Benzene and NO2 Exposure during Pregnancy and Preterm Birth in Two Philadelphia Hospitals, 2013–2017
Abstract
:1. Introduction
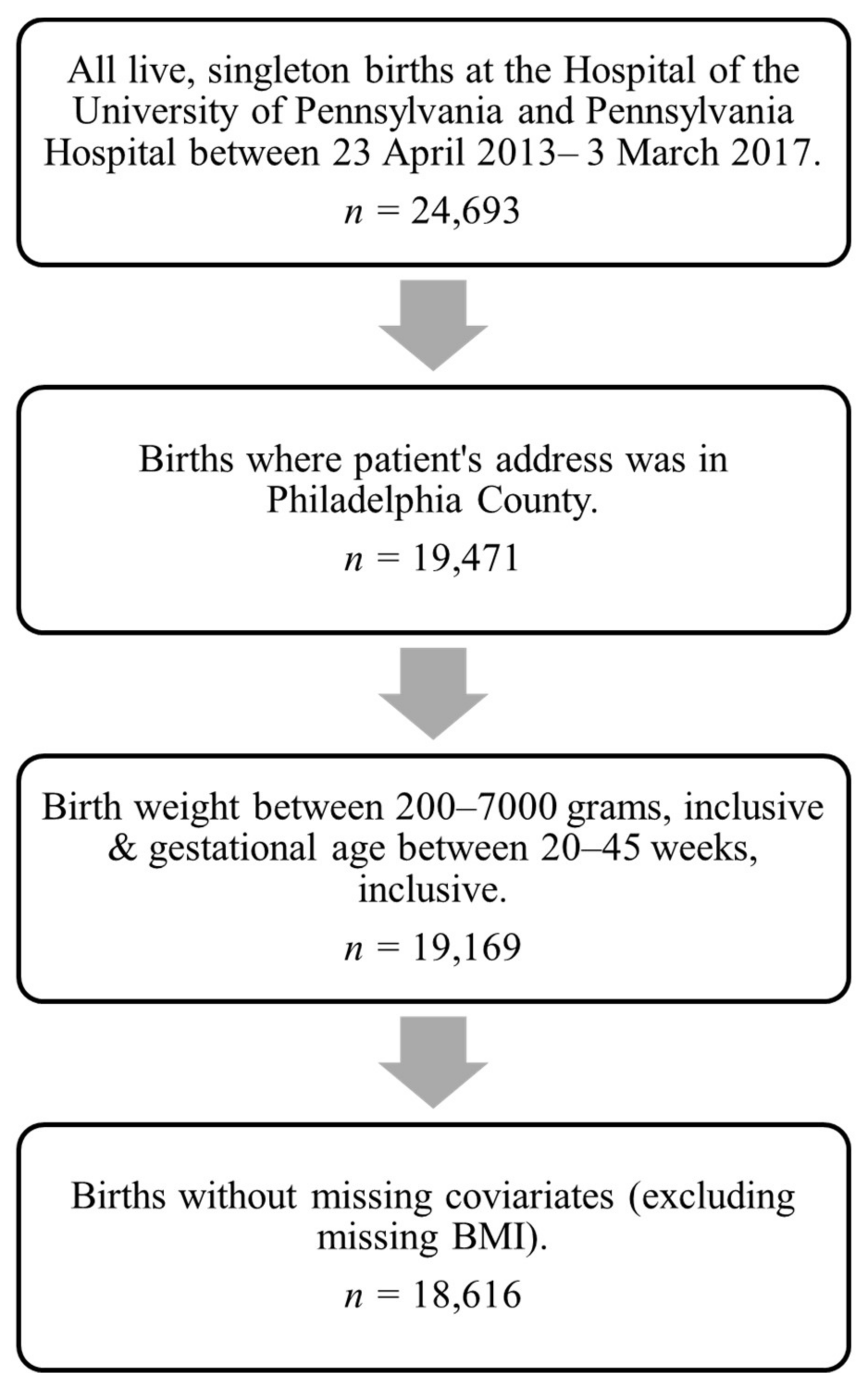
2. Materials and Methods
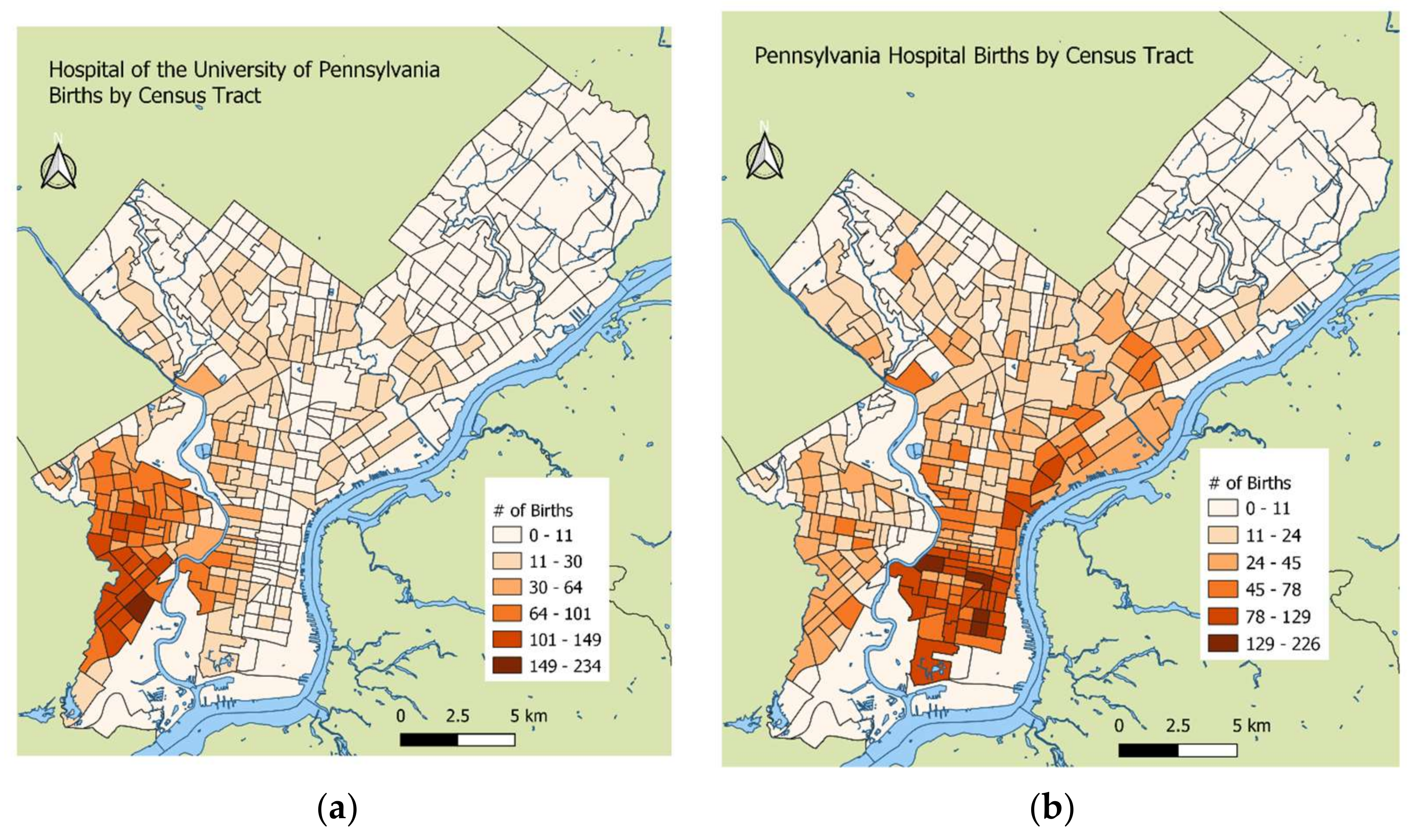
2.1. Study Population
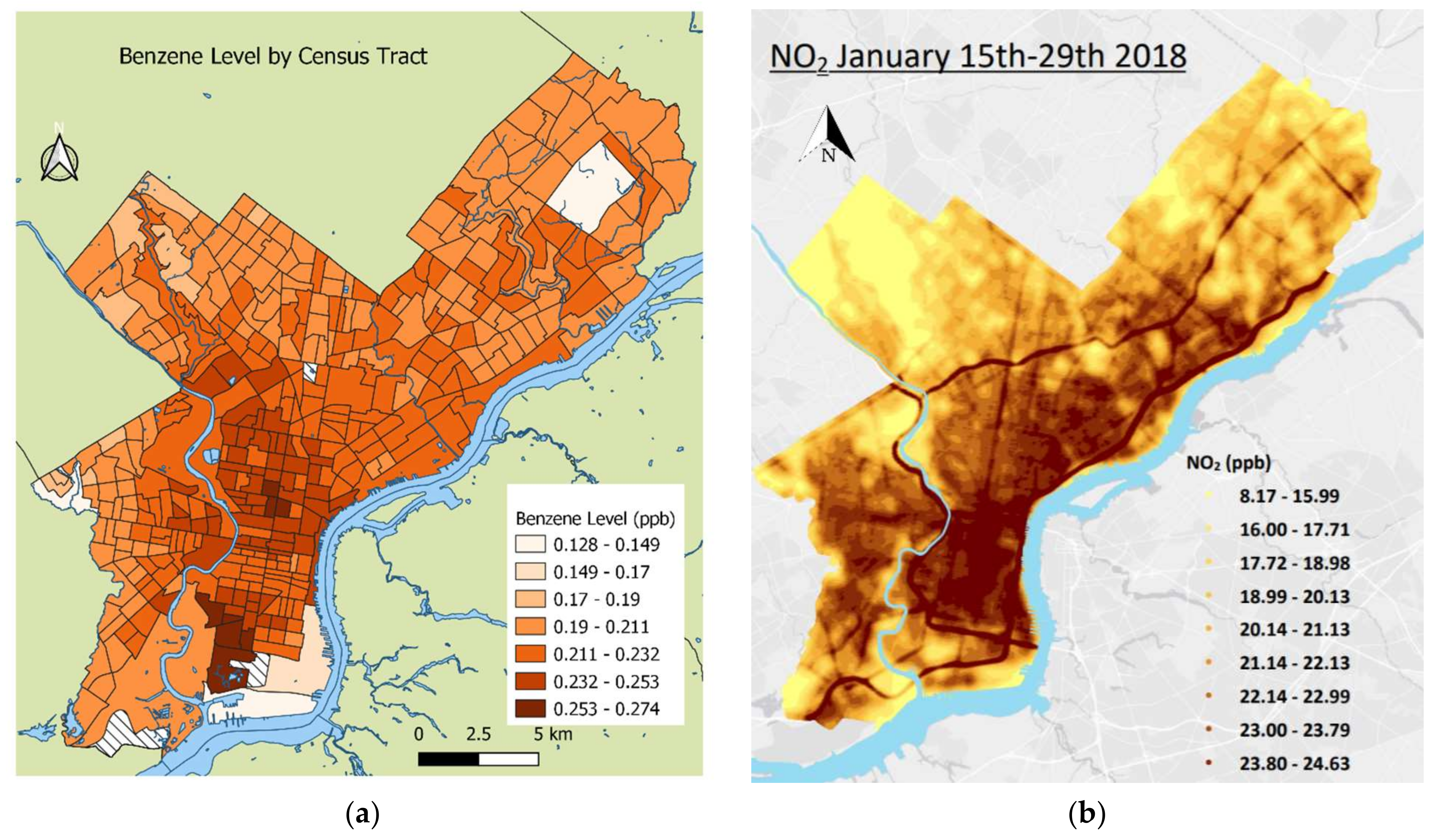
2.2. Exposure Estimates
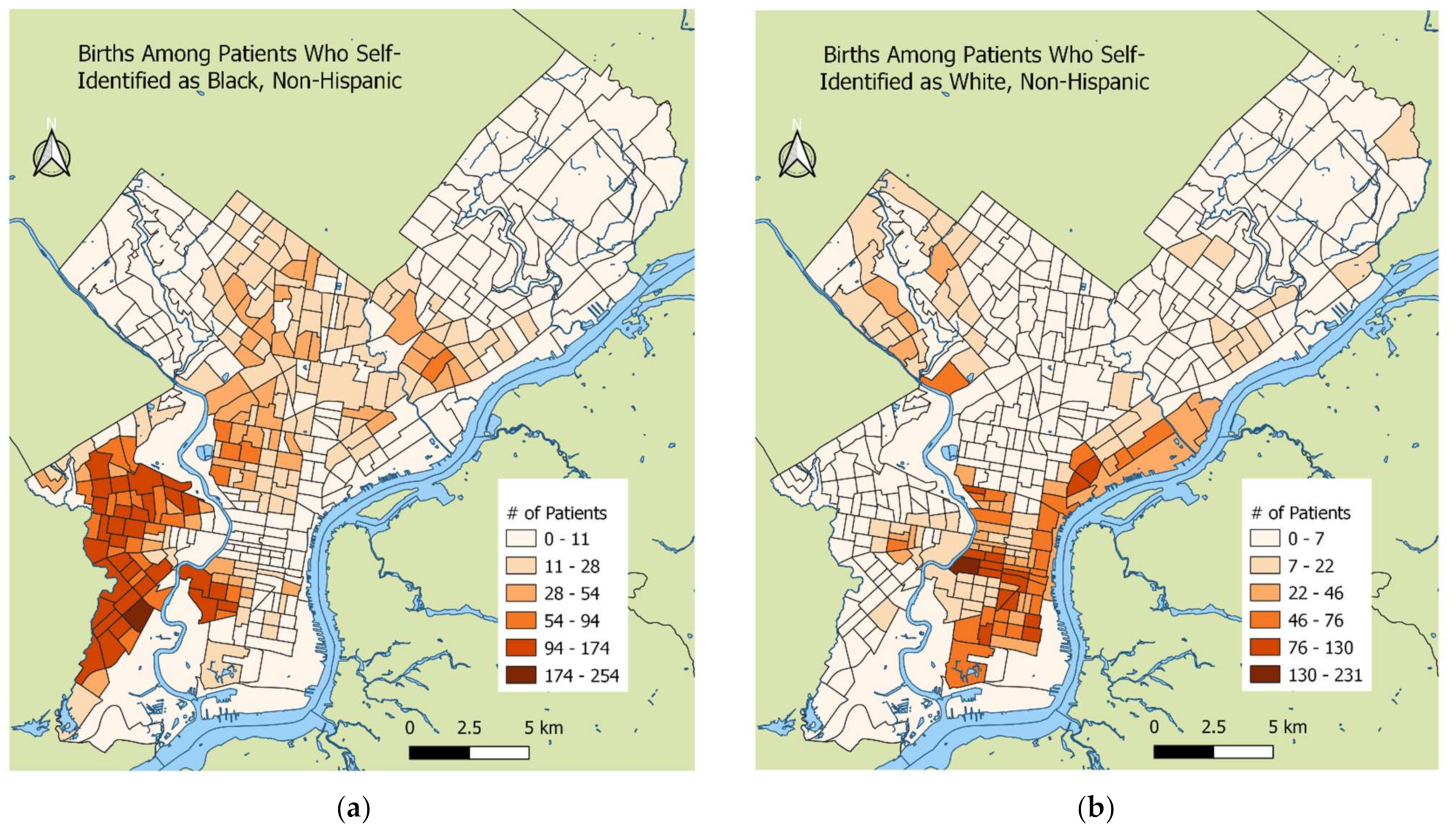
2.3. Birth Outcomes and Covariate Ascertainment
2.4. Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Birth Outcome: PTB OR (95% CI) | Birth Outcome: sPTB OR (95% CI) | Birth Outcome: mPTB OR (95% CI) | |
|---|---|---|---|
| HUP | |||
| Unadjusted Benzene | 1.01 (0.944, 1.09) | 1.07 (0.982, 1.17) | 0.920 (0.817, 1.03) |
| Adjusted Benzene | 1.02 (0.933, 1.11) | 1.06 (0.953, 1.19) | 0.931 (0.819, 1.06) |
| Unadjusted NO2 | 1.04 (0.964, 1.12) | 1.06 (0.970, 1.17) | 1.02 (0.891, 1.18) |
| Adjusted NO2 | 1.03 (0.942, 1.12) | 1.03 (0.921, 1.14) | 1.02 (0.900, 1.16) |
| PAH | |||
| Unadjusted Benzene | 0.971 (0.902, 1.05) | 1.01 (0.915, 1.10) | 0.920 (0.817, 1.03) |
| Adjusted Benzene | 1.02 (0.945, 1.11) | 1.03 (0.935, 1.15) | 1.01 (0.888, 1.14) |
| Unadjusted NO2 | 0.909 (0.850, 0.973) | 0.949 (0.870, 1.04) | 0.974 (0.828, 1.15) |
| Adjusted NO2 | 0.939 (0.868, 1.02) | 0.941 (0.851, 1.04) | 0.935 (0.826, 1.06) |
References
- Centers for Disease Control and Prevention. Preterm Birth. Reproductive Health 2020. Available online: https://www.cdc.gov/reproductivehealth/maternalinfanthealth/pretermbirth.htm (accessed on 8 October 2021).
- Hack, M.; Flannery, D.J.; Schluchter, M.; Cartar, L.; Borawski, E.; Klein, N. Outcomes in Young Adulthood for Very-Low-Birth-Weight Infants. N. Engl. J. Med. 2002, 346, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Zwicker, J.G.; Harris, S.R. Quality of Life of Formerly Preterm and Very Low Birth Weight Infants from Preschool Age to Adulthood: A Systematic Review. Pediatrics 2008, 121, e366–e376. [Google Scholar] [CrossRef] [PubMed]
- Osterman, M.J.; Hamilton, B.E.; Martin, J.A.; Driscoll, A.K.; Valenzuela, C.P. Births: Final data for 2020. In National Vital Statistics Report; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2021; pp. 1–50. [Google Scholar]
- Burris, H.H.; Lorch, S.A.; Kirpalani, H.; Pursley, D.M.; Elovitz, M.; Clougherty, J.E. Racial disparities in preterm birth in USA: A biosensor of physical and social environmental exposures. Arch. Dis. Child. 2019, 104, 931–935. [Google Scholar] [CrossRef] [PubMed]
- Jelliffe-Pawlowski, L.; Baer, R.; Blumenfeld, Y.; Ryckman, K.; O’Brodovich, H.; Gould, J.; Druzin, M.; El-Sayed, Y.; Lyell, D.; Stevenson, D.; et al. Maternal characteristics and mid-pregnancy serum biomarkers as risk factors for subtypes of preterm birth. BJOG Int. J. Obstet. Gynaecol. 2015, 122, 1484–1493. [Google Scholar] [CrossRef] [PubMed]
- Berkowitz, G.S.; Blackmore-Prince, C.; Lapinski, R.H.; Savitz, D.A. Risk Factors for Preterm Birth Subtypes. Epidemiology 1998, 9, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Henderson, J.J.; McWilliam, O.A.; Newnham, J.; Pennell, C. Preterm birth aetiology 2004–2008. Maternal factors associated with three phenotypes: Spontaneous preterm labour, preterm pre-labour rupture of membranes and medically indicated preterm birth. J. Matern. Neonatal Med. 2012, 25, 642–647. [Google Scholar] [CrossRef]
- Ananth, C.V.; Vintzileos, A.M. Epidemiology of preterm birth and its clinical subtypes. J. Matern. Neonatal Med. 2006, 19, 773–782. [Google Scholar] [CrossRef]
- Fuchs, F.; Monet, B.; Ducruet, T.; Chaillet, N.; Audibert, F. Effect of maternal age on the risk of preterm birth: A large cohort study. PLoS ONE 2018, 13, e0191002. [Google Scholar] [CrossRef]
- Cassidy-Bushrow, A.E.; Burmeister, C.; Lamerato, L.; Lemke, L.D.; Mathieu, M.; O’Leary, B.F.; Sperone, F.G.; Straughen, J.K.; Reiners, J.J. Prenatal airshed pollutants and preterm birth in an observational birth cohort study in Detroit, Michigan, USA. Environ. Res. 2020, 189, 109845. [Google Scholar] [CrossRef]
- Llop, S.; Ballester, F.; Estarlich, M.; Esplugues, A.; Rebagliato, M.; Iñiguez, C. Preterm birth and exposure to air pollutants during pregnancy. Environ. Res. 2010, 110, 778–785. [Google Scholar] [CrossRef]
- Brauer, M.; Lencar, C.; Tamburic, L.; Koehoorn, M.; Demers, P.; Karr, C. A Cohort Study of Traffic-Related Air Pollution Impacts on Birth Outcomes. Environ. Health Perspect. 2008, 116, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.; Bobb, J.F.; Ito, K.; Savitz, D.A.; Elston, B.; Shmool, J.L.; Dominici, F.; Ross, Z.; Clougherty, J.E.; Matte, T. Ambient Fine Particulate Matter, Nitrogen Dioxide, and Preterm Birth in New York City. Environ. Health Perspect. 2016, 124, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.S.; Balkhair, T. Air pollution and birth outcomes: A systematic review. Environ. Int. 2011, 37, 498–516. [Google Scholar] [CrossRef] [PubMed]
- Badham, H.J.; Renaud, S.J.; Wan, J.; Winn, L.M. Benzene-initiated oxidative stress: Effects on embryonic signaling pathways. Chem. Biol. Interact. 2010, 184, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Erickson, A.C.; Arbour, L. The Shared Pathoetiological Effects of Particulate Air Pollution and the Social Environment on Fetal-Placental Development. J. Environ. Public Health 2014, 2014, 901017. [Google Scholar] [CrossRef]
- Kannan, S.; Misra, D.P.; Dvonch, J.T.; Krishnakumar, A. Exposures to Airborne Particulate Matter and Adverse Perinatal Outcomes: A Biologically Plausible Mechanistic Framework for Exploring Potential Effect Modification by Nutrition. Environ. Health Perspect. 2006, 114, 1636–1642. [Google Scholar] [CrossRef]
- Handley, S.C.; Mullin, A.M.; Elovitz, M.A.; Gerson, K.D.; Montoya-Williams, D.; Lorch, S.A.; Burris, H.H. Changes in Preterm Birth Phenotypes and Stillbirth at 2 Philadelphia Hospitals During the SARS-CoV-2 Pandemic, March–June 2020. JAMA 2021, 325, 87–89. [Google Scholar] [CrossRef]
- Montoya-Williams, D.; Mullin, A.M.; Handley, S.C.; Flannery, D.D.; Lorch, S.A.; Elovitz, M.A.; Burris, H.H. Disparities in SARS-CoV-2 positivity among pregnant patients with limited English proficiency. J. Perinatol. 2021, 41, 2564–2565. [Google Scholar] [CrossRef]
- Environmental Protection Agency. National Air Toxics Assessment: 2014 NATA: Assessment Results. 2018. Available online: https://www.epa.gov/national-air-toxics-assessment/2014-nata-assessment-results#pollutant (accessed on 15 January 2020).
- Environmental Protection Agency. National Air Toxics Assessment: 2014 NATA: Assessment Methods. 2018. Available online: https://www.epa.gov/national-air-toxics-assessment/2014-nata-assessment-methods (accessed on 15 January 2020).
- R Core Team. R: A Language and Environment for Statistical Computing. 2019. Available online: https://www.R-project.org (accessed on 11 August 2021).
- RStudio Team. RStudio: Integrated Development Environment for R. 2019. Available online: http://www.rstudio.com (accessed on 11 August 2021).
- QGIS.org. QGIS Geographic Information System. 2021. Available online: http://www.qgis.org (accessed on 7 June 2021).
- McDonald, S.D.; Han, Z.; Mulla, S.; Beyene, J.; Knowledge Synthesis Group. Overweight and obesity in mothers and risk of preterm birth and low birth weight infants: Systematic review and meta-analyses. BMJ 2010, 341, c3428. [Google Scholar] [CrossRef]
- Ion, R.; Bernal, A.L. Smoking and Preterm Birth. Reprod. Sci. 2015, 22, 918–926. [Google Scholar] [CrossRef]
- Köck, K.; Köck, F.; Klein, K.; Bancher-Todesca, D.; Helmer, H. Diabetes mellitus and the risk of preterm birth with regard to the risk of spontaneous preterm birth. J. Matern. Fetal Neonatal Med. 2010, 23, 1004–1008. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Villar, J.; Sun, W.; Merialdi, M.; Abdel-Aleem, H.; Mathai, M.; Ali, M.; Yu, K.F.; Zavaleta, N.; Purwar, M.; et al. Blood pressure dynamics during pregnancy and spontaneous preterm birth. Am. J. Obstet. Gynecol. 2007, 197, 162.e1–162.e6. [Google Scholar] [CrossRef]
- Stieb, D.M.; Chen, L.; Eshoul, M.; Judek, S. Ambient air pollution, birth weight and preterm birth: A systematic review and meta-analysis. Environ. Res. 2012, 117, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Meng, X.; Liu, C.; Chen, R.; Ge, Y.; Kan, L.; Fu, Q.; Li, W.; Tse, L.A.; Kan, H. Nitrogen dioxide air pollution and preterm birth in Shanghai, China. Environ. Res. 2019, 169, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Pennsylvania Department of Health. Birth Statistics. Occurrent Live Births by Hospital and Method of Delivery. Available online: https://www.health.pa.gov/topics/HealthStatistics/VitalStatistics/BirthStatistics/Pages/birth-statistics.aspx (accessed on 20 May 2022).
- Darrall, K.G.; Figgins, J.A.; Brown, R.D. Determination of benzene and associated volatile compounds in mainstream cigarette smoke. Analyst 1998, 123, 1095–1101. [Google Scholar] [CrossRef]
- Drake, P.; Driscoll, A.K.; Mathews, T.J. Cigarette Smoking During Pregnancy: United States, 2016. NCHS Data Brief 2018, 305, 1–8. [Google Scholar]
| Overall | Term | PTB | sPTB | mPTB | |
|---|---|---|---|---|---|
| Characteristics | n = 19,169 | n = 17,461 | n = 1708 | n = 1035 | n = 657 |
| Maternal Age | |||||
| <25 | 26.9% | 26.7% | 29.1% | 33.2% | 22.7% |
| 25–34 | 55.3% | 55.5% | 53.0% | 51.5% | 55.4% |
| ≥35 | 17.8% | 17.8% | 17.9% | 15.5% | 21.9% |
| Maternal Race | |||||
| White Non-Hispanic | 28.2% | 29.0% | 19.4% | 21.0% | 17.0% |
| Black Non-Hispanic | 52.6% | 51.4% | 64.4% | 61.2% | 69.7% |
| Asian | 6.3% | 6.4% | 4.7% | 6.3% | 2.1% |
| Hispanic | 8.4% | 8.5% | 7.6% | 7.3% | 7.6% |
| Mixed/Other/Unknown | 4.2% | 4.6% | 3.9% | 4.3% | 3.5% |
| BMI 1 | |||||
| <25 | 49.5% | 50.2% | 42.3% | 49.2% | 32.4% |
| 25–<30 | 23.5% | 23.5% | 23.3% | 23.2% | 23.8% |
| ≥30 | 27.0% | 26.3% | 34.4% | 27.7% | 43.8% |
| Private Insurance 2 | 45.1% | 46.1% | 35.8% | 36.3% | 35.4% |
| Nulliparity 3 | 44.5% | 44.8% | 41.3% | 41.1% | 42.0% |
| Census Tract: Percentage of Poverty | 28.0 (14.7) | 27.7 (14.8) | 30.35 (14.1) | 29.9 (14.2) | 30.9 (13.8) |
| Hospital of Birth | |||||
| Pennsylvania Hospital | 54.8% | 55.6% | 47.2% | 46.4% | 47.5% |
| Hospital of the University of Pennsylvania | 45.2% | 44.4% | 52.8% | 53.6% | 52.5% |
| Air Pollutants | |||||
| NO2 (ppb) | 17.2 (2.3) | 17.2 (2.3) | 17.1 (2.6) | 17.2 (2.6) | 17.0 (2.6) |
| Benzene (ppb) | 0.22 (0.02) | 0.22 (0.02) | 0.22 (0.02) | 0.22 (0.02) | 0.22 (0.02) |
| Birth Outcome: PTB OR (95% CI) | Birth Outcome: sPTB OR (95% CI) | Birth Outcome: mPTB OR (95% CI) | |
|---|---|---|---|
| Overall | |||
| Unadjusted Benzene | 0.96 (0.91, 1.01) | 1.00 (0.94, 1.06) | 0.89 (0.82, 0.97) * |
| Adjusted Benzene | 1.02 (0.96, 1.08) | 1.05 (0.97, 1.13) | 0.97 (0.89, 1.06) |
| Unadjusted NO2 | 0.95 (0.90, 0.99) * | 0.98 (0.92, 1.04) | 0.90 (0.84, 0.97) * |
| Adjusted NO2 | 0.975 (0.92, 1.03) | 0.972 (0.90, 1.05) | 0.97 (0.89, 1.06) |
| Maternal race/ethnicity: Asian NH | |||
| Unadjusted Benzene | 1.06 (0.844, 1.33) | 1.01 (0.78, 1.30) | 1.26 (0.745, 2.04) |
| Adjusted Benzene | 1.05 (0.817, 1.34) | 0.974 (0.735, 1.29) | 1.36 (0.80, 2.30) |
| Unadjusted NO2 | 1.11 (0.878, 1.42) | 1.16 (0.891, 1.52) | 0.925 (0.564, 1.63) |
| Adjusted NO2 | 1.12 (0.857, 1.46) | 1.20 (0.892, 1.62) | 0.833 (0.47, 1.47) |
| Maternal race/ethnicity: Black NH | |||
| Unadjusted Benzene | 1.04 (0.98, 1.10) | 1.05 (0.97, 1.14) | 1.01 (0.92, 1.11) |
| Adjusted Benzene | 1.01 (0.94, 1.09) | 1.04 (0.94, 1.15) | 0.97 (0.88, 1.08) |
| Unadjusted NO2 | 1.01 (0.95, 1.09) | 1.03 (0.94, 1.13) | 1.00 (0.90, 1.11) |
| Adjusted NO2 | 0.99 (0.92, 1.07) | 0.99 (0.90, 1.10) | 1.00 (0.893, 1.12) |
| Maternal race/ethnicity: Hispanic | |||
| Unadjusted Benzene | 0.986 (0.782, 1.23) | 1.09 (0.82, 1.43) | 0.856 (0.58, 1.22) |
| Adjusted Benzene | 1.05 (0.816, 1.35) | 1.19 (0.861, 1.64) | 0.929 (0.623, 1.39) |
| Unadjusted NO2 | 0.953 (0.78, 1.17) | 0.980 (0.759, 1.29) | 0.930 (0.684, 1.30) |
| Adjusted NO2 | 0.905 (0.715, 1.15) | 0.855 (0.625, 1.17) | 0.966 (0.677, 1.38) |
| Maternal race/ethnicity: White NH | |||
| Unadjusted Benzene | 0.96 (0.85, 1.08) | 1.03 (0.89, 1.19) | 0.82 (0.66, 1.00) |
| Adjusted Benzene | 0.99 (0.87, 1.13) | 1.02 (0.88, 1.19) | 0.94 (0.75, 1.18) |
| Unadjusted NO2 | 0.95 (0.86, 1.05) | 0.99 (0.88, 1.13) | 0.87 (0.75, 1.02) |
| Adjusted NO2 | 0.95 (0.85, 1.05) | 0.983 (0.858, 1.13) | 0.88 (0.74, 1.05) |
| Maternal race/ethnicity: other | |||
| Unadjusted Benzene | 1.05 (0.815, 1.34) | 1.02 (0.753, 1.38) | 1.10 (0.719, 1.65) |
| Adjusted Benzene | 1.12 (0.852, 1.48) | 1.22 (0.875, 1.70) | 0.962 (0.594, 1.56) |
| Unadjusted NO2 | 1.15 (0.911, 1.46) | 1.03 (0.785, 1.37) | 1.47 (0.971, 2.31) |
| Adjusted NO2 | 1.11 (0.850, 1.45) | 0.964 (0.698, 1.33) | 1.58 (0.962, 2.58) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escoto, K.M.; Mullin, A.M.; Ledyard, R.; Rovit, E.; Yang, N.; Tripathy, S.; Burris, H.H.; Clougherty, J.E. Benzene and NO2 Exposure during Pregnancy and Preterm Birth in Two Philadelphia Hospitals, 2013–2017. Int. J. Environ. Res. Public Health 2022, 19, 10365. https://doi.org/10.3390/ijerph191610365
Escoto KM, Mullin AM, Ledyard R, Rovit E, Yang N, Tripathy S, Burris HH, Clougherty JE. Benzene and NO2 Exposure during Pregnancy and Preterm Birth in Two Philadelphia Hospitals, 2013–2017. International Journal of Environmental Research and Public Health. 2022; 19(16):10365. https://doi.org/10.3390/ijerph191610365
Chicago/Turabian StyleEscoto, Kathleen M., Anne M. Mullin, Rachel Ledyard, Elizabeth Rovit, Nancy Yang, Sheila Tripathy, Heather H. Burris, and Jane E. Clougherty. 2022. "Benzene and NO2 Exposure during Pregnancy and Preterm Birth in Two Philadelphia Hospitals, 2013–2017" International Journal of Environmental Research and Public Health 19, no. 16: 10365. https://doi.org/10.3390/ijerph191610365
APA StyleEscoto, K. M., Mullin, A. M., Ledyard, R., Rovit, E., Yang, N., Tripathy, S., Burris, H. H., & Clougherty, J. E. (2022). Benzene and NO2 Exposure during Pregnancy and Preterm Birth in Two Philadelphia Hospitals, 2013–2017. International Journal of Environmental Research and Public Health, 19(16), 10365. https://doi.org/10.3390/ijerph191610365







