28, 507–512. [Google Scholar] [CrossRef]
- Örnek, K.; Karel, F.; Büyükbingöl, Z. May nitric oxide molecule have a role in the pathogenesis of human cataract? Exp. Eye Res. 2002, 76, 23–27. [Google Scholar] [CrossRef]
- Paik, D.C.; Dillon, J. The Nitrite/Alpha Crystallin Reaction: A Possible Mechanism in Lens Matrix Damage. Exp. Eye Res. 2000, 70, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Donma, O.; Yorulmaz, E.; Pekel, H.; Suyugül, N. Blood and lens lipid peroxidation and antioxidant status in normal individuals, senile and diabetic cataractous patients. Curr. Eye Res. 2002, 25, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Shumiya, S. Establishment of the hereditary cataract rat strain (SCR) and genetic analysis. Lab. Anim. Sci. 1995, 45, 671–673. [Google Scholar]
- Shumiya, S.; Nagase, S. Breeding of hereditary cataract rat. Proc. Jpn. Assoc. Anim. Model Hum. Dis. 1988, 4, 30. [Google Scholar]
- Burge, W.E. Analysis of the ash of the normal and the cataractous lens. Arch. Ophthalmol. 1909, 38, 435–450. [Google Scholar]
- Chen, H.; Zhou, J. Effects of Sodium Selenite on Oxidative Damage in the Liver, Kidney and Brain in a Selenite Cataract Rat Model. Biol. Trace Elem. Res. 2020, 197, 533–543. [Google Scholar] [CrossRef]
- Rasi, V.; Costantini, S.; Moramarco, A.; Giordano, R.; Giustolisi, R.; Balacco Gabrieli, C. Inorganic element concentra-tions in cataractous human lenses. Ann. Ophthalmol. 1992, 24, 459–464. [Google Scholar]
- Jedziniak, J.L. On the calcium concentration of cataractous and normal human lenses and protein fractions of catarac-tous lenses. Exp. Eye Res. 1976, 23, 325–332. [Google Scholar] [CrossRef]
- Duncan, G.; van Heyningen, R. Distribution of non-diffusible calcium and sodium in normal and cataractous human lenses. Exp. Eye Res. 1977, 25, 183–193. [Google Scholar] [CrossRef]
- Hightower, K.R.; Reddy, V.N. Calcium content and distribution in human cataract. Exp. Eye Res. 1982, 34, 413–421. [Google Scholar] [CrossRef]
- Ringvold, A.; Sagen, E.; Bjerve, K.S.; Folling, I. The calcium and magnesium content of the human lens and aqueous humour. Acta Ophthalmol. 1988, 66, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Duncan, G.; Jacob, T. Calcium and the Physiology of Cataract. Ciba Found Symp 2008, 106, 132–162. [Google Scholar] [CrossRef]
- Borchman, D.; Patenon, C.A.; Delamere, N.A. Ca2+-ATPase activity in the human lens. Curr. Eye Res. 1989, 8, 1049–1054. [Google Scholar] [CrossRef]
- Paterson, C.A.; Zeng, J.; Husseini, Z.; Borchman, D.; Delamere, N.; Garland, D.; Jimenez-Asensio, J. Calcium ATPase activity and membrane structure in clear and cataractous human lenses. Curr. Eye Res. 1997, 16, 333–338. [Google Scholar] [CrossRef]
- Lucas, V.A.; Duncan, G.; Davies, P.D. Membrane permeability characteristics of perfused human senile cataractous lenses. Exp. Eye Res. 1986, 42, 151–165. [Google Scholar] [CrossRef]
- Duncan, G.; Jacob, T.J. Human cataract formation. CIBA Found Symp. 1984, 106, 132–148. [Google Scholar]
- Tang, D.; Borchman, D.; Yappert, M.C.; Vrensen, G.F.J.M.; Rasi, V. Influence of Age, Diabetes, and Cataract on Calcium, Lipid-Calcium, and Protein-Calcium Relationships in Human Lenses. Investig. Opthalmol. Vis. Sci. 2003, 44, 2059–2066. [Google Scholar] [CrossRef]
- Adams, D.R. The rôle of calcium in senile cataract. Biochem. J. 1929, 23, 902–912. [Google Scholar] [CrossRef]
- Delamere, N.; Paterson, C.A.; Holmes, D.L. Hypocalcemic cataract. I. An animal model and cation distribution study. Metab. Pediatr. Ophthalmol. 1981, 5, 77–82. [Google Scholar]
- Bernardini, G.; Peracchia, C. Gap junction crystallization in lens fibers after an increase in cell calcium. Investig. Ophthalmol. Vis. Sci. 1981, 21, 291–299. [Google Scholar]
- Spector, A.; Adams, D.; Krul, K. Calcium and high molecular weight protein aggregates in bovine and human lens. Investig. Ophthalmol. 1974, 13, 982–990. [Google Scholar]
- Van Heyningen, R. The human lens. I. A comparison of cataracts extracted in Oxford (England) and Shikarpur (W. Pakistan). Exp. Eye Res. 1972, 13, 136–147. [Google Scholar] [CrossRef]
- Clayton, R.M.; Cuthbert, J.; Seth, J.; Phillips, C.I.; Duffy, J.; Bartholomew, R.S.; Reid, J.M. Epidemiological and other studies in the assessment of factors contributing to cataractogenesis. Ciba Found Symp 1981, 106, 25–40. [Google Scholar]
- Nemet, A.Y.; Hanhart, J.; Kaiserman, I.; Vinker, S. Are cataracts associated with osteoporosis? Clin. Ophthalmol. 2013, 7, 2079–2084. [Google Scholar] [CrossRef]
- Gutteridge, J.M.C.; Rowley, D.A.; Halliwell, B. Superoxide-dependent formation of hydroxyl radicals in the presence of iron salts. Detection of ‘free’ iron in biological systems by using bleomycin-dependent degradation of DNA. Biochem. J. 1981, 199, 263–265. [Google Scholar] [CrossRef]
- Lou, M.F. Redox regulation in the lens. Prog. Retin. Eye Res. 2003, 22, 657–682. [Google Scholar] [CrossRef]
- Spector, A. Oxidative stress-induced cataract: Mechanism of action. FASEB J. 1995, 9, 1173–1182. [Google Scholar] [CrossRef]
- Spector, A. Review: Oxidative Stress and Disease. J. Ocul. Pharmacol. Ther. 2000, 16, 193–201. [Google Scholar] [CrossRef]
- Truscott, R.J. Age-related nuclear cataract—Oxidation is the key. Exp. Eye Res. 2005, 80, 709–725. [Google Scholar] [CrossRef]
- Kleiman, N.; Wang, R.-R.; Spector, A. Hydrogen peroxide-induced DNA damage in bovine lens epithelial cells. Mutat. Res. Toxicol. 1990, 240, 35–45. [Google Scholar] [CrossRef]
- Garland, D. Role of site-specific, metal-catalyzed oxidation in lens aging and cataract: A hypothesis. Exp. Eye Res. 1990, 50, 677–682. [Google Scholar] [CrossRef]
- McDermott, M.J.; Chiesa, R.; Spector, A. Fe2+ oxidation of α-crystallin produces a 43,000 Da aggregate composed of A and B chains cross-linked by non-reducible covalent bonds. Biochem. Biophys. Res. Commun. 1988, 157, 626–631. [Google Scholar] [CrossRef]
- McGahan, M. Does the lens serve as a ‘sink’ for iron during ocular inflammation? Exp. Eye Res. 1992, 54, 525–530. [Google Scholar] [CrossRef]
- Goralska, M.; Ferrell, J.; Harned, J.; Lall, M.; Nagar, S.; Fleisher, L.; McGahan, M. Iron metabolism in the eye: A review. Exp. Eye Res. 2009, 88, 204–215. [Google Scholar] [CrossRef]
- García-Castiñeiras, S. Iron, the retina and the lens: A focused review. Exp. Eye Res. 2010, 90, 664–678. [Google Scholar] [CrossRef]
- Nemeth, E. Iron regulation and erythropoiesis. Curr. Opin. Hematol. 2008, 15, 169–175. [Google Scholar] [CrossRef]
- Dawczynski, J.; Blum, M.; Winnefeld, K.; Strobel, J. Increased Content of Zinc and Iron in Human Cataractous Lenses. Biol. Trace Element Res. 2002, 90, 15–24. [Google Scholar] [CrossRef]
- Garner, B.; Roberg, K.; Qian, M.; Eaton, J.W.; Truscott, R.J. Distribution of Ferritin and Redox-active Transition Metals in Normal and Cataractous Human Lenses. Exp. Eye Res. 2000, 71, 599–607. [Google Scholar] [CrossRef]
- Leske, M.C.; Wu, S.Y.; Hyman, L.; Sperduto, R.; Underwood, B.; Chylack, L.T.; Milton, R.C.; Srivastava, S.; Ansari, N. Biochemical factors in the lens opacities. Case-control study. The Lens Opacities Case-Control Study Group. Arch. Ophthalmol. 1995, 113, 1113–1119. [Google Scholar] [CrossRef]
- Aydin, E.; Cumurcu, T.; Özuğurlu, F.; Ozyurt, H.; Sahinoglu, S.; Mendil, D.; Hasdemir, E. Levels of Iron, Zinc, and Copper in Aqueous Humor, Lens, and Serum in Nondiabetic and Diabetic Patients: Their Relation to Cataract. Biol. Trace Element Res. 2005, 108, 33–41. [Google Scholar] [CrossRef]
- Dai, J.; Liu, H.; Zhou, J.; Huang, K. Selenoprotein R Protects Human Lens Epithelial Cells against D-Galactose-Induced Apoptosis by Regulating Oxidative Stress and Endoplasmic Reticulum Stress. Int. J. Mol. Sci. 2016, 17, 231. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Lu, Y. Selenium supplementation can slow the development of naphthalene cataract. Curr. Eye Res. 2012, 37, 163–169. [Google Scholar] [CrossRef]
- Arnér, E.S. Selenoproteins—What unique properties can arise with selenocysteine in place of cysteine? Exp. Cell Res. 2010, 316, 1296–1303. [Google Scholar] [CrossRef]
- Karaküçük, S.; Mirza, G.E.; Ekinciler, F.; Saraymen, R.; Karaküçük, I.; Üstdal, M. Selenium concentrations in serum, lens and aqueous humour of patients with senile cataract. Acta Ophthalmol. Scand. 2009, 73, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Flohé, L. Selenium, Selenoproteins and Vision. Dev. Ophthalmol. 2005, 38, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Duffield, A.J.; Thomson, C.D.; Hill, K.E.; Williams, S. An estimation of selenium requirements for New Zealanders. Am. J. Clin. Nutr. 1999, 70, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Lemire, M.; Fillion, M.; Frenette, B.; Mayer, A.; Philibert, A.; Passos, C.J.S.; Guimaraes, J.R.D.; Barbosa, F.; Mergler, N. Selenium and Mercury in the Brazilian Amazon: Opposing Influences on Age-Related Cataracts. Environ. Health Perspect. 2010, 118, 1584–1589. [Google Scholar] [CrossRef]
- Post, M.; Lubiński, W.; Lubiński, J.; Krzystolik, K.; Baszuk, P.; Muszyńska, M.; Marciniak, W. Serum selenium levels are associated with age-related cataract. Ann. Agric. Environ. Med. 2018, 25, 443–448. [Google Scholar] [CrossRef]
- Dawczynski, J.; Winnefeld, K.; Königsdörffer, E.; Augsten, R.; Blum, M.; Strobel, J. Selen und Katarakt Risikofaktor oder sinnvolle Nahrungsergänzung? [Selenium and cataract–risk factor or useful dietary supplement?]. Klin. Monbl. Augenheilkd. 2006, 223, 675–680. [Google Scholar] [CrossRef]
- Srivastava, V.K.; Varshney, N.; Pandey, D.C. Role of trace elements in senile cataract. Acta Ophthalmol. 2009, 70, 839–841. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S. Clinical and biochemical manifestations of zinc deficiency in human subjects. J. Am. Coll. Nutr. 1985, 4, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, V.K.; Chaturvedi, N.; Garg, M.; Maq, Z.; Padney, D.C. Copper and zinc in human senile cataract. Curr. Sci. 1988, 57, 1288. [Google Scholar]
- Soares, F.M.; Nogueira, N.D.N.; Marreiro, D.D.N.; De Carvalho, C.M.R.G.; Monte, S.J.H.D.; Neto, J.M.M.; Rocha, V.D.S.; Cardoso, B.V.S. Concentrações plasmáticas e eritrocitárias de zinco em idosos portadores e não-portadores de catarata senil em um serviço oftalmológico especializado de Teresina-Piauí. [Plasma and erythrocyte zinc concentrations in elderly patients with and without senile cataract in a tertiary eye care center at Teresina-Piauí]. Arq. Bras. Oftalmol. 2008, 71, 674–678. [Google Scholar] [CrossRef]
- Gündüz, G.; Gündüz, F.; Yucel, I.; Şentürk, K. Levels of Zinc and Magnesium in Senile and Diabetic Senile Cataractous Lenses. Biol. Trace Element Res. 2003, 95, 107–112. [Google Scholar] [CrossRef]
- Sperduto, R.D.; Hu, T.-S.; Milton, R.C.; Zhao, J.-L.; Everett, D.F.; Cheng, Q.-F.; Blot, W.J.; Bing, L.; Taylor, P.R.; Jun-Yao, L.; et al. The Linxian Cataract Studies. Two nutrition intervention trials. Arch. Ophthalmol. 1993, 111, 1246–1253. [Google Scholar] [CrossRef]
- Lin, J. Pathophysiology of cataracts: Copper ion and peroxidation in diabetics. Jpn. J. Ophthalmol. 1997, 41, 130–137. [Google Scholar] [CrossRef]
- Nath, R.; Srivastava, S.K.; Singh, K. Copper levels in human cataract lens. Ind. J. Exp. Biol. 1969, 7, 25–28. [Google Scholar]
- Balaji, M.; Sasikala, K.; Ravindran, T. Copper levels in human mixed, nuclear brunescance, and posterior subcapsular cataract. Br. J. Ophthalmol. 1992, 76, 668–669. [Google Scholar] [CrossRef][Green Version]
- Racz, P.; Erdöhelyi, A. Cadmium, Lead and Copper Concentrations in Normal and Senile Cataractous Human Lenses. Ophthalmic Res. 1988, 20, 10–13. [Google Scholar] [CrossRef]
- Cekic, O. Copper, lead, cadmium and calcium in cataractous lenses. Ophthalmic Res. 1998, 30, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Swanson, A.A.; Truesdale, A.W. Elemental analysis in normal and cataractous human lens tissue. Biochem. Biophys. Res. Commun. 1971, 45, 1488–1496. [Google Scholar] [CrossRef]
- Cook, C.S.; McGahan, M.C. Copper concentration in cornea, iris, normal and cataractous lenses and intraocular fluid of vertebrates. Curr. Eye Res. 1986, 5, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Dolar-Szczasny, J.; Święch, A.; Flieger, J.; Tatarczak-Michalewska, M.; Niedzielski, P.; Proch, J.; Majerek, D.; Kawka, J.; Mackiewicz, J. Levels of Trace Elements in the Aqueous Humor of Cataract Patients Measured by the Inductively Coupled Plasma Optical Emission Spectrometry. Molecules 2019, 24, 4127. [Google Scholar] [CrossRef] [PubMed]
- Schaumberg, D.A.; Mendes, F.; Balaram, M.; Dana, M.R.; Sparrow, D.; Hu, H. Accumulated Lead Exposure and Risk of Age-Related Cataract in Men. JAMA 2004, 292, 2750–2754, Erratum in JAMA 2005, 293, 425. [Google Scholar] [CrossRef] [PubMed]
- Neal, R.; Cooper, K.; Gurer-Orhan, H.; Ercal, N. Effects of N-acetylcysteine and 2,3-dimercaptosuccinic acid on lead induced oxidative stress in rat lenses. Toxicology 1998, 130, 167–174. [Google Scholar] [CrossRef][Green Version]
- Walczyk, T.; Wick, J.Y. The ketogenic diet: Making a comeback. Consult Pharm. 2017, 32, 388–396. [Google Scholar] [CrossRef]
- Zhu, H.; Bi, D.; Zhang, Y.; Kong, C.; Du, J.; Wu, X.; Wei, Q.; Qin, H. Ketogenic diet for human diseases: The underlying mechanisms and potential for clinical implementations. Sig. Transduct. Target. Ther. 2022, 7, 11. [Google Scholar] [CrossRef]
- Huttenlocher, P. Ketonemia and seizures: Metabolic and anticonvulsant effects of two ketogenic diets in childhood epilepsy. Pediatr. Res. 1976, 10, 536–540. [Google Scholar] [CrossRef]
- Liu, Y.-M.C.; Williams, S.; Basualdo-Hammond, C.; Stephens, D.; Curtis, R. A prospective study: Growth and nutritional status of children treated with the ketogenic diet. J. Am. Diet. Assoc. 2003, 103, 707–712. [Google Scholar] [CrossRef]
- Crosby, L.; Davis, B.; Joshi, S.; Jardine, M.; Paul, J.; Neola, M.; Barnard, N.D. Ketogenic Diets and Chronic Disease: Weighing the Benefits Against the Risks. Front Nutr. 2021, 8, 702802. [Google Scholar] [CrossRef] [PubMed]
- Prudencio, M.B.; de Lima, P.A.; Murakami, D.K.; de Brito Sampaio, L.P.; Damasceno, N.R.T. Micronutrient supplementation needs more attention in patients with refractory epilepsy under ketogenic diet treatment. Nutrition 2021, 86, 111158. [Google Scholar] [CrossRef] [PubMed]
- Christodoulides, S.S.; Neal, E.G.; Fitzsimmons, G.; Chaffe, H.M.; Jeanes, Y.M.; Aitkenhead, H.; Cross, J.H. The effect of the classical and medium chain triglyceride ketogenic diet on vitamin and mineral levels. J. Hum. Nutr. Diet 2011, 25, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Zupec-Kania, B.; Zupanc, M.L. Long-term management of the ketogenic diet: Seizure monitoring, nutrition, and supplementation. Epilepsia 2008, 49, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Bergqvist, A.; Chee, C.; Lutchka, L.; Rychik, J.; Stallings, V. Selenium Deficiency Associated with Cardiomyopathy: A Complication of the Ketogenic Diet. Epilepsia 2003, 44, 618–620. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Pinho, J.; Borges, C.; Teixeira Santos, M.; Santos, A.; Graça, P. Guidelines for a Healthy Vegetarian Diet; Direção-Geral da Saúde: Lisbon, Portugal, 2015. [Google Scholar]
- Neufingerl, N.; Eilander, A. Nutrient Intake and Status in Adults Consuming Plant-Based Diets Compared to Meat-Eaters: A Systematic Review. Nutrients 2022, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Bakaloudi, D.R.; Halloran, A.; Rippin, H.L.; Oikonomidou, A.C.; Dardavesis, T.I.; Williams, J.; Wickramasinghe, K.; Breda, J.; Chourdakis, M. Intake and adequacy of the vegan diet. A systematic review of the evidence. Clin. Nutr. 2021, 40, 3503–3521. [Google Scholar] [CrossRef]
- Allès, B.; Baudry, J.; Méjean, C.; Touvier, M.; Péneau, S.; Hercberg, S.; Kesse-Guyot, E. Comparison of Sociodemographic and Nutritional Characteristics between Self-Reported Vegetarians, Vegans, and Meat-Eaters from the NutriNet-Santé Study. Nutrients 2017, 9, 1023. [Google Scholar] [CrossRef]
- Davey, G.; Spencer, E.; Appleby, P.; Allen, N.; Knox, K.; Key, T. EPIC–Oxford: Lifestyle characteristics and nutrient intakes in a cohort of 33 883 meat-eaters and 31 546 non meat-eaters in the UK. Public Health Nutr. 2003, 6, 259–268. [Google Scholar] [CrossRef]
- Johnstone, A.M. Safety and efficacy of high-protein diets for weight loss. Proc. Nutr. Soc. 2012, 71, 339–349. [Google Scholar] [CrossRef]
- Gwin, J.A.; Karl, J.P.; Lutz, L.J.; Gaffney-Stomberg, E.; McClung, J.P.; Pasiakos, S.M. Higher Protein Density Diets Are Associated With Greater Diet Quality and Micronutrient Intake in Healthy Young Adults. Front. Nutr. 2019, 6, 59. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.R.; Johnson, L.K.; Fariba Roughead, Z.K. Dietary protein and calcium interact to influence calcium retention: A controlled feeding study. Am. J. Clin. Nutr. 2009, 89, 1357–1365. [Google Scholar] [CrossRef] [PubMed]
- Braakhuis, A.J.; Donaldson, C.I.; Lim, J.C.; Donaldson, P.J. Nutritional Strategies to Prevent Lens Cataract: Current Status and Future Strategies. Nutrients 2019, 11, 1186. [Google Scholar] [CrossRef] [PubMed]
- Christen, W.G.; Glynn, R.J.; Gaziano, J.M.; Darke, A.K.; Crowley, J.J.; Goodman, P.J.; Lippman, S.M.; Lad, T.E.; Bearden, J.D.; Goodman, G.E.; et al. Age-related cataract in men in the selenium and vitamin e cancer prevention trial eye endpoints study: A randomized clinical trial. JAMA Ophthalmol. 2015, 133, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Milton, R.C.; Sperduto, R.D.; Clemons, T.E.; Ferris, F.L., 3rd. Age-Related Eye Disease Study Research Group. Centrum use and progression of age-related cataract in the Age-Related Eye Disease Study: A propensity score approach. AREDS report No. 21. Ophthalmology 2006, 113, 1264–1270. [Google Scholar] [PubMed]
| References | Examined Factor | Likely Impact on Risk of Developing Cataracts |
|---|---|---|
| [12,23,29,30,31,32,33,34] | High sodium intake | ↑ |
| [33,45] | Low magnesium intake | ↓ |
| [64,65] | Low calcium intake | ↑ |
| [80] | Low iron intake | ↑ |
| [86] | High selenium intake | no evidence |
| [96] | Low zinc intake | ↑ |
| [105] | High lead exposure | ↑ |
| Element | References | Examined Factor | Risk of Developing Cataracts |
|---|---|---|---|
| Sodium | [24,25,26,27,28] | High lens level | ↑ |
| [12,23,29,30,31,32,33,34] | High plasma level | ↑ | |
| [29] | High aqueous humour level | ↑ | |
| Potassium | [26,27,28] | Low lens level | ↑ |
| [35] | Low/High plasma level | ↑ | |
| Magnesium | [27,36,37,38] | Low lens level | ↑ |
| Calcium | [24,26,27,28,46,47,48,49,50,51,52,57,61,62,63] | High lens level | ↑ |
| [60] | Low lens level | ↑ | |
| [59] | High plasma and aqueous humour level | ↑ | |
| Iron | [76,77,78] | High lens level | ↑ |
| Selenium | [81,86,87,88] | Low lens level | ↑ |
| [90] | High lens level | ↑ | |
| [89] | Low plasma level | ↑ | |
| Zinc | [48,89] | High lens level | ↑ |
| [26] | Low aqueous humour level | ↑ | |
| [94] | Low plasma level | no impact | |
| Copper | [28,81,97,98,99,100,101] | High lens level | ↑ |
| Toxic elements | [104] | High aqueous humour thallium, tellurium, caesium, lead, aluminium, phosphorus level | ↑ |
| [28,98,99,105,106,107] | High lens lead level | ↑ |
| Compartment | Examined Factor | References | Risk of Developing Cataracts |
|---|---|---|---|
| Lens | High sodium level | [24,25,26,27,28] | ↑ |
| Low potassium level | [26,27,28] | ↑ | |
| Low magnesium level | [27,36,37,38] | ↑ | |
| High calcium level | [24,26,27,28,46,47,48,49,50,51,52,57,61,62,63] | ↑ | |
| Low calcium level | [60] | ↑ | |
| High iron level | [76,77,78] | ↑ | |
| Low selenium level | [81,86,87,88] | ↑ | |
| High selenium level | [90] | ↑ | |
| High zinc level | [48,89] | ↑ | |
| High copper level | [28,81,97,98,99,100,101] | ↑ | |
| High lead level | [28,98,99,105,106,107] | ↑ | |
| Plasma | High sodium level | [12,23,29,30,31,32,33,34] | ↑ |
| Low/High potassium level | [35] | ↑ | |
| High calcium level | [59] | ↑ | |
| Low selenium level | [89] | ↑ | |
| Low zinc level | [94] | no impact | |
| Aqueous humour | High sodium level | [29] | ↑ |
| High calcium level | [59] | ↑ | |
| Low zinc level | [26] | ↑ | |
| High thallium, tellurium, caesium, lead, aluminium, phosphorus level | [104] | ↑ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Micun, Z.; Falkowska, M.; Młynarczyk, M.; Kochanowicz, J.; Socha, K.; Konopińska, J. Levels of Trace Elements in the Lens, Aqueous Humour, and Plasma of Cataractous Patients—A Narrative Review. Int. J. Environ. Res. Public Health 2022, 19, 10376. https://doi.org/10.3390/ijerph191610376
Micun Z, Falkowska M, Młynarczyk M, Kochanowicz J, Socha K, Konopińska J. Levels of Trace Elements in the Lens, Aqueous Humour, and Plasma of Cataractous Patients—A Narrative Review. International Journal of Environmental Research and Public Health. 2022; 19(16):10376. https://doi.org/10.3390/ijerph191610376
Chicago/Turabian StyleMicun, Zuzanna, Martyna Falkowska, Maryla Młynarczyk, Jan Kochanowicz, Katarzyna Socha, and Joanna Konopińska. 2022. "Levels of Trace Elements in the Lens, Aqueous Humour, and Plasma of Cataractous Patients—A Narrative Review" International Journal of Environmental Research and Public Health 19, no. 16: 10376. https://doi.org/10.3390/ijerph191610376
APA StyleMicun, Z., Falkowska, M., Młynarczyk, M., Kochanowicz, J., Socha, K., & Konopińska, J. (2022). Levels of Trace Elements in the Lens, Aqueous Humour, and Plasma of Cataractous Patients—A Narrative Review. International Journal of Environmental Research and Public Health, 19(16), 10376. https://doi.org/10.3390/ijerph191610376








