Removal of Methyl Red from Aqueous Solution Using Polyethyleneimine Crosslinked Alginate Beads with Waste Foundry Dust as a Magnetic Material
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of WFD/SA-PEI
2.3. Batch Experiments
2.4. Data Analysis
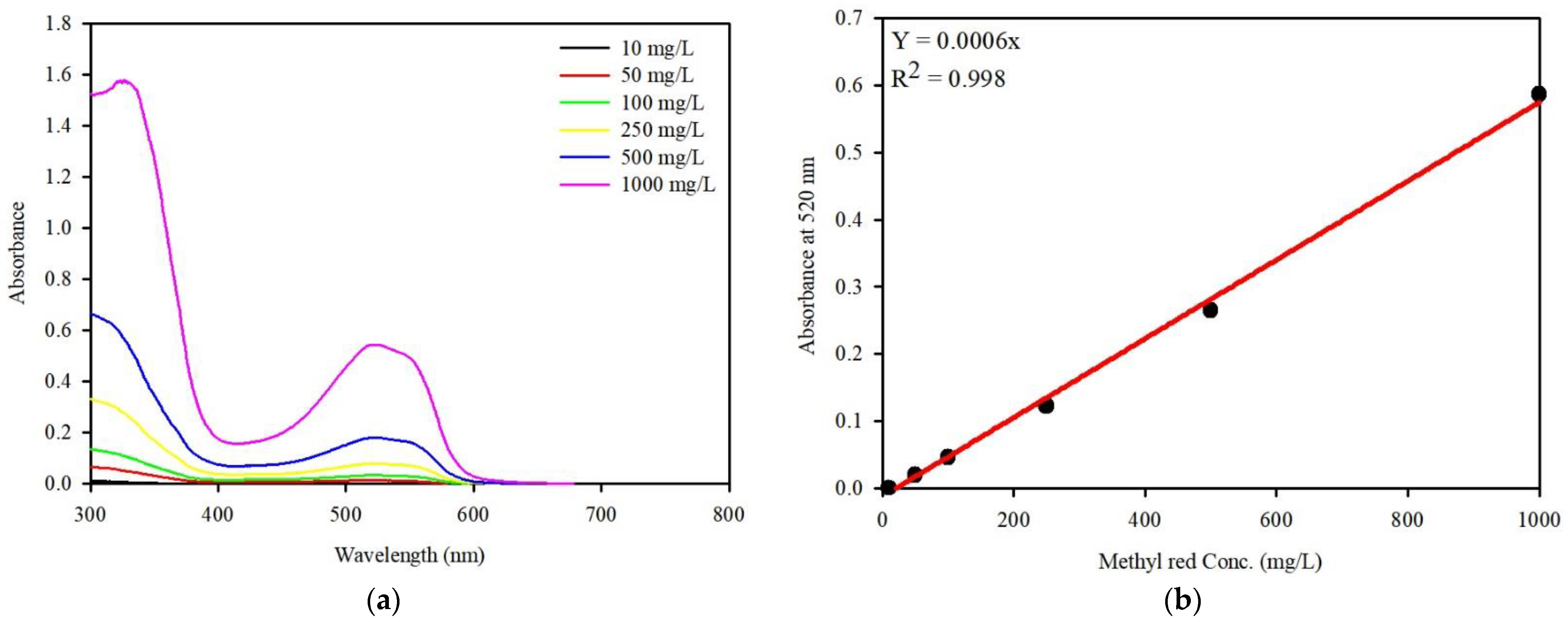
2.5. Analytical Methods
3. Results and Discussion
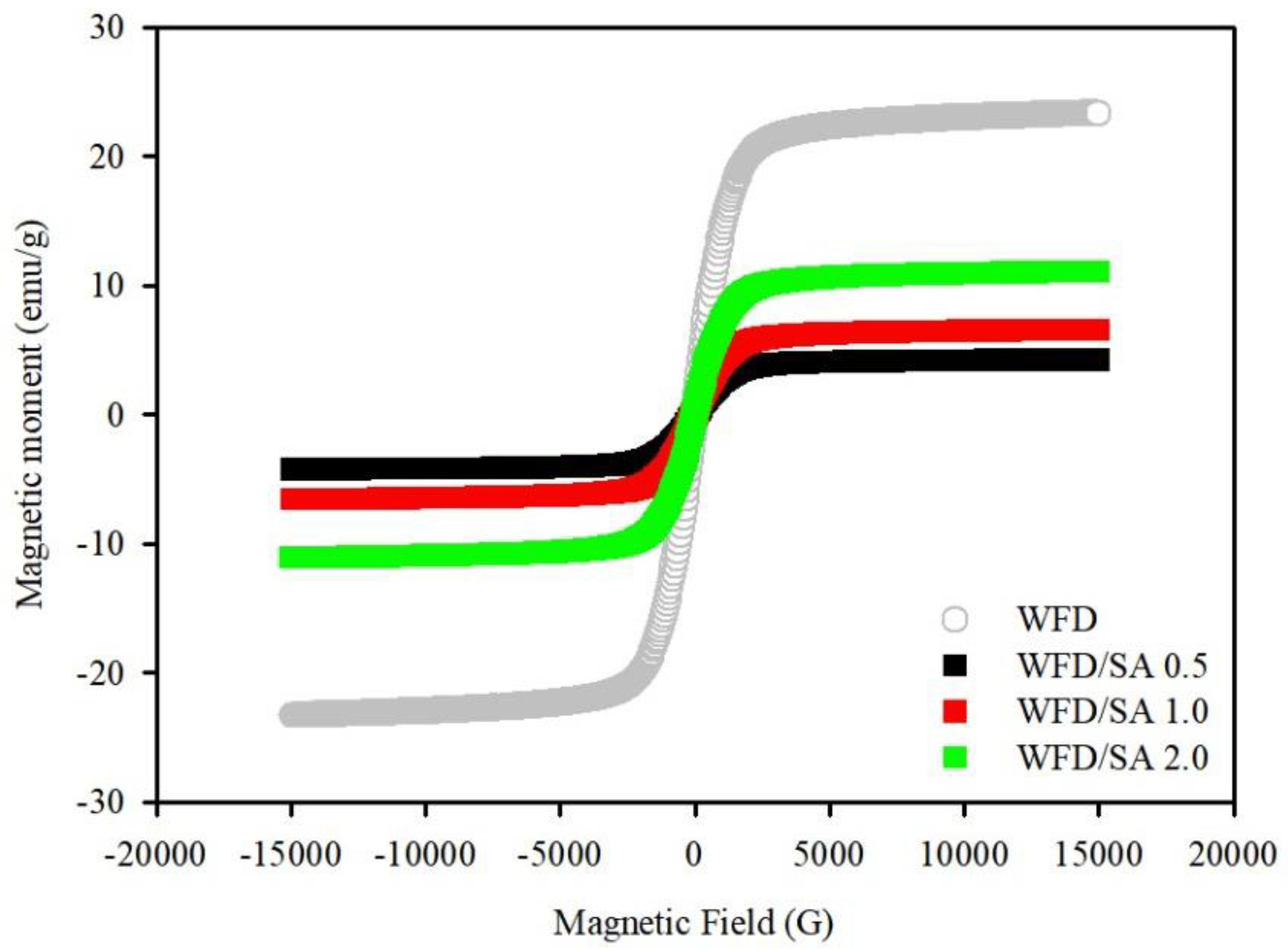
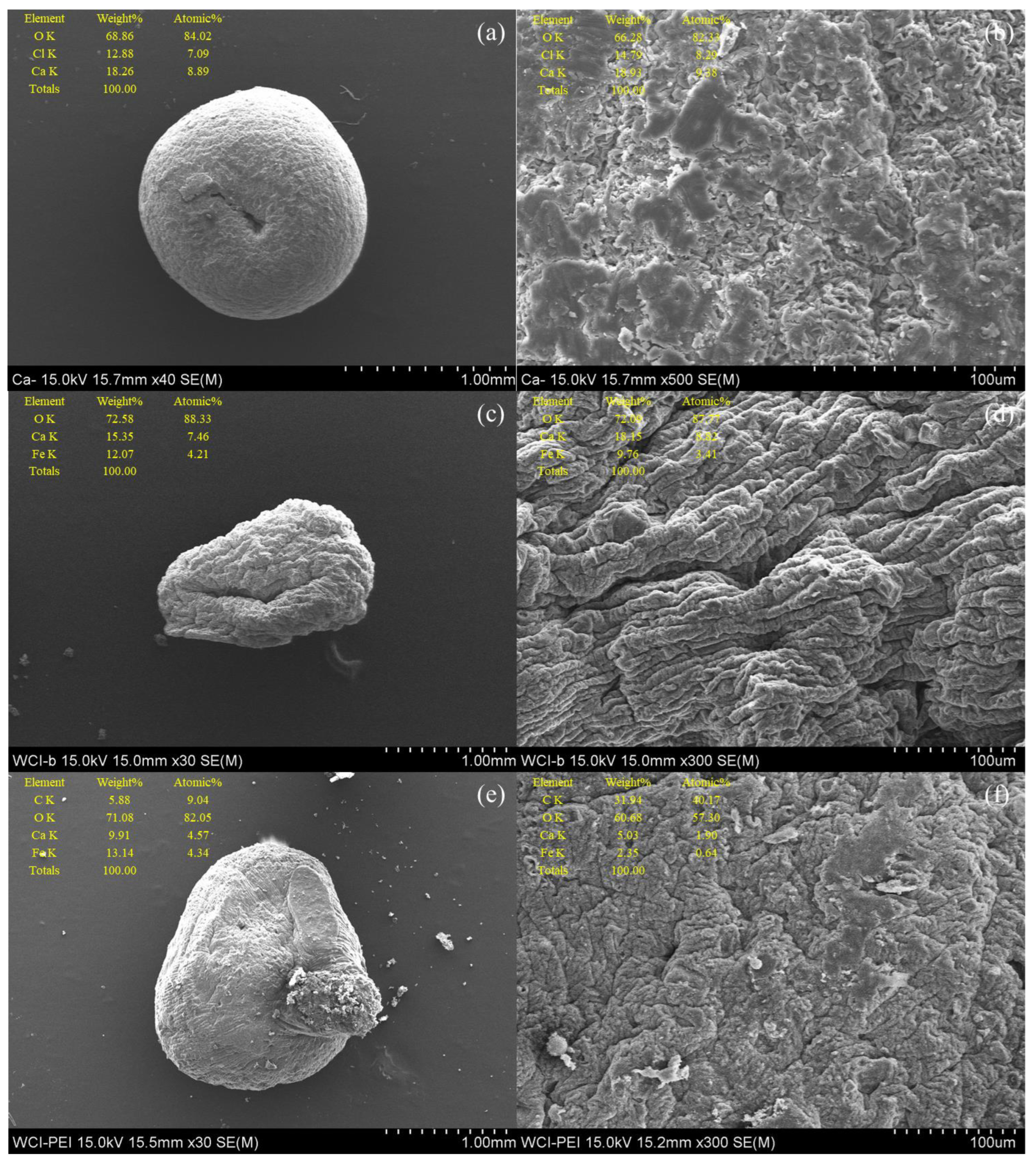
3.1. Characterization of WFD
3.2. Characterization of WFD/SA Bead and WFD/SA-PEI Bead
3.3. MR Removal under Batch Conditions
3.3.1. Effect of Initial pH on the Adsorption
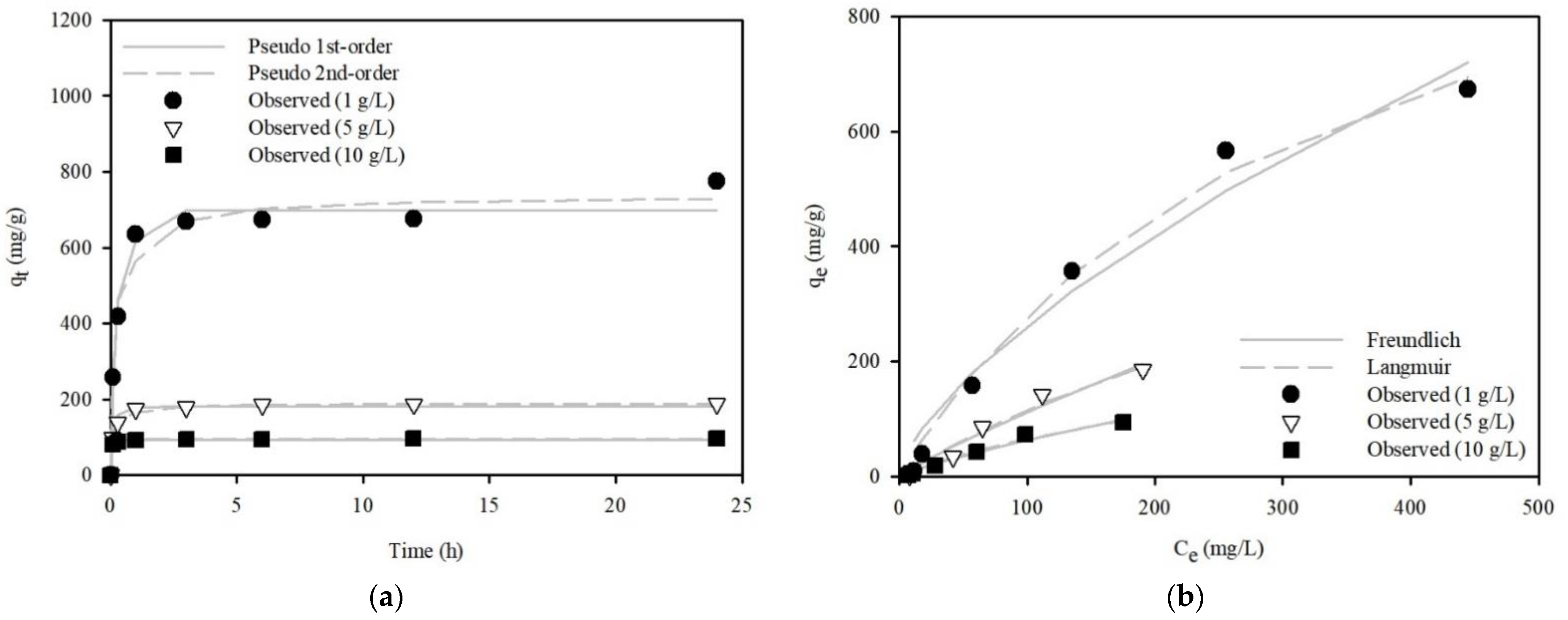
3.3.2. Kinetic and Equilibrium Model Analyses
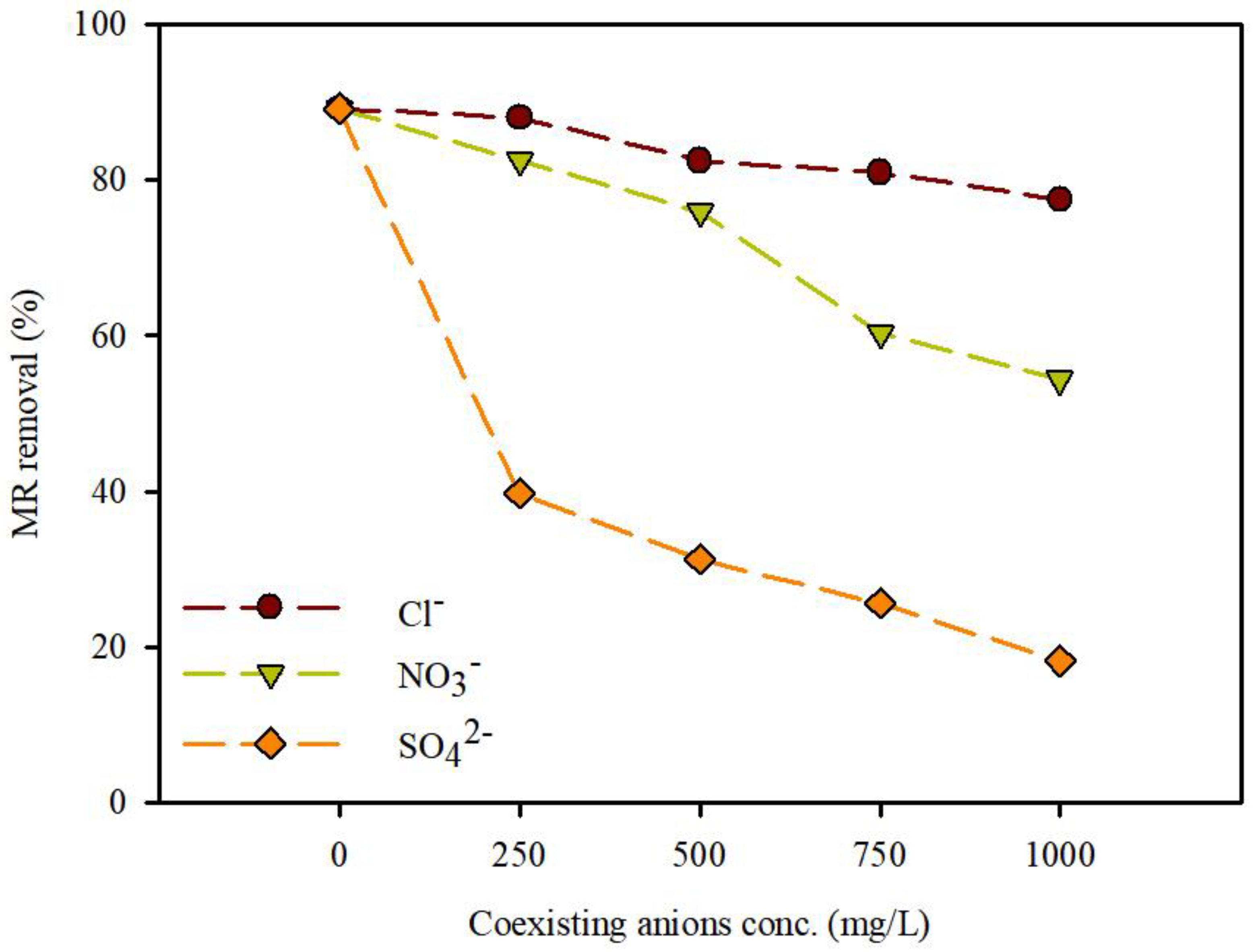
3.3.3. Effect of Coexisting Ions for the Removal of MR
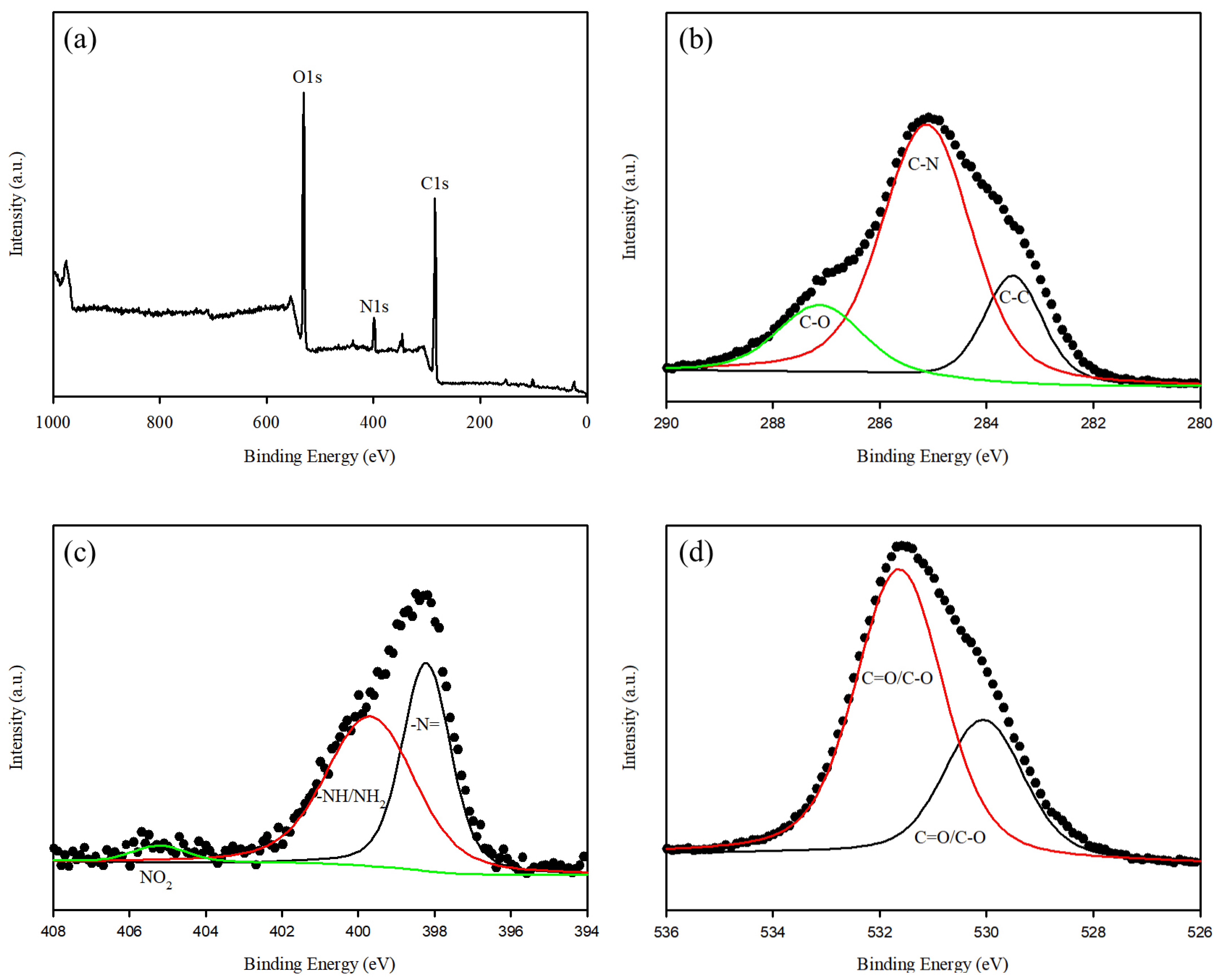
3.4. MR Removal Mechanisms of WFD/SA-PEI Bead
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gong, X.; Jiang, W.; Hu, S.; Yang, Z.; Liu, X.; Fan, Z. Comprehensive utilization of foundry dust: Coal powder and clay minerals separation by ultrasonic-assisted flotation. J. Hazard. Mater. 2021, 402, 124124. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.; Mahajani, S.M.; Jadhav, G.N.; Vishwakarma, R.; Malgaonkar, V.; Mandre, S. Mechanical and thermal methods for reclamation of waste foundry sand. J. Environ. Manag. 2021, 279, 111628. [Google Scholar] [CrossRef]
- Kim, E.; Spooren, J.; Broos, K.; Nielsen, P.; Horckmans, L.; Vrancken, K.C.; Quaghebeur, M. New method for selective Cr recovery from stainless steel slag by NaOCl assisted alkaline leaching and consecutive BaCrO4 precipitation. Chem. Eng. J. 2016, 295, 542–551. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, H.; Jiang, H.; Zhang, W.; Mao, L. Recycling municipal solid waste incineration fly ash in fired bricks: An evaluation of physical-mechanical and environmental properties. Constr. Build. Mater. 2021, 294, 123476. [Google Scholar] [CrossRef]
- Bhardwaj, B.; Kumar, P. Waste foundry sand in concrete: A review. Constr. Build. Mater. 2017, 156, 661–674. [Google Scholar] [CrossRef]
- Zhang, Y.; Sappinen, T.; Korkiala-Tanttu, L.; Vilenius, M.; Juuti, E. Investigations into stabilized waste foundry sand for applications in pavement structures. Resour. Conserv. Recycl. 2021, 170, 105585. [Google Scholar] [CrossRef]
- Górka-Kostrubiec, B.; Magiera, T.; Dudzisz, K.; Dytłow, S.; Wawer, M.; Winkler, A. Integrated Magnetic Analyses for the Discrimination of Urban and Industrial Dusts. Minerals 2020, 10, 1056. [Google Scholar] [CrossRef]
- Coronado, M.; Andrés, A.; Cheeseman, C.R. Acid gas emissions from structural clay products containing secondary resources: Foundry sand dust and Waelz slag. J. Clean. Prod. 2016, 115, 191–202. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Gao, M.; Wang, M.; Wu, C.; Wang, Q.; Wang, Y. Chloride removal from municipal solid waste incineration fly ash using lactic acid fermentation broth. Waste Manag. 2021, 130, 23–29. [Google Scholar] [CrossRef]
- Rha, S.; Jo, H.Y. Waste foundry dust (WFD) as a reactive material for removing As(III) and Cr(VI) from aqueous solutions. J. Hazard. Mater. 2021, 412, 125290. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Xu, D.; Li, Y.; Ou, Z.; Howard, A. Synthesis of a new porous geopolymer from foundry dust to remove Pb2+ and Ni2+ from aqueous solutions. J. Clean. Prod. 2022, 349, 131488. [Google Scholar] [CrossRef]
- Khan, F.S.A.; Mubarak, N.M.; Tan, Y.H.; Khalid, M.; Karri, R.R.; Walvekar, R.; Abdullah, E.C.; Nizamuddin, S.; Mazari, S.A. A comprehensive review on magnetic carbon nanotubes and carbon nanotube-based buckypaper for removal of heavy metals and dyes. J. Hazard. Mater. 2021, 413, 125375. [Google Scholar] [CrossRef]
- Pan, J.; Zhou, L.; Chen, H.; Liu, X.; Hong, C.; Chen, D.; Pan, B. Mechanistically understanding adsorption of methyl orange, indigo carmine, and methylene blue onto ionic/nonionic polystyrene adsorbents. J. Hazard. Mater. 2021, 418, 126300. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Srivastava, S.K.; Gupta, B.; Gupta, A.K. Hollow Polyaniline Microsphere/MnO2/Fe3O4 Nanocomposites in Adsorptive Removal of Toxic Dyes from Contaminated Water. ACS Appl. Mater. Interfaces 2021, 13, 54324–54338. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Yadav, A.; Bagotia, N.; Sharma, A.K.; Kumar, S. Adsorptive potential of modified plant-based adsorbents for sequestration of dyes and heavy metals from wastewater—A review. J. Water Proc. Eng. 2021, 42, 102148. [Google Scholar] [CrossRef]
- Pap, S.; Bezanovic, V.; Radonic, J.; Babic, A.; Saric, S.; Adamovic, D.; Turk Sekulic, M. Synthesis of highly-efficient functionalized biochars from fruit industry waste biomass for the removal of chromium and lead. J. Mol. Liq. 2018, 268, 315–325. [Google Scholar] [CrossRef]
- Zhang, H.; Li, R.; Zhang, Z. A versatile EDTA and chitosan bi-functionalized magnetic bamboo biochar for simultaneous removal of methyl orange and heavy metals from complex wastewater. Environ. Pollut. 2022, 293, 118517. [Google Scholar] [CrossRef]
- Liu, D.M.; Dong, C.; Zhong, J.; Ren, S.; Chen, Y.; Qiu, T. Facile preparation of chitosan modified magnetic kaolin by one-pot coprecipitation method for efficient removal of methyl orange. Carbohydr. Polym. 2020, 245, 116572. [Google Scholar] [CrossRef]
- Hamedi, A.; Zarandi, M.B.; Nateghi, M.R. Highly efficient removal of dye pollutants by MIL-101(Fe) metal-organic framework loaded magnetic particles mediated by Poly L-Dopa. J. Environ. Chem. Eng. 2019, 7, 102882. [Google Scholar] [CrossRef]
- Kamari, S.; Shahbazi, A. Biocompatible Fe3O4@SiO2-NH2 nanocomposite as a green nanofiller embedded in PES-nanofiltration membrane matrix for salts, heavy metal ion and dye removal: Long-term operation and reusability tests. Chemosphere 2020, 243, 125282. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y.; Liu, X.; Jiang, X.; Zhang, Z.; Zhang, T.; Zhang, L. The investigation of synergistic and competitive interaction between dye Congo red and methyl blue on magnetic MnFe2O4. Chem. Eng. J. 2014, 246, 88–96. [Google Scholar] [CrossRef]
- Kondaveeti, S.; Cornejo, D.R.; Petri, D.F. Alginate/magnetite hybrid beads for magnetically stimulated release of dopamine. Colloids Surf. B Biointerfaces 2016, 138, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Khoobi, M.; Motevalizadeh, S.F.; Asadgol, Z.; Forootanfar, H.; Shafiee, A.; Faramarzi, M.A. Polyethyleneimine-modified superparamagnetic Fe3O4 nanoparticles for lipase immobilization: Characterization and application. Mater. Chem. Phys. 2015, 149-150, 77–86. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, H.; Xu, J.; Du, X.; Cheng, X.; Du, Z.; Wang, H. Fabrication of MXene/PEI functionalized sodium alginate aerogel and its excellent adsorption behavior for Cr(VI) and Congo Red from aqueous solution. J. Hazard. Mater. 2021, 416, 125777. [Google Scholar] [CrossRef] [PubMed]
- Qamar, S.A.; Qamar, M.; Basharat, A.; Bilal, M.; Cheng, H.; Iqbal, H.M.N. Alginate-based nano-adsorbent materials—Bioinspired solution to mitigate hazardous environmental pollutants. Chemosphere 2022, 288, 132618. [Google Scholar] [CrossRef]
- Elgarahy, A.M.; Elwakeel, K.Z.; Mohammad, S.H.; Elshoubaky, G.A. A critical review of biosorption of dyes, heavy metals and metalloids from wastewater as an efficient and green process. Clean. Eng. Technol. 2021, 4, 100209. [Google Scholar] [CrossRef]
- Gogoi, H.; Leiviska, T.; Ramo, J.; Tanskanen, J. Production of aminated peat from branched polyethylenimine and glycidyltrimethylammonium chloride for sulphate removal from mining water. Environ. Res. 2019, 175, 323–334. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, W. Polyethylenimine-functionalized polyacrylonitrile anion exchange fiber as a novel adsorbent for rapid removal of nitrate from wastewater. Chemosphere 2020, 258, 127373. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, Y.; Song, J.; Yang, J.; Pan, C.; Xu, T.; Zhang, L. Novel Balanced Charged Alginate/PEI Polyelectrolyte Hydrogel that Resists Foreign-Body Reaction. ACS Appl. Mater. Interfaces 2018, 10, 6879–6886. [Google Scholar] [CrossRef]
- Godiya, C.B.; Xiao, Y.; Lu, X. Amine functionalized sodium alginate hydrogel for efficient and rapid removal of methyl blue in water. Int. J. Biol. Macromol. 2020, 144, 671–681. [Google Scholar] [CrossRef]
- Wu, Y.; Zeng, J.; Huang, X.; Yuan, Z.; Liu, G.; Ding, J. Styrene-maleic anhydride/polyethersulfone blending membranes modified by PEI functionalized TiO2 to enhance separation and antifouling properties: Dye purification. J. Environ. Chem. Eng. 2021, 9, 106040. [Google Scholar] [CrossRef]
- Dangi, Y.R.; Bediako, J.K.; Lin, X.; Choi, J.W.; Lim, C.R.; Song, M.H.; Han, M.; Yun, Y.S. Polyethyleneimine impregnated alginate capsule as a high capacity sorbent for the recovery of monovalent and trivalent gold. Sci Rep. 2021, 11, 17836. [Google Scholar] [CrossRef]
- Yue, H.; Shang, Z.; Xu, P.; Feng, D.; Li, X. Preparation of EDTA modified chitooligosaccharide/sodium alginate/Ca2+ physical double network hydrogel by using of high-salinity oilfield produced water for adsorption of Zn2+, Ni2+ and Mn2+. Sep. Purif. Technol. 2022, 280, 119767. [Google Scholar] [CrossRef]
- Fan, L.; Lu, Y.; Yang, L.Y.; Huang, F.; Ouyang, X.K. Fabrication of polyethylenimine-functionalized sodium alginate/cellulose nanocrystal/polyvinyl alcohol core-shell microspheres ((PVA/SA/CNC)@PEI) for diclofenac sodium adsorption. J. Colloid Interface Sci. 2019, 554, 48–58. [Google Scholar] [CrossRef]
- Luo, H.; Zeng, X.; Liao, P.; Rong, H.; Zhang, T.C.; Jason Zhang, Z.; Meng, X. Phosphorus removal and recovery from water with macroporous bead adsorbent constituted of alginate-Zr(4+) and PNIPAM-interpenetrated networks. Int. J. Biol. Macromol. 2019, 126, 1133–1144. [Google Scholar] [CrossRef]
- Vu, H.C.; Dwivedi, A.D.; Le, T.T.; Seo, S.-H.; Kim, E.-J.; Chang, Y.-S. Magnetite graphene oxide encapsulated in alginate beads for enhanced adsorption of Cr(VI) and As(V) from aqueous solutions: Role of crosslinking metal cations in pH control. Chem. Eng. J. 2017, 307, 220–229. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Saleh, M.M.; Zaki, M.M.; Nabil, G.M. A sustainable nanocomposite for removal of heavy metals from water based on crosslinked sodium alginate with iron oxide waste material from steel industry. J. Environ. Chem. Eng. 2020, 8, 104015. [Google Scholar] [CrossRef]
- Li, L.; Zhang, M.; Jiang, W.; Yang, P. Study on the efficacy of sodium alginate gel particles immobilized microorganism SBBR for wastewater treatment. J. Environ. Chem. Eng. 2022, 10, 107134. [Google Scholar] [CrossRef]
- Voo, W.-P.; Lee, B.-B.; Idris, A.; Islam, A.; Tey, B.-T.; Chan, E.-S. Production of ultra-high concentration calcium alginate beads with prolonged dissolution profile. RSC Adv. 2015, 5, 36687–36695. [Google Scholar] [CrossRef]
- Uyen, N.T.T.; Hamid, Z.A.A.; Tram, N.X.T.; Ahmad, N. Fabrication of alginate microspheres for drug delivery: A review. Int. J. Biol. Macromol. 2020, 153, 1035–1046. [Google Scholar] [CrossRef]
- Gao, C.; Wang, X.L.; An, Q.D.; Xiao, Z.Y.; Zhai, S.R. Synergistic preparation of modified alginate aerogel with melamine/chitosan for efficiently selective adsorption of lead ions. Carbohydr. Polym. 2021, 256, 117564. [Google Scholar] [CrossRef]
- Bediako, J.K.; Lin, S.; Sarkar, A.K.; Zhao, Y.; Choi, J.-W.; Song, M.-H.; Wei, W.; Reddy, D.H.K.; Cho, C.-W.; Yun, Y.-S. Benignly-fabricated crosslinked polyethylenimine/calcium-alginate fibers as high-performance adsorbents for effective recovery of gold. J. Clean. Prod. 2020, 252, 119389. [Google Scholar] [CrossRef]
- Xu, R.; Su, C.; Cui, L.; Zhang, K.; Li, J. Preparing Sodium Alginate/Polyethyleneimine Spheres for Potential Application of Killing Tumor Cells by Reducing the Concentration of Copper Ions in the Lesions of Colon Cancer. Materials 2019, 12, 1570. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.; Yang, R.; Yang, F.; Sun, L.; Li, Y.; Xu, J. Fabrication of polyethylenimine functionalized magnetic cellulose nanofibers for the sorption of Ni(II), Cu(II) and Cd(II) in single-component and multi-component systems. Int. J. Biol. Macromol. 2021, 184, 68–78. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, L.; Wang, Z.; Yang, S.; Li, P.; Song, P.; Ban, M. A highly permeable loose nanofiltration membrane prepared via layer assembled in-situ mineralization. J. Membr. Sci. 2019, 587, 117159. [Google Scholar] [CrossRef]
- Guo, D.-M.; An, Q.-D.; Xiao, Z.-Y.; Zhai, S.-R.; Shi, Z. Polyethylenimine-functionalized cellulose aerogel beads for efficient dynamic removal of chromium(vi) from aqueous solution. RSC Adv. 2017, 7, 54039–54052. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Chen, T.; Liu, J.; Li, D. Adsorption of tetracycline antibiotics from an aqueous solution onto graphene oxide/calcium alginate composite fibers. RSC Adv. 2018, 8, 2616–2621. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; An, Q.; Xiao, Z.; Zheng, W.; Zhai, S. Flexible core-shell/bead-like alginate@PEI with exceptional adsorption capacity, recycling performance toward batch and column sorption of Cr(VI). Chem. Eng. J. 2017, 313, 475–486. [Google Scholar] [CrossRef]
- Kang, J.-K.; Lee, S.-C.; Kim, S.-B. Enhancement of selective Cu(II) sorption through preparation of surface-imprinted mesoporous silica SBA-15 under high molar concentration ratios of chloride and copper ions. Microporous Mesoporous Mater. 2018, 272, 193–201. [Google Scholar] [CrossRef]
- Zaheer, Z.; Al-Asfar, A.; Aazam, E.S. Adsorption of methyl red on biogenic Ag@Fe nanocomposite adsorbent: Isotherms, kinetics and mechanisms. J. Mol. Liq. 2019, 283, 287–298. [Google Scholar] [CrossRef]
- Roik, N.V.; Belyakova, L.A.; Dziazko, M.O. Selective sorptive removal of Methyl Red from individual and binary component solutions by mesoporous organosilicas of MCM-41 type. J. Environ. Sci. 2021, 99, 59–71. [Google Scholar] [CrossRef]
- Saleh, T.A.; Al-Absi, A.A. Kinetics, isotherms and thermodynamic evaluation of amine functionalized magnetic carbon for methyl red removal from aqueous solutions. J. Mol. Liq. 2017, 248, 577–585. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Ahmed, N.A.B.; Adegoke, K.A.; Bello, O.S. Sorption studies of methyl red dye removal using lemon grass (Cymbopogon citratus). Chem. Data Collect. 2019, 22, 100249. [Google Scholar] [CrossRef] [Green Version]
- Dadfarnia, S.; Haji Shabani, A.M.; Moradi, S.E.; Emami, S. Methyl red removal from water by iron based metal-organic frameworks loaded onto iron oxide nanoparticle adsorbent. Appl. Surf. Sci. 2015, 330, 85–93. [Google Scholar] [CrossRef]
- Mozaffari, M.; Emami, M.R.S.; Binaeian, E. A novel thiosemicarbazide modified chitosan (TSFCS) for efficiency removal of Pb (II) and methyl red from aqueous solution. Int. J. Biol. Macromol. 2019, 123, 457–467. [Google Scholar] [CrossRef]
- Romdhane, D.F.; Satlaoui, Y.; Nasraoui, R.; Charef, A.; Azouzi, R. Adsorption, Modeling, Thermodynamic, and Kinetic Studies of Methyl Red Removal from Textile-Polluted Water Using Natural and Purified Organic Matter Rich Clays as Low-Cost Adsorbent. J. Chem. 2020, 2020, 4376173. [Google Scholar] [CrossRef]
- Lafi, R.; Abdellaoui, L.; Montasser, I.; Mabrouk, W.; Hafiane, A. The effect of head group of surfactant on the adsorption of methyl red onto modified coffee residues. J. Mol. Struct. 2022, 1249, 131527. [Google Scholar] [CrossRef]
- Masengo, J.L.; Mulopo, J. Synthesis and performance evaluation of adsorbents derived from sewage sludge blended with waste coal for nitrate and methyl red removal. Sci. Rep. 2022, 12, 1670. [Google Scholar] [CrossRef]
- Ghorbani, F.; Kamari, S. Core–shell magnetic nanocomposite of Fe3O4@SiO2@NH2 as an efficient and highly recyclable adsorbent of methyl red dye from aqueous environments. Environ. Technol. Innov. 2019, 14, 100333. [Google Scholar] [CrossRef]
- Chen, M.; Hankins, N.P. Interaction among branched polyethylenimine (PEI), sodium dodecyl sulfate (SDS) and metal cations during copper recovery from water using polymer-surfactant aggregates. J. Water Proc. Eng. 2020, 34, 101170. [Google Scholar] [CrossRef]





| Dye | Color | pKa | Change Range of pH | Molecular Weight (g/mol) | Maximum Adsorption Wavelength (nm) | Chemical Structure |
|---|---|---|---|---|---|---|
| Methyl red | Red | 5.1 | 4.4 < pH < 6.0 | 269.3 | 520 | 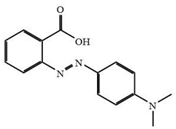 |
| Compound | Fe2O3 | SiO2 | ZrO2 | Al2O3 | SO3 |
| Conc. (%) | 40.03 | 25.45 | 10.09 | 7.28 | 4.03 |
| Compound | CuO | ZnO | PdO | K2O | CaO |
| Conc. (%) | 2.45 | 1.99 | 1.87 | 1.48 | 1.44 |
| Sample | Final pH | Al | As | Ba | Ca | Cd | Co | Cr | Cu |
| pH1 | 1.53 | 143.36 | 0.58 | 0.13 | 503.10 | N.D | N.D | 0.08 | 205.94 |
| pH3 | 5.24 | 11.52 | N.D | N.D | 258.98 | N.D | N.D | N.D | 1.90 |
| pH5 | 5.58 | 6.55 | N.D | N.D | 202.73 | N.D | N.D | N.D | 1.14 |
| pH7 | 5.67 | 6.23 | N.D | N.D | 199.75 | N.D | N.D | N.D | 0.88 |
| pH9 | 5.68 | 6.50 | N.D | N.D | 217.35 | N.D | N.D | N.D | 1.05 |
| Sample | Fe | K | Mg | Mn | Mo | Na | Ni | Pb | V |
| pH1 | 156.51 | 74.58 | 38.41 | 95.00 | 0.34 | 94.13 | N.D | 15.77 | 0.97 |
| pH3 | N.D | 38.13 | 27.58 | 32.48 | 0.06 | 80.48 | N.D | N.D | N.D |
| pH5 | N.D | 23.66 | 15.78 | 17.70 | N.D | 47.93 | N.D | N.D | N.D |
| pH7 | N.D | 23.45 | 14.81 | 17.21 | N.D | 45.79 | N.D | N.D | N.D |
| pH9 | N.D | 28.07 | 20.32 | 23.01 | N.D | 59.79 | N.D | N.D | N.D |
| Adsorbent Dose (g/L) | Pseudo-First-Order Model | ||||
| qe (mg/g) | k1 (1/h) | R2 | SSE | ||
| 1 | 698.41 | 2.15 | 0.97 | 2.64 × 101 | 1.23 × 104 |
| 5 | 180.97 | 4.14 | 0.97 | 4.32 × 100 | 6.46 × 102 |
| 10 | 93.14 | 11.77 | 0.99 | 5.93 × 10−1 | 5.51 × 101 |
| Adsorbent Dose (g/L) | Pseudo-Second-Order Model | ||||
| qe (mg/g) | k2 (g/mg/h) | R2 | SSE | ||
| 1 | 737.79 | 0.004 | 0.97 | 1.90 × 101 | 1.14 × 104 |
| 5 | 190.11 | 0.03 | 0.99 | 9.38 × 10−1 | 1.47 × 102 |
| 10 | 95.05 | 1.09 | 0.98 | 1.56 × 100 | 1.42 × 102 |
| Adsorbent Dose (g/L) | Freundlich Model | ||||
| KF (L/g) | 1/n | R2 | SSE | ||
| 1 | 11.83 | 0.67 | 0.96 | 8.78 × 101 | 1.351 × 104 |
| 5 | 1.94 | 0.87 | 0.96 | 2.04 × 101 | 9.924 × 102 |
| 10 | 1.45 | 0.81 | 0.97 | 1.00 × 101 | 2.04 × 102 |
| Adsorbent Dose (g/L) | Langmuir Model | ||||
| Qm (mg/g) | KL (L/mg) | R2 | SSE | ||
| 1 | 1203.91 | 0.003 | 0.99 | 4.00 × 101 | 3.93 × 103 |
| 5 | 704.10 | 0.001 | 0.97 | 1.74 × 101 | 7.66 × 102 |
| 10 | 262.15 | 0.003 | 0.98 | 7.14 × 100 | 1.13 × 102 |
| Adsorbent | Removal Capacity (mg/g) | Reference |
|---|---|---|
| BPEI-modified magnetic activated carbon | 526 | [52] |
| Lemongrass leaf-based activated carbon | 72.3 | [53] |
| Fe3O4@MIL-100(Fe) | 686.3 | [54] |
| Thiosemicarbazide-modified chitosan (TSFCS) | 17.3 | [55] |
| Natural and Purified Organic Matter Rich Clays | 397 | [56] |
| N, N-Dimethyldodecylamine N-oxide(DDAO)–coffee residues(CR) | 76.7 | [57] |
| Sewage sludge blended with waste coal | 312.7 | [58] |
| Fe3O4@SiO2@NH2, amorphous silica from rice husk | 81.3 | [59] |
| WFD/SA-PEI | 672.7 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Purev, O.; Myung, E.; Choi, N.; Cho, K. Removal of Methyl Red from Aqueous Solution Using Polyethyleneimine Crosslinked Alginate Beads with Waste Foundry Dust as a Magnetic Material. Int. J. Environ. Res. Public Health 2022, 19, 9030. https://doi.org/10.3390/ijerph19159030
Kim H, Purev O, Myung E, Choi N, Cho K. Removal of Methyl Red from Aqueous Solution Using Polyethyleneimine Crosslinked Alginate Beads with Waste Foundry Dust as a Magnetic Material. International Journal of Environmental Research and Public Health. 2022; 19(15):9030. https://doi.org/10.3390/ijerph19159030
Chicago/Turabian StyleKim, Hyunsoo, Oyunbileg Purev, Eunji Myung, Nagchoul Choi, and Kanghee Cho. 2022. "Removal of Methyl Red from Aqueous Solution Using Polyethyleneimine Crosslinked Alginate Beads with Waste Foundry Dust as a Magnetic Material" International Journal of Environmental Research and Public Health 19, no. 15: 9030. https://doi.org/10.3390/ijerph19159030
APA StyleKim, H., Purev, O., Myung, E., Choi, N., & Cho, K. (2022). Removal of Methyl Red from Aqueous Solution Using Polyethyleneimine Crosslinked Alginate Beads with Waste Foundry Dust as a Magnetic Material. International Journal of Environmental Research and Public Health, 19(15), 9030. https://doi.org/10.3390/ijerph19159030





