Application of Advanced Oxidation Technology in Sludge Conditioning and Dewatering: A Critical Review
Abstract
1. Introduction
2. Application of Hydrogen Peroxide in Sludge Dewatering
3. Application of Persulfate in Sludge Dewatering
4. Application of Ozone in Sludge Dewatering
5. Application of Permanganate and Ferrate in Sludge Dewatering
6. Application of Other Technologies in Sludge Dewatering
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lin, W.; Liu, X.; Ding, A.; Ngo, H.H.; Zhang, R.; Nan, J.; Ma, J.; Li, G. Advanced oxidation processes (AOPs)-based sludge conditioning for enhanced sludge dewatering and micropollutants removal: A critical review. J. Water Process Eng. 2022, 45, 102468. [Google Scholar] [CrossRef]
- Toutian, V.; Barjenbruch, M.; Loderer, C.; Remy, C. Impact of process parameters of thermal alkaline pretreatment on biogas yield and dewaterability of waste activated sludge. Water Res. 2021, 202, 117465. [Google Scholar] [CrossRef]
- Izydorczyk, G.; Mikula, K.; Skrzypczak, D.; Trzaska, K.; Moustakas, K.; Witek-Krowiak, A.; Chojnacka, K. Agricultural and non-agricultural directions of bio-based sewage sludge valorization by chemical conditioning. Environ. Sci. Pollut. Res. 2021, 28, 47725–47740. [Google Scholar] [CrossRef]
- Jiang, X.; Li, Y.; Tang, X.; Jiang, J.; He, Q.; Xiong, Z.; Zheng, H. Biopolymer-based flocculants: A review of recent technologies. Environ. Sci. Pollut. Res. 2021, 28, 46934–46963. [Google Scholar] [CrossRef]
- Hatinoğlu, M.D.; Sanin, F.D. Sewage sludge as a source of microplastics in the environment: A review of occurrence and fate during sludge treatment. J. Environ. Manag. 2021, 295, 113028. [Google Scholar] [CrossRef]
- Liu, H.; Basar, I.A.; Nzihou, A.; Eskicioglu, C. Hydrochar derived from municipal sludge through hydrothermal processing: A critical review on its formation, characterization, and valorization. Water Res. 2021, 199, 117186. [Google Scholar] [CrossRef]
- Nabaterega, R.; Kumar, V.; Khoei, S.; Eskicioglu, C. A review on two-stage anaerobic digestion options for optimizing municipal wastewater sludge treatment process. J. Environ. Chem. Eng. 2021, 9, 105502. [Google Scholar] [CrossRef]
- Lu, J.; Xu, S. Post-treatment of food waste digestate towards land application: A review. J. Clean. Prod. 2021, 303, 127033. [Google Scholar] [CrossRef]
- Cao, B.; Zhang, T.; Zhang, W.; Wang, D. Enhanced technology based for sewage sludge deep dewatering: A critical review. Water Res. 2021, 189, 116650. [Google Scholar] [CrossRef]
- Langone, M.; Basso, D. Process waters from hydrothermal carbonization of sludge: Characteristics and possible valorization pathways. Int. J. Environ. Res. Public Health 2020, 17, 6618. [Google Scholar] [CrossRef]
- Wu, B.; Dai, X.; Chai, X. Critical review on dewatering of sewage sludge: Influential mechanism, conditioning technologies and implications to sludge re-utilizations. Water Res. 2020, 180, 115912. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Edraki, M.; Fawell, P.; Berry, L. Improved water recovery: A review of clay-rich tailings and saline water interactions. Powder Technol. 2020, 364, 604–621. [Google Scholar] [CrossRef]
- Brisolara, K.F.; Bourgeois, J. Biosolids and sludge management. Water Environ. Res. 2019, 91, 1168–1176. [Google Scholar] [CrossRef]
- Ibrahim, A.B.A.; Akilli, H. Supercritical water gasification of wastewater sludge for hydrogen production. Int. J. Hydrogen Energy 2019, 44, 10328–10349. [Google Scholar] [CrossRef]
- Brisolara, K.F.; Qi, Y.; Kendrick, Q.; Davis, Y. Biosolids and sludge management. Water Environ. Res. 2018, 90, 978–1006. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wen, Q.; Ma, M.; Hou, H.; Yu, W.; Gui, G.; Wu, Q.; Xiao, K.; Zhu, Y.; Tao, S.; Liang, S.; et al. Recirculation of reject water in deep-dewatering process to influent of wastewater treatment plant and dewaterability of sludge conditioned with Fe2+/H2O2, Fe2+/Ca(ClO)2, and Fe2+/Na2S2O8: From bench to pilot-scale study. Environ. Res. 2022, 203, 111825. [Google Scholar] [CrossRef] [PubMed]
- Tunçal, T.; Mujumdar, A.S. Modern techniques for sludge dewaterability improvement. Dry. Technol. 2022. [Google Scholar] [CrossRef]
- Xia, J.; Rao, T.; Ji, J.; He, B.; Liu, A.; Sun, Y. Enhanced dewatering of activated sludge by Skeleton-Assisted flocculation process. Int. J. Environ. Res. Public Health 2022, 19, 6540. [Google Scholar] [CrossRef]
- Deng, P.; Liu, C.; Wang, M.; Lan, G.; Zhong, Y.; Wu, Y.; Fu, C.; Shi, H.; Zhu, R.; Zhou, L. Effect of dewatering conditioners on phosphorus removal efficiency of sludge biochar. Environ. Technol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zhang, T.; Sun, J.; Zhu, J.; Yan, W.; Tian, S.; Xiong, Y. Improvement of sewage sludge dewatering by piezoelectric effect driven directly with pressure from pressure filtration: Towards understanding piezo-dewatering mechanism. Water Res. 2022, 209, 117922. [Google Scholar] [CrossRef]
- Wang, L.; Shi, Y.; Chai, J.; Huang, L.; Wang, Y.; Wang, S.; Pi, K.; Gerson, A.R.; Liu, D. Transfer of microplastics in sludge upon Fe(II)-persulfate conditioning and mechanical dewatering. Sci. Total Environ. 2022, 838, 156316. [Google Scholar] [CrossRef]
- Li, Y.; Liu, L.; Li, X.; Xie, J.; Guan, M.; Wang, E.; Lu, D.; Dong, T.; Zhang, X. Influence of alternating electric field on deep dewatering of municipal sludge and changes of extracellular polymeric substance during dewatering. Sci. Total Environ. 2022, 842, 156839. [Google Scholar] [CrossRef] [PubMed]
- Guan, R.; Yuan, X.; Wu, Z.; Jiang, L.; Li, Y.; Zeng, G. Principle and application of hydrogen peroxide based advanced oxidation processes in activated sludge treatment: A review. Chem. Eng. J. 2018, 339, 519–530. [Google Scholar] [CrossRef]
- Liang, J.; Zhou, Y. Iron-based advanced oxidation processes for enhancing sludge dewaterability: State of the art, challenges, and sludge reuse. Water Res. 2022, 218, 118499. [Google Scholar] [CrossRef]
- Barati Rashvanlou, R.; Pasalari, H.; Moserzadeh, A.A.; Farzadkia, M. A combined ultrasonic and chemical conditioning process for upgrading the sludge dewaterability. Int. J. Environ. Anal. Chem. 2022, 102, 1613–1626. [Google Scholar] [CrossRef]
- Liu, H.; Yang, J.; Shi, Y.; Li, Y.; He, S.; Yang, C.; Yao, H. Conditioning of sewage sludge by Fenton’s reagent combined with skeleton builders. Chemosphere 2012, 88, 235–239. [Google Scholar] [CrossRef]
- Yu, W.; Yang, J.; Shi, Y.; Song, J.; Shi, Y.; Xiao, J.; Li, C.; Xu, X.; He, S.; Liang, S.; et al. Roles of iron species and pH optimization on sewage sludge conditioning with Fenton’s reagent and lime. Water Res. 2016, 95, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Yang, J.; Hou, H.; Liang, S.; Xiao, K.; Qiu, J.; Hu, J.; Liu, B.; Yu, W.; Deng, H. Enhanced sludge dewatering via homogeneous and heterogeneous Fenton reactions initiated by Fe-rich biochar derived from sludge. Chem. Eng. J. 2019, 372, 977–996. [Google Scholar] [CrossRef]
- Zeng, X.; Twardowska, I.; Wei, S.; Sun, L.; Wang, J.; Zhu, J.; Cai, J. Removal of trace metals and improvement of dredged sediment dewaterability by bioleaching combined with Fenton-like reaction. J. Hazard. Mater. 2015, 288, 51–59. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, A. Removal of steroid estrogens from waste activated sludge using Fenton oxidation: Influencing factors and degradation intermediates. Chemosphere 2014, 105, 24–30. [Google Scholar] [CrossRef]
- He, D.; Sun, M.; Bao, B.; Chen, J.; Luo, H.; Li, J. Rethinking the timing of flocculant addition during activated sludge dewatering. J. Water Process Eng. 2022, 47, 102744. [Google Scholar] [CrossRef]
- Liang, J.; Huang, S.; Dai, Y.; Li, L.; Sun, S. Dewaterability of five sewage sludges in Guangzhou conditioned with Fenton’s reagent/lime and pilot-scale experiments using ultrahigh pressure filtration system. Water Res. 2015, 84, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, J.; Yu, W.; Luo, S.; Peng, L.; Shen, X.; Shi, Y.; Zhang, S.; Song, J.; Ye, N.; et al. Mechanism of red mud combined with Fenton’s reagent in sewage sludge conditioning. Water Res. 2014, 59, 239–247. [Google Scholar] [CrossRef]
- Song, K.; Zhou, X.; Liu, Y.; Gong, Y.; Zhou, B.; Wang, D.; Wang, Q. Role of oxidants in enhancing dewaterability of anaerobically digested sludge through Fe (II) activated oxidation processes: Hydrogen peroxide versus persulfate. Sci. Rep. 2016, 6, 24800. [Google Scholar] [CrossRef]
- Hu, L.; Wang, P.; Shen, T.; Wang, Q.; Wang, X.; Xu, P.; Zheng, Q.; Zhang, G. The application of microwaves in sulfate radical-based advanced oxidation processes for environmental remediation: A review. Sci. Total Environ. 2020, 722, 137831. [Google Scholar] [CrossRef] [PubMed]
- Karadoğan, Ü.; Çevikbilen, G.; Korkut, S.; Pasaoglu, M.E.; Teymur, B. Dewatering of Golden Horn sludge with geotextile tube and determination of optimum operating conditions: A novel approach. Mar. Georesources Geotechnol. 2022, 40, 782–794. [Google Scholar] [CrossRef]
- Sha, L.; Wu, Z.; Ling, Z.; Liu, X.; Yu, X.; Zhang, S. Investigation on the improvement of activated sludge dewaterability using different iron forms (ZVI vs. Fe(II))/peroxydisulfate combined vertical electro-dewatering processes. Chemosphere 2022, 292, 133416. [Google Scholar] [CrossRef]
- Zhen, G.; Lu, X.; Su, L.; Kobayashi, T.; Kumar, G.; Zhou, T.; Xu, K.; Li, Y.; Zhu, X.; Zhao, Y. Unraveling the catalyzing behaviors of different iron species (Fe2+ vs. FeO) in activating persulfate-based oxidation process with implications to waste activated sludge dewaterability. Water Res. 2018, 134, 101–114. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, Q.; Jiang, G.; Liu, P.; Yuan, Z. A novel conditioning process for enhancing dewaterability of waste activated sludge by combination of zero-valent iron and persulfate. Bioresour. Technol. 2015, 185, 416–420. [Google Scholar] [CrossRef]
- Song, K.; Zhou, X.; Liu, Y.; Xie, G.; Wang, D.; Zhang, T.; Liu, C.; Liu, P.; Zhou, B.; Wang, Q. Improving dewaterability of anaerobically digested sludge by combination of persulfate and zero valent iron. Chem. Eng. J. 2016, 295, 436–442. [Google Scholar] [CrossRef]
- Liu, M.; Yuan, C.; Ru, S.; Li, J.; Lei, Z.; Zhang, Z.; Shimizu, K.; Yuan, T.; Li, F. Combined organic reagents for co-conditioning of sewage sludge: High performance in deep dewatering and significant contribution to the floc property. J. Water Process Eng. 2022, 48, 102855. [Google Scholar] [CrossRef]
- Fan, X.; Wang, Y.; Zhang, D.; Guo, Y.; Gao, S.; Li, E.; Zheng, H. Effects of acid, acid-ZVI/PMS, Fe(II)/PMS and ZVI/PMS conditioning on the wastewater activated sludge (WAS) dewaterability and extracellular polymeric substances (EPS). J. Environ. Sci. 2020, 91, 73–84. [Google Scholar] [CrossRef]
- Zhou, X.; Jin, W.; Wang, L.; Che, L.; Chen, C.; Li, S.; Wang, X.; Tu, R.; Han, S.; Feng, X.; et al. Alum sludge conditioning with ferrous iron/peroxymonosulfate oxidation: Characterization and mechanism. Korean J. Chem. Eng. 2020, 37, 663–669. [Google Scholar] [CrossRef]
- Chacana, J.; Labelle, M.; Laporte, A.; Gadbois, A.; Barbeau, B.; Comeau, Y. Ozonation of Primary Sludge and Digested Sludge to Increase Methane Production in a Chemically Enhanced Primary Treatment Facility. Ozone Sci. Eng. 2017, 39, 148–158. [Google Scholar] [CrossRef]
- Lin, N.; Zhu, W.; You, X.; Wang, X.; Zhong, J.; Sun, J.; Sun, T.; Cao, J.; Li, L. Verification of the mechanism and effect of secondary advanced dewatering promoted by selective oxidative decomposition: On pilot scale. J. Environ. Chem. Eng. 2022, 10, 107217. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Tian, Y.; Li, N.; Kong, L.; Sun, L.; Yu, M.; Zuo, W. Changes of physicochemical properties of sewage sludge during ozonation treatment: Correlation to sludge dewaterability. Chem. Eng. J. 2016, 301, 238–248. [Google Scholar] [CrossRef]
- Li, L.; Peng, C.; Deng, L.; Zhang, F.; Wu, D.; Ma, F.; Liu, Y. Understanding the synergistic mechanism of PAM-FeCl3 for improved sludge dewaterability. J. Environ. Manag. 2022, 301, 113926. [Google Scholar] [CrossRef]
- Srinivasan, A.; Young, C.; Liao, P.H.; Lo, K.V. Radiofrequency-oxidation treatment of sewage sludge. Chemosphere 2015, 141, 212–218. [Google Scholar] [CrossRef]
- Yu, H.; Gu, L.; Zhang, D.; Wen, H.; Wang, M.; Zhu, N. Enhancement of sludge dewaterability by three-dimensional electrolysis with sludge-based particle electrodes. Sep. Purif. Technol. 2022, 287, 120599. [Google Scholar] [CrossRef]
- Ge, D.; Bian, C.; Yuan, H.; Zhu, N. An in-depth study on the deep-dewatering mechanism of waste activated sludge by ozonation pre-oxidation and chitosan re-flocculation conditioning. Sci. Total Environ. 2020, 714, 136627. [Google Scholar] [CrossRef]
- Zeng, R.; Li, Y.; Sha, L.; Liu, X.; Zhang, S. Electro-dewatering of steel industrial sludge: Performance and metal speciation. J. Water Process Eng. 2022, 46, 102600. [Google Scholar] [CrossRef]
- Bień, B.; Bień, J.D. Analysis of reject water formed in the mechanical dewatering process of digested sludge conditioned by physical and chemical methods. Energies 2022, 15, 1678. [Google Scholar] [CrossRef]
- Liu, Y.F.; Dong, L.M.; Cui, G.N.; Hu, X.Y.; Yu, Z.Q.; Liang, S. Low-field nuclear magnetic resonance detection of changes in the water distribution in citric acid biosludge during dewatering. Int. J. Environ. Sci. Technol. 2022, 19, 4153–4166. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, S.; Ye, M.; Huang, J.; Yang, X.; Li, S.; Huang, S.; Sun, S. Improving sewage sludge dewaterability with rapid and cost-effective in-situ generation of Fe2+ combined with oxidants. Chem. Eng. J. 2020, 380, 122499. [Google Scholar] [CrossRef]
- Yuan, L.; Liu, H.; Lu, Y.; Lu, Y.; Wang, D. Enhancing the dewaterability of waste activated sludge by the combined ascorbic acid and zero-valent iron/persulfate system. Chemosphere 2022, 303, 135104. [Google Scholar] [CrossRef]
- Rai, P.K.; Lee, J.; Kailasa, S.K.; Kwon, E.E.; Tsang, Y.F.; Ok, Y.S.; Kim, K.H. A critical review of ferrate(VI)-based remediation of soil and groundwater. Environ. Res. 2018, 160, 420–448. [Google Scholar] [CrossRef]
- Han, Q.; Dong, W.; Wang, H.; Liu, T.; Tian, Y.; Song, X. Degradation of tetrabromobisphenol A by ferrate(VI) oxidation: Performance, inorganic and organic products, pathway and toxicity control. Chemosphere 2018, 198, 92–102. [Google Scholar] [CrossRef]
- Wu, S.; Liu, H.; Lin, Y.; Yang, C.; Lou, W.; Sun, J.; Du, C.; Zhang, D.; Nie, L.; Yin, K.; et al. Insights into mechanisms of UV/ferrate oxidation for degradation of phenolic pollutants: Role of superoxide radicals. Chemosphere 2020, 244, 125490. [Google Scholar] [CrossRef] [PubMed]
- Ai, J.; Zhang, W.; Chen, F.; Liao, G.; Li, D.; Xia, H.; Wang, D.; Ma, T. Catalytic pyrolysis coupling to enhanced dewatering of waste activated sludge using KMnO4-Fe(II) conditioning for preparing multi-functional material to treat groundwater containing combined pollutants. Water Res. 2019, 158, 424–437. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Qi, Y.; Zhang, X.; Zhang, G.; Yang, H.; Nattabi, F. Experimental investigation of sludge dewatering for single- and double-drainage conditions with a vacuum negative pressure load at the bottom. PLoS ONE 2021, 16, e253806. [Google Scholar] [CrossRef]
- Urrea, J.L.; Collado, S.; Oulego, P.; Díaz, M. Formation and Degradation of Soluble Biopolymers during Wet Oxidation of Sludge. ACS Sustain. Chem. Eng. 2017, 5, 3011–3018. [Google Scholar] [CrossRef]
- Zhang, X.; Tong, G.; Zhou, Y.; Li, G.; Zhang, H. Enhancing paper sludge dewatering by waste polyester fiber and FeCl3 for preparation of Fe-rich biochar. BioResources 2021, 16, 2326–2345. [Google Scholar] [CrossRef]
- Xiao, Y.; Lu, Y.; Zheng, G.; Zhou, L. Impact of initial sludge pH on enhancing the dewaterability of waste activated sludge by zero-valent iron-activated peroxydisulphate. Environ. Technol. 2021, 42, 2573–2586. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Zhang, G.; Wang, H. Current state of sludge production, management, treatment and disposal in China. Water Res. 2015, 78, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Bień, B.; Bień, J.D. Conditioning of sewage sludge with physical, chemical and dual methods to improve sewage sludge dewatering. Energies 2021, 14, 5079. [Google Scholar] [CrossRef]
- Liu, C.; Zhou, X.; Zhou, L.; Wei, Y.; Liu, J. Enhancement of sludge electro-dewatering by anthracite powder modification. Environ. Res. 2021, 201, 111510. [Google Scholar] [CrossRef]
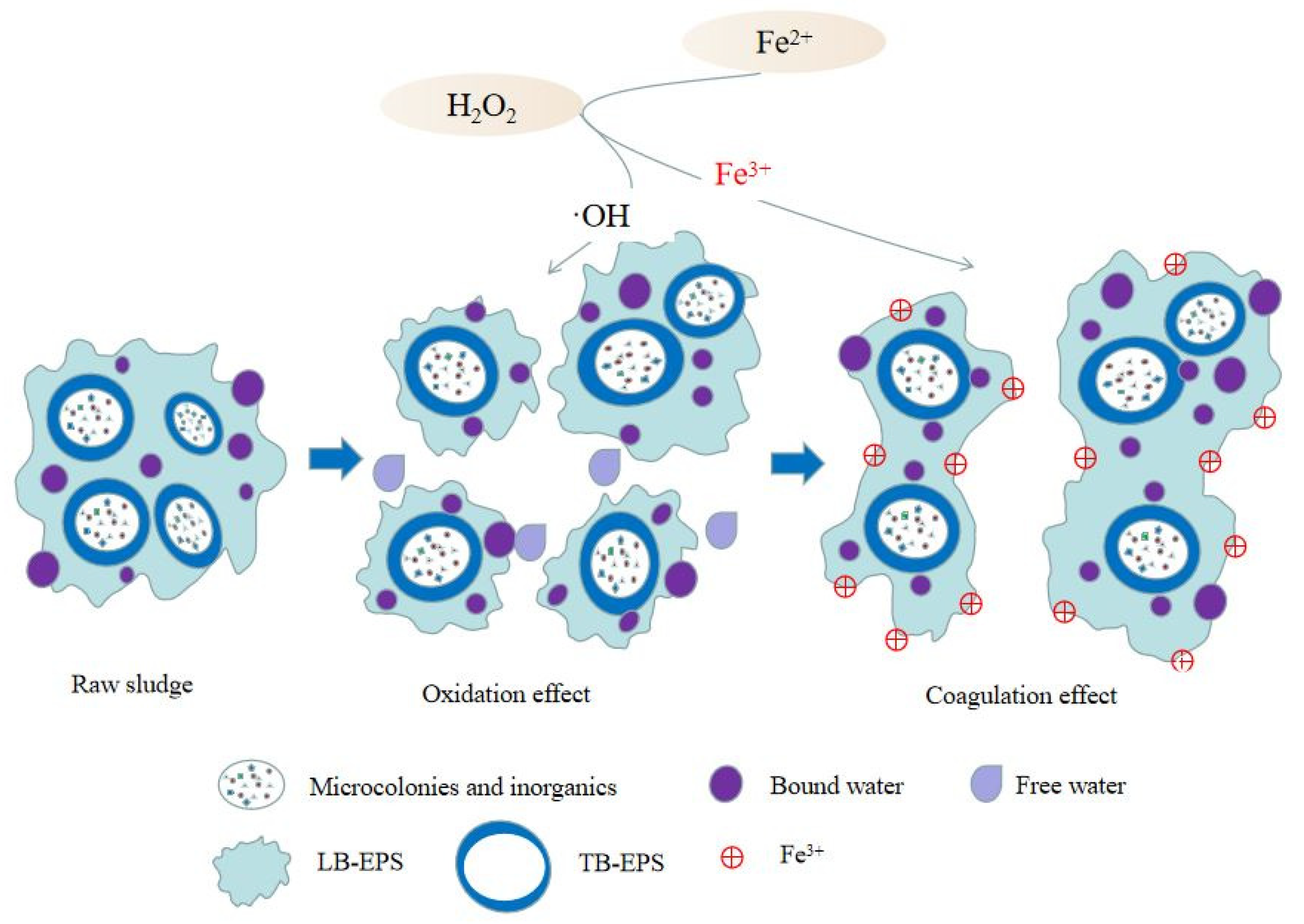

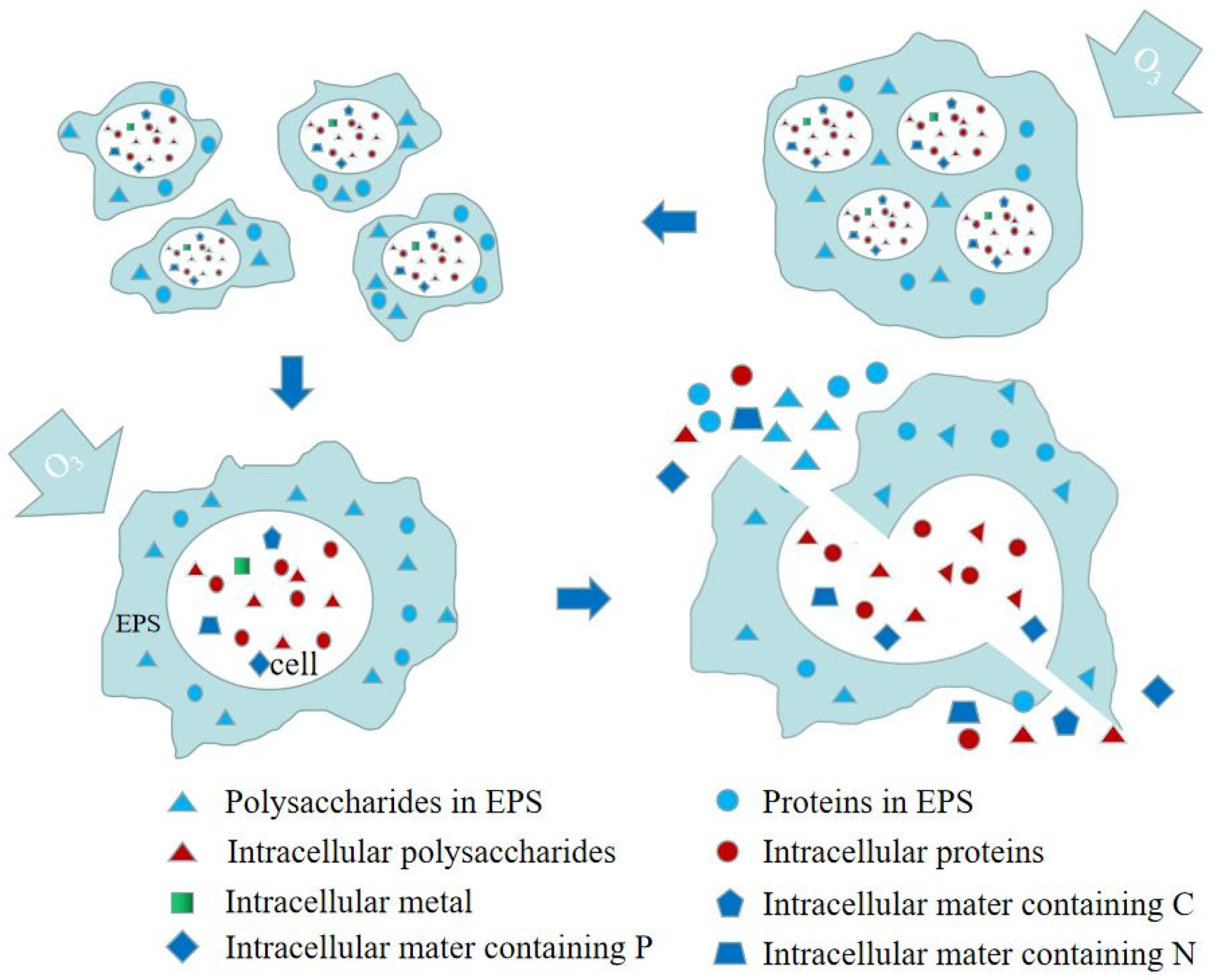
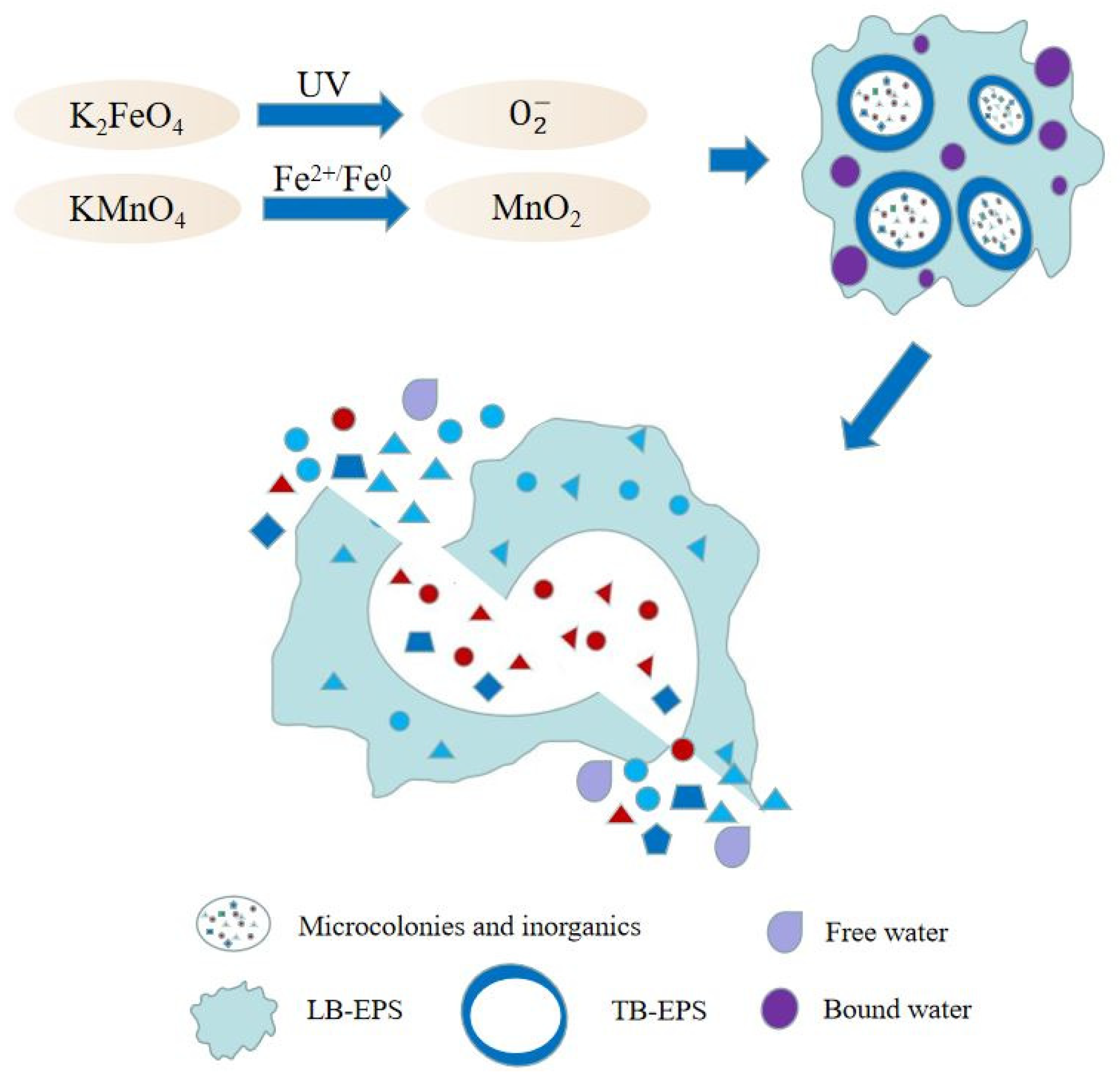
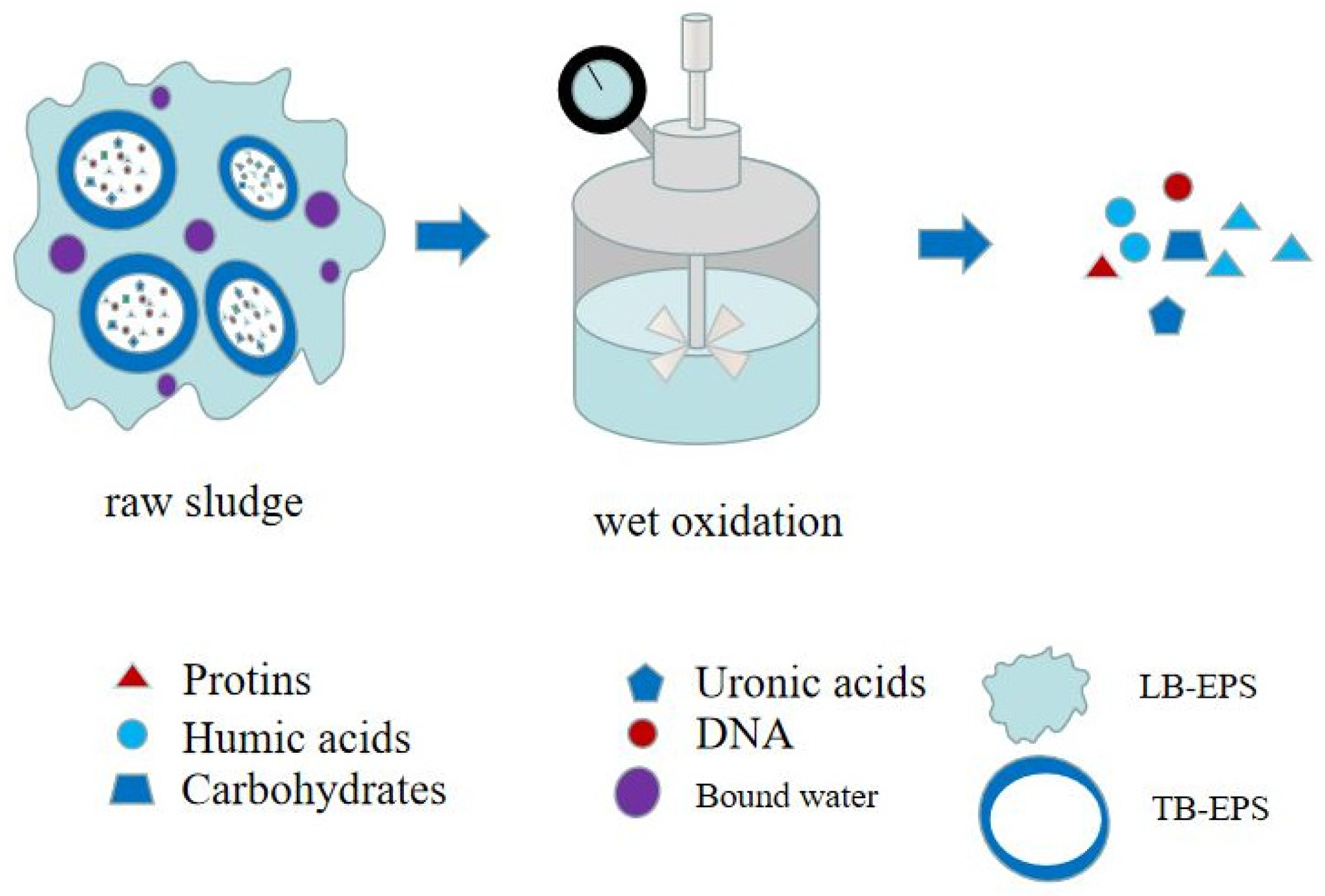
| Preprocessing Method | Optimal Pretreatment Conditions | Conditioning Dehydration Effect | Ref. |
|---|---|---|---|
| Fenton | Fe2+: 5000 mg/L; H2O2: 6000 mg/L | CST reduction: 48.5%; SRF reduction: 93.3% | [25] |
| Fenton | Fe2+: 25 mg/g DS; H2O2: 50 mg/g DS | CST reduction: 95.0% | [34] |
| Lime + Fenton | Fe2+: 40 mg/g DS; H2O2: 32 mg/g DS; pH: 5; Lime: 400 mg/g DS | SRF reduction: 95.0% | [26] |
| Lime + Fenton | Fe2+: 47.9 mg/g DS; H2O2: 34.3 mg/g DS; pH: 6.6 ± 0.2; Lime: 43.2 mg/g DS | Water content: 55.8 ± 0.6% | [27] |
| Lime + Fenton | Fe2+/H2O2: 50/30 mg/g DS; pH: 3; Lime: 50 mg/g DS; t: 10 min | SRF reduction: 90.0% | [27,32] |
| Red mud + Fenton | Fe2+: 31.9 mg/g DS; H2O2: 33.7 mg/g DS; Red mud: 275.1 mg/g DS | Water content: 59.8 ± 0.4% | [33] |
| Bioleaching + Fenton | H2O2: 2.0 g/L; t: 5 d | CST reduction: 89.8%; SRF reduction: 83.8% | [29] |
| Iron rich biochar + Fenton | Biochar: 0.792 g/g VS; H2O2: 0.072 g/g VS | CST reduction: 90.2%; SRF reduction: 98.0%; Water content: 46.0% | [28] |
| Preprocessing Method | Optimal Pretreatment Conditions | Conditioning Dehydration Effect | Ref. |
|---|---|---|---|
| Fe2+/S2O82− | Fe2+: 1.5 mmol/g; S2O82−: 1.2 mmol/g | CST reduction: 88.8% | [36] |
| Fe2+/S2O82− | Fe2+: 1.5 mmol/g; S2O82−: 1.2 mmol/g; T: 80 °C | CST reduction: 97.0% | [37] |
| Fe0/S2O82− | Fe0: 15.0 g/L; S2O82−: 4.0 g/L | CST reduction: 50.2% | [39] |
| Fe0/S2O82− | Fe0: 2.0 g/L; S2O82−: 0.5 g/L | CST reduction: 90.0% | [40] |
| Electrolysis + Fe2+/S2O82− | Fe2+: 0.5 mmol/g; S2O82−: 0.4 mmol/g; | Water content: 82.5% | [41] |
| Acid-ZVI/PMS | ZVI: 0.15 g/g DS; Oxone: 0.07 g/g DS; pH: 3 | CST reduction: 19.6%; Water content: 76.3% | [42] |
| Fe2+/PMS | PMS 0.1 g/g TSS; Fe2+ 0.5 g/g TSS | CST reduction: 66.0%; SRF reduction: 88.0% | [43] |
| Preprocessing Method | Optimal Pretreatment Conditions | Conditioning Dehydration Effect | Ref. |
|---|---|---|---|
| O3 | O3: 37.8 mg/g SS | SS reduction: 13.5%;VSS reduction 11.9% | [46] |
| O3 | O3: 0.5 g/g TS | SS reduction 77.8%; VSS reduction 71.6% | [45] |
| O3 | O3: 0.1 g/g COD | Methane growth rate: 180% | [47] |
| O3 | O3: 0.1 g/g DS | Methane growth rate: 25% | [49] |
| O3 | O3: 50 mg/g DS | SS reduction: 49.1%; VSS reduction: 45.7% | [52] |
| O3 + CT | O3: 60 g/g TS; CT 20 mg/g TS | Water content: 56.5% | [29,50] |
| Preprocessing Method | Optimal Pretreatment Conditions | Conditioning Dehydration Effect | Ref. |
|---|---|---|---|
| TA + Fe0/KMnO4 | KMnO4 0.4 mM/g DS; Fe0 2.5 mMg/g DS; T: 45 °C | Water Content: 60.1% | [54] |
| Ferrate (VI) | VI: 25.3 mmol/L; T: 25 °C; pH: 7.0 | tetrabromobisphenol A reduction: 99.1% | [57] |
| K2FeO4 | K2FeO4: 1200 mg/L; pH: 3 | SRF reduction: 30.5%; Water content: 73.4% | [55] |
| UV + Ferrate (VI) | VI: 0.1 g/L; pH: 7.0; UV: 0.198 mW/cm2 | 2,4-DCP degradation growth rate: 1020% | [58] |
| KMnO4 + Fe2+ | KMnO4/Fe2+ = 3/1 (mass ration) | SRF reduction: 83.8% | [59] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, J.; Ji, J.; Hu, Z.; Rao, T.; Liu, A.; Ma, J.; Sun, Y. Application of Advanced Oxidation Technology in Sludge Conditioning and Dewatering: A Critical Review. Int. J. Environ. Res. Public Health 2022, 19, 9287. https://doi.org/10.3390/ijerph19159287
Xia J, Ji J, Hu Z, Rao T, Liu A, Ma J, Sun Y. Application of Advanced Oxidation Technology in Sludge Conditioning and Dewatering: A Critical Review. International Journal of Environmental Research and Public Health. 2022; 19(15):9287. https://doi.org/10.3390/ijerph19159287
Chicago/Turabian StyleXia, Jiahua, Juan Ji, Zhiqiang Hu, Ting Rao, Ankang Liu, Jingqian Ma, and Yongjun Sun. 2022. "Application of Advanced Oxidation Technology in Sludge Conditioning and Dewatering: A Critical Review" International Journal of Environmental Research and Public Health 19, no. 15: 9287. https://doi.org/10.3390/ijerph19159287
APA StyleXia, J., Ji, J., Hu, Z., Rao, T., Liu, A., Ma, J., & Sun, Y. (2022). Application of Advanced Oxidation Technology in Sludge Conditioning and Dewatering: A Critical Review. International Journal of Environmental Research and Public Health, 19(15), 9287. https://doi.org/10.3390/ijerph19159287







