Highly Efficient Transfer Hydrogenation of Biomass-Derived Furfural to Furfuryl Alcohol over Mesoporous Zr-Containing Hybrids with 5-Sulfosalicylic Acid as a Ligand
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of 5-Sulfosalicylic Acid-Derived Zr-Hybrids
2.3. Characterization
2.4. MPV Reduction of Furfural to Furfuryl Alcohol
3. Results and Discussion
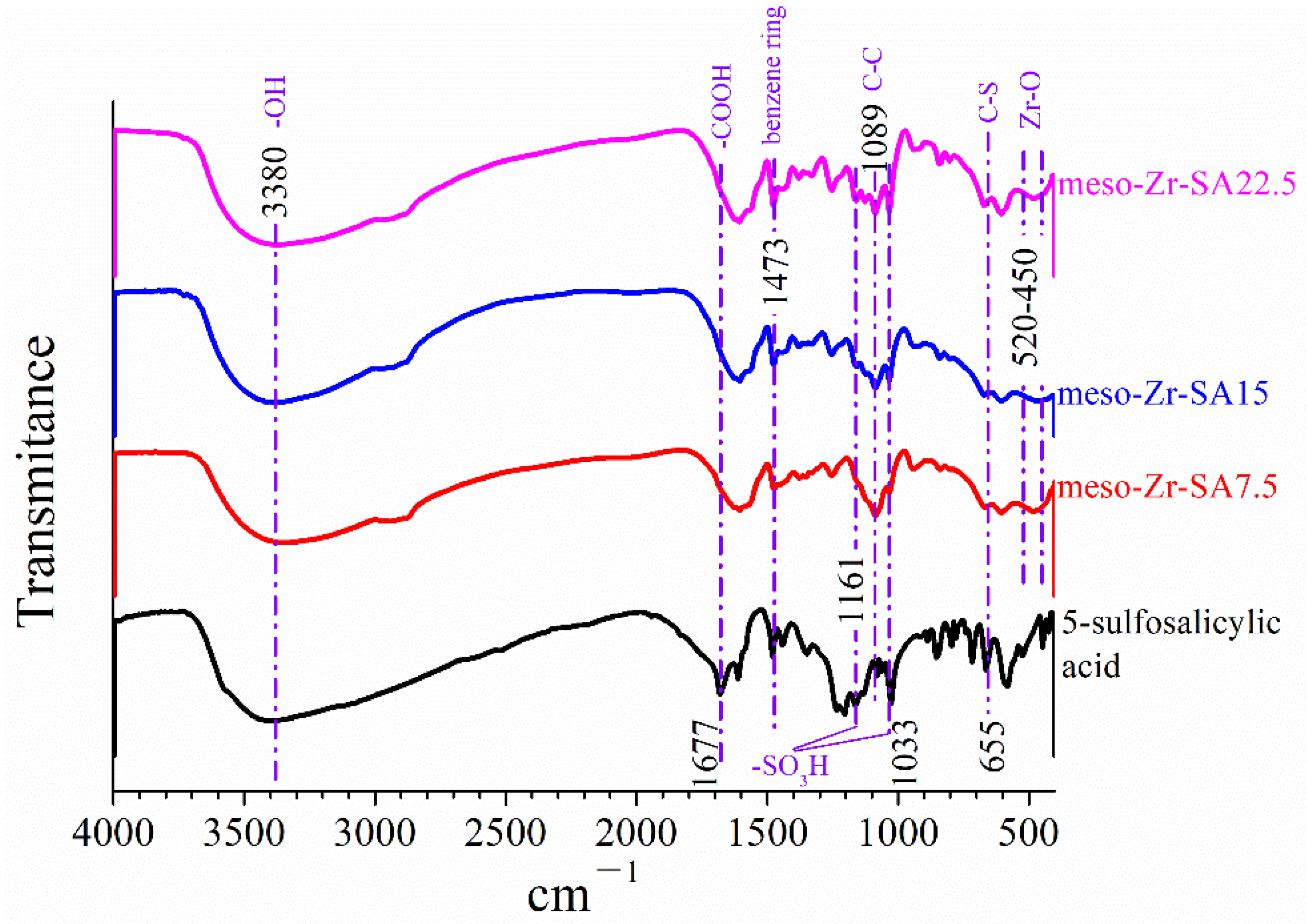
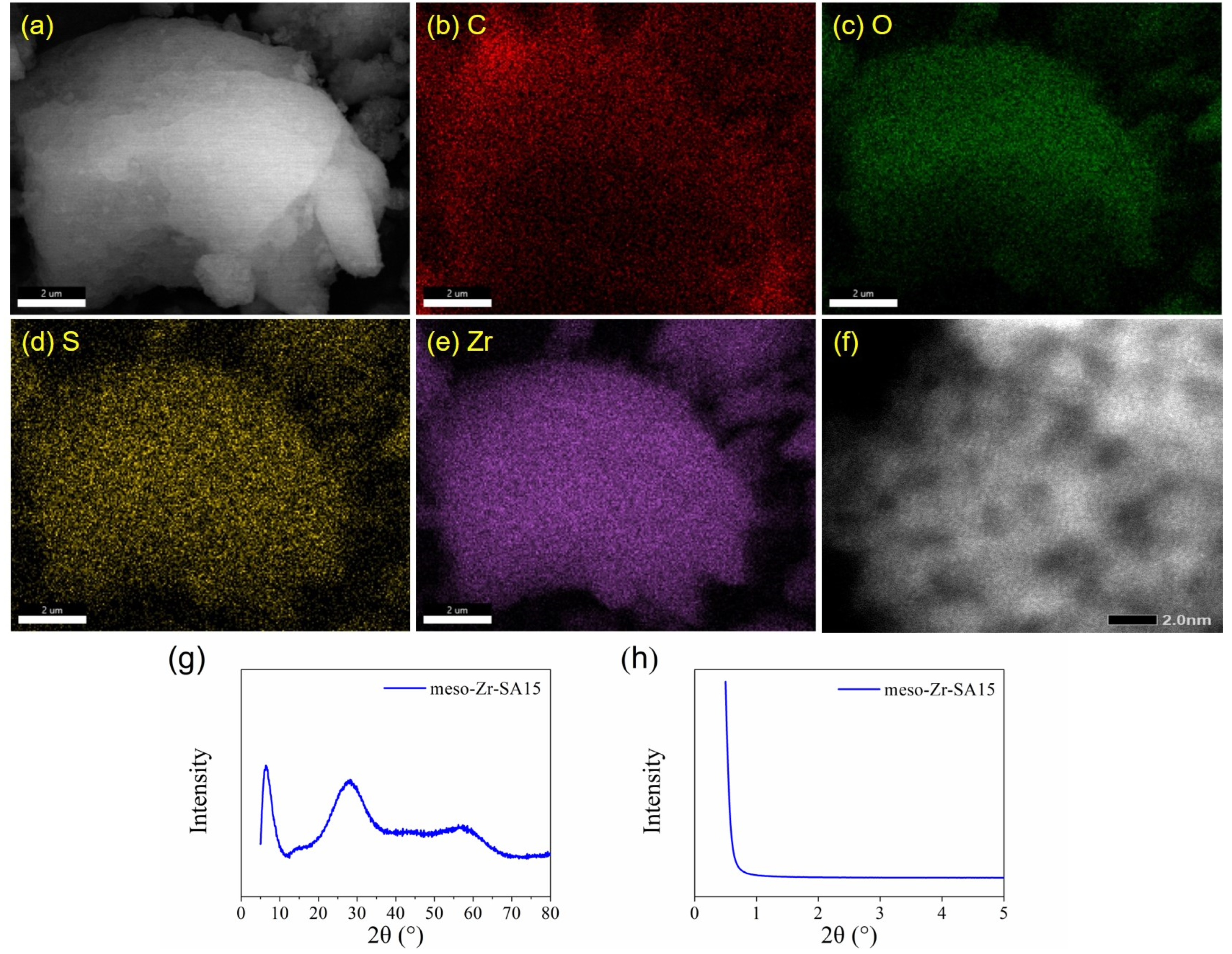
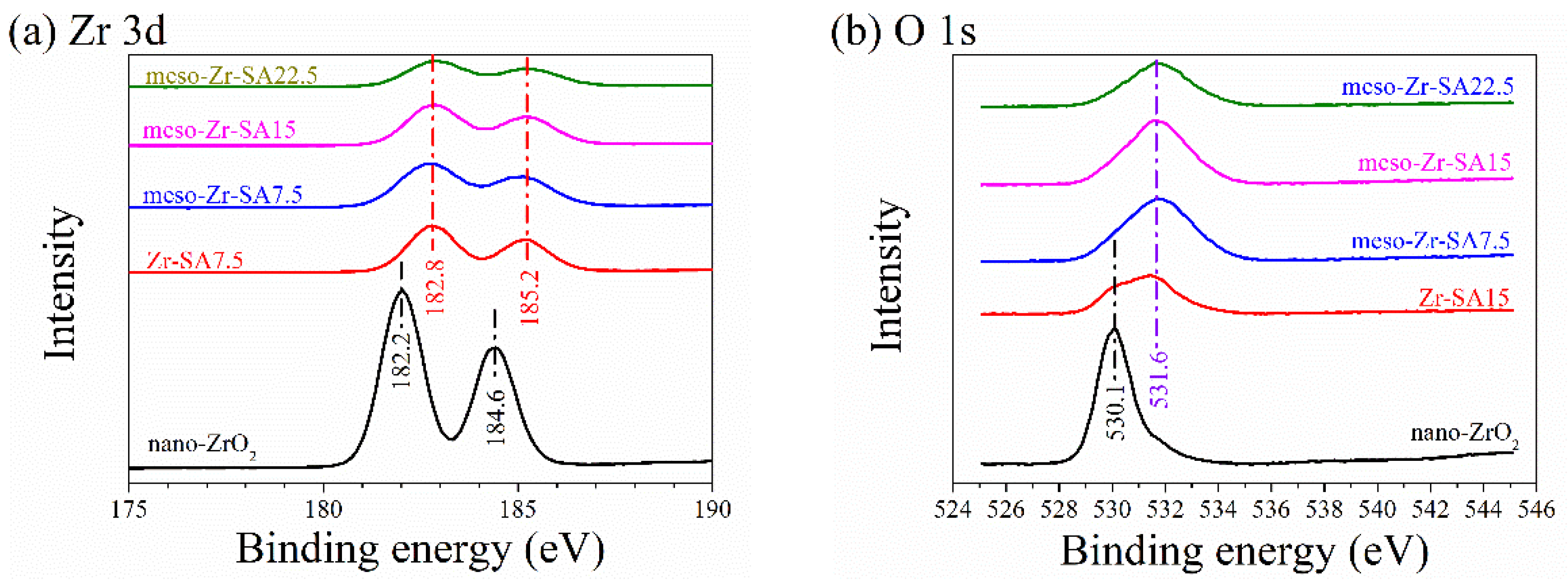
3.1. Characterization Results
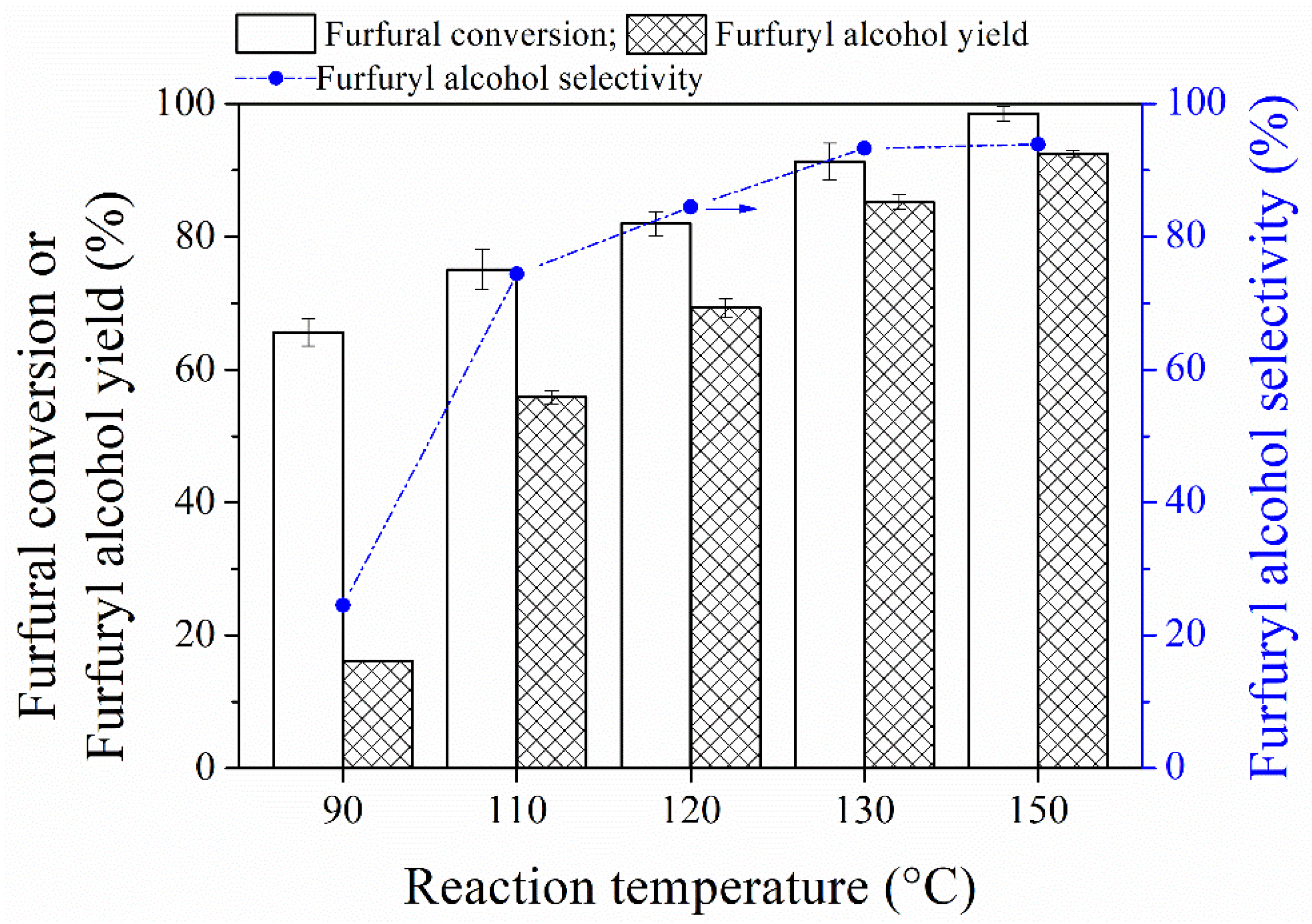
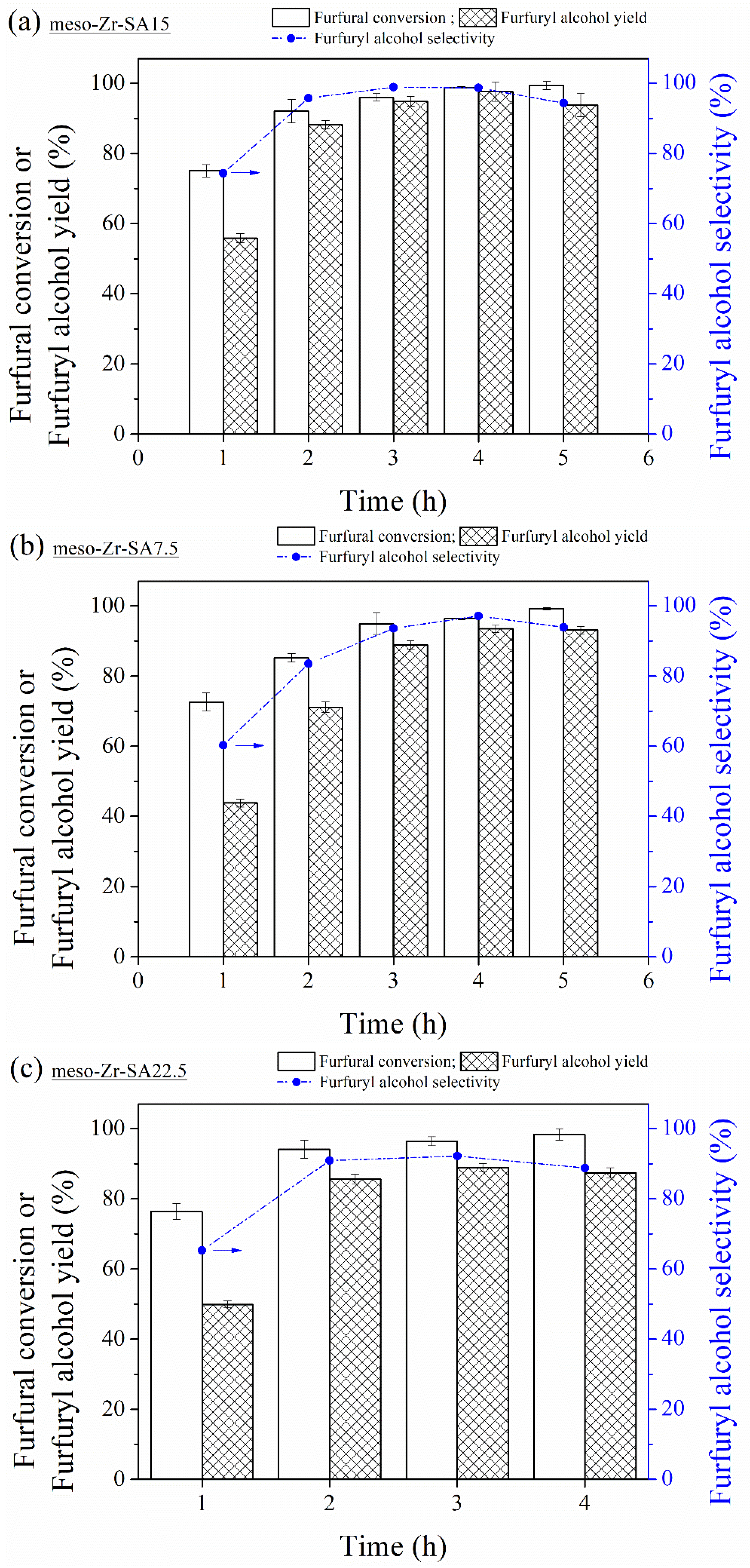
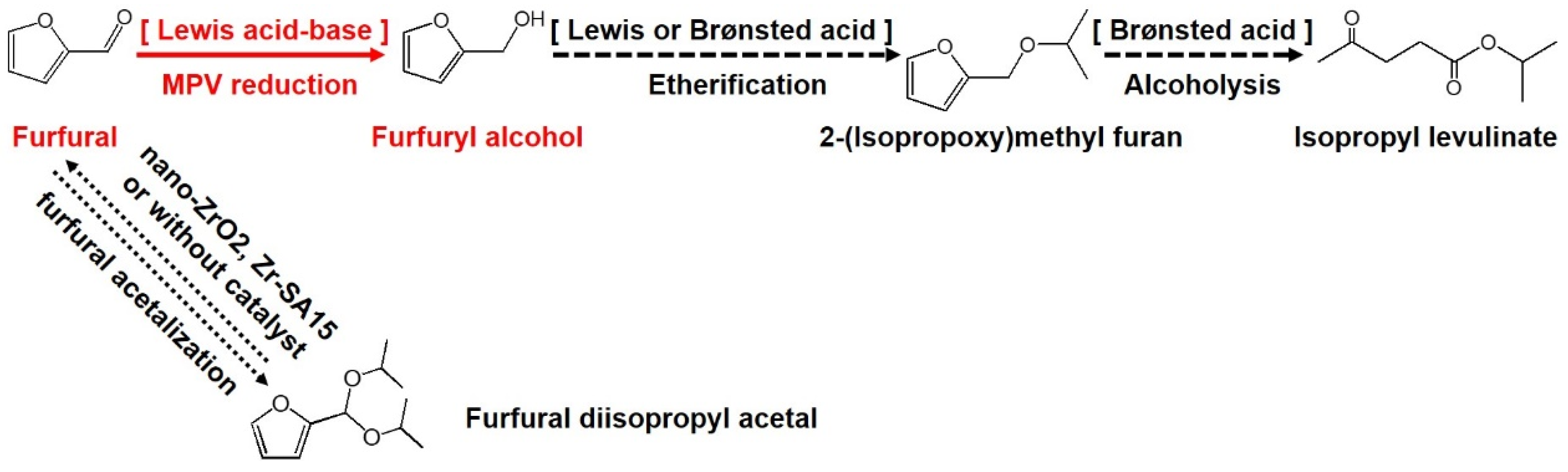
3.2. MPV Reduction of Furfural to Furfuryl Alcohol
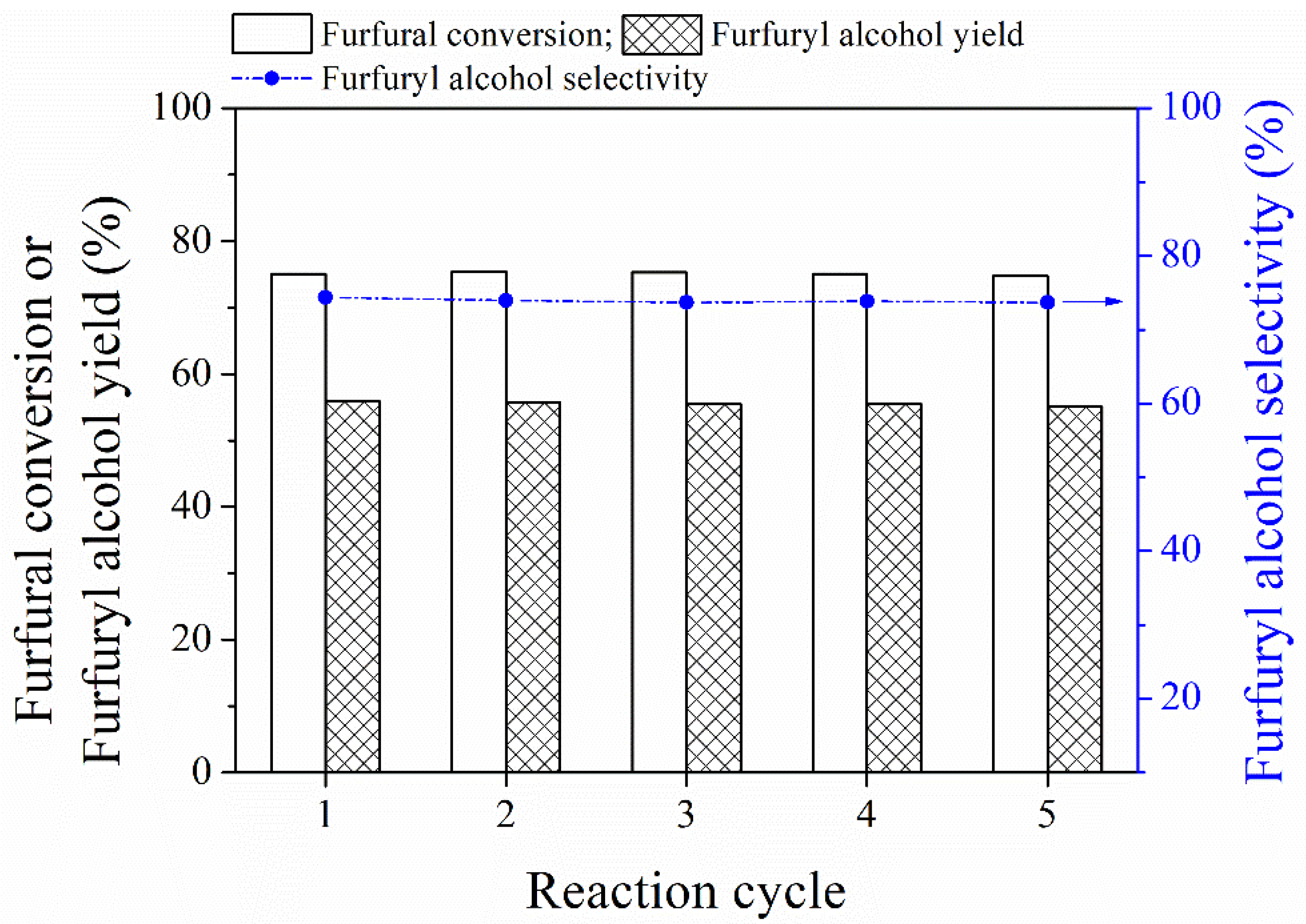
3.3. Catalyst Recyclability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, Y.; Li, M. Hydrothermal liquefaction of lignocellulose for value-added products: Mechanism, parameter and production application. Bioresour. Technol. 2021, 342, 126035. [Google Scholar] [CrossRef]
- Banek, N.A.; Abele, D.T.; McKenzie, K.R.; Wagner, M.J. Sustainable conversion of lignocellulose to high-purity, highly crystalline flake potato graphite. ACS Sustain. Chem. Eng. 2018, 6, 13199–13207. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Li, F.; Huang, Z.; Yuan, G. Hydrogen-transfer conversion of furfural into levulinate esters as potential biofuel feedstock. J. Energy Chem. 2016, 25, 888–894. [Google Scholar] [CrossRef]
- Gómez Bernal, H.; Benito, P.; Rodríguez-Castellón, E.; Raspolli Galletti, A.M.; Funaioli, T. Synthesis of isopropyl levulinate from furfural: Insights on a cascade production perspective. Appl. Catal. A Gen. 2019, 575, 111–119. [Google Scholar] [CrossRef]
- Mariscal, R.; Mairelestorres, P.; Ojeda, M.; Sadaba, I.; Granados, M.L. Furfural: A renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
- Sener, C.; Motagamwala, A.H.; Alonso, D.M.; Dumesic, J.A. Enhanced furfural yields from xylose dehydration in the γ-valerolactone/water solvent system at elevated temperatures. ChemSusChem 2018, 11, 2321–2331. [Google Scholar] [CrossRef] [PubMed]
- Peleteiro, S.; Rivas, S.; Alonso, J.L.; Santos, V.; Parajó, J.C. Furfural production using ionic liquids: A review. Bioresour. Technol. 2016, 202, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Dohade, M.G.; Dhepe, P.L. Efficient hydrogenation of concentrated aqueous furfural solutions into furfuryl alcohol under ambient conditions in presence of PtCo bimetallic catalyst. Green Chem. 2017, 19, 1144–1154. [Google Scholar] [CrossRef]
- Gao, G.; Remón, J.; Jiang, Z.; Yao, L.; Hu, C. Selective hydrogenation of furfural to furfuryl alcohol in water under mild conditions over a hydrotalcite-derived Pt-based catalyst. Appl. Catal. B Environ. 2022, 309, 121260. [Google Scholar] [CrossRef]
- Cao, D.; Xia, S.; Pan, P.; Zeng, H.; Li, C.; Peng, Y. Light-driven MPV-type reduction of aryl ketones/aldehydes to alcohols with isopropanol under mild conditions. Green Chem. 2021, 23, 7539–7543. [Google Scholar] [CrossRef]
- Zhang, J.; Dong, K.; Luo, W.; Guan, H. Selective transfer hydrogenation of furfural into furfuryl alcohol on Zr-containing catalysts using lower alcohols as hydrogen donors. ACS Omega 2018, 3, 6206–6216. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Sun, K.; Zhang, L.; Xu, Q.; Zhang, Z.; Li, Q.; Zhang, S.; Wang, Y.; Liu, Q.; Hu, X. Balanced distribution of Brønsted acidic sites and Lewis acidic sites for highly selective conversion of xylose into levulinic acid/ester over Zr-beta catalysts. Green Chem. 2019, 21, 6634–6645. [Google Scholar] [CrossRef]
- Qiu, M.; Guo, T.; Xi, R.; Li, D.; Qi, X. Highly efficient catalytic transfer hydrogenation of biomass-derived furfural to furfuryl alcohol using UiO-66 without metal catalysts. Appl. Catal. A Gen. 2020, 602, 117719. [Google Scholar] [CrossRef]
- Song, J.; Zhou, B.; Zhou, H.; Wu, L.; Meng, Q.; Liu, Z.; Han, B. Porous zirconium-phytic acid hybrid: A highly efficient catalyst for Meerwein-Ponndorf-Verley reductions. Angew. Chem. Int. Ed. 2015, 54, 9399–9403. [Google Scholar] [CrossRef]
- Sha, Y.; Xiao, Z.; Zhou, H.; Yang, K.; Song, Y.; Li, N.; He, R.; Zhi, K.; Liu, Q. Direct use of humic acid mixtures to construct efficient Zr-containing catalysts for Meerwein-Ponndorf-Verley reactions. Green Chem. 2017, 19, 4829–4837. [Google Scholar] [CrossRef]
- Li, H.; Liu, X.; Yang, T.; Zhao, W.; Saravanamurugan, S.; Yang, S. Porous zirconium-furandicarboxylate microspheres for efficient redox conversion of biofuranics. ChemSusChem 2017, 10, 1761–1770. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Dai, F.; Xiang, Z.; Song, T.; Liu, D.; Lu, F.; Qi, H. Zirconium-lignosulfonate polyphenolic polymer for highly efficient hydrogen transfer of biomass-derived oxygenates under mild conditions. Appl. Catal. B Environ. 2019, 248, 31–43. [Google Scholar] [CrossRef]
- Leng, Y.; Shi, L.; Du, S.; Jiang, J.; Jiang, P. A tannin-derived zirconium-containing porous hybrid for efficient Meerwein-Ponndorf-Verley reduction under mild conditions. Green Chem. 2020, 22, 180–186. [Google Scholar] [CrossRef]
- Li, H.; Yang, T.; Fang, Z. Biomass-derived mesoporous Hf-containing hybrid for efficient Meerwein-Ponndorf-Verley reduction at low temperatures. Appl. Catal. B Environ. 2018, 227, 79–89. [Google Scholar] [CrossRef]
- Faustini, M.; Nicole, L.; Ruizhitzky, E.; Sanchez, C. History of organic-inorganic hybrid materials: Prehistory, art, science, and advanced applications. Adv. Funct. Mater. 2018, 28, 1704158. [Google Scholar] [CrossRef]
- Song, J.; Hua, M.; Huang, X.; Visa, A.; Wu, T.; Fan, H.; Hou, M.; Zhang, Z.; Han, B. Highly efficient Meerwein-Ponndorf-Verley reductions over a robust zirconium-organoboronic acid hybrid. Green Chem. 2021, 23, 1259–1265. [Google Scholar] [CrossRef]
- Hao, J.; Han, L.; Sha, Y.; Yu, X.; Liu, H.; Ma, X.; Yang, Y.; Zhou, H.; Liu, Q. Facile use of lignite as robust organic ligands to construct Zr-based catalysts for the conversion of biomass derived carbonyl platforms into alcohols. Fuel 2019, 239, 1304–1314. [Google Scholar] [CrossRef]
- Wang, T.; Hu, A.; Xu, G.; Liu, C.; Wang, H.; Xia, Y. Porous Zr-thiophenedicarboxylate hybrid for catalytic transfer hydrogenation of bio-based furfural to furfuryl alcohol. Catal. Lett. 2019, 149, 1845–1855. [Google Scholar] [CrossRef]
- Li, H.; He, J.; Riisager, A.; Saravanamurugan, S.; Song, B.; Yang, S. Acid-base bifunctional Zirconium N-alkyltriphosphate nanohybrid for hydrogen transfer of biomass-derived carboxides. ACS Catal. 2016, 6, 7722–7727. [Google Scholar] [CrossRef]
- Corma, A.; García, H.; Llabrés i Xamena, F.X. Engineering metal organic frameworks for heterogeneous catalysis. Chem. Rev. 2010, 110, 4606–4655. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Aktara, M.N.; Sahoo, N.K.; Jha, P.K.; Hossain, M. Sensitive and robust colorimetric assay of Hg2+ and S2− in aqueous solution directed by 5-sulfosalicylic acid-stabilized silver nanoparticles for wide range application in real samples. J. Environ. Chem. Eng. 2017, 5, 5645–5654. [Google Scholar] [CrossRef]
- Wang, W.; Tang, B.; Wu, S.; Gao, Z.; Ju, B.; Teng, X.; Zhang, S. Controllable 5-sulfosalicylic acid assisted solvothermal synthesis of monodispersed superparamagnetic Fe3O4 nanoclusters with tunable size. J. Magn. Magn. Mater. 2017, 423, 111–117. [Google Scholar] [CrossRef]
- Xie, H.; Du, K.; Hu, G.; Duan, J.; Peng, Z.; Zhang, Z.; Cao, Y. Synthesis of LiNi0.8Co0.15Al0.05O2 with 5-sulfosalicylic acid as a chelating agent and its electrochemical properties. J. Mater. Chem. A 2015, 3, 20236–20243. [Google Scholar] [CrossRef]
- An, Z.; Li, J. Recent advances in the catalytic transfer hydrogenation of furfural to furfuryl alcohol over heterogeneous catalysts. Green Chem. 2022, 24, 1780–1808. [Google Scholar] [CrossRef]
- Szczęśniak, B.; Phuriragpitikhon, J.; Choma, J.; Jaroniec, M. Recent advances in the development and applications of biomass-derived carbons with uniform porosity. J. Mater. Chem. A 2020, 8, 18464–18491. [Google Scholar] [CrossRef]
- Wang, X.; Qiu, M.; Smith, R.L.; Yang, J.; Shen, F.; Qi, X. Ferromagnetic lignin-derived ordered mesoporous carbon for catalytic hydrogenation of furfural to furfuryl alcohol. ACS Sustain. Chem. Eng. 2020, 8, 18157–18166. [Google Scholar] [CrossRef]
- Varghese, H.T.; Panicker, C.Y.; Philip, D. IR, Raman and SERS spectra of 5-sulphosalicylic acid dihydrate. J. Raman Spectrosc. 2007, 38, 309–315. [Google Scholar] [CrossRef]
- Ko, Y.S.; Kwon, Y.U. Mesoporous zirconia thin films with three-dimensional pore structures and their application to electrochemical glucose detection. ACS Appl. Mater. Interfaces 2013, 5, 3599–3606. [Google Scholar] [CrossRef]
- Ward, D.A.; Ko, E.I. Preparing catalytic materials by the sol-gel method. Ind. Eng. Chem. Res. 1995, 34, 421–433. [Google Scholar] [CrossRef]
- Huo, S.; Zhao, H.; Dong, J.; Xu, J. Facile synthesis of ordered mesoporous zirconia for electrochemical enrichment and detection of organophosphorus pesticides. Electroanalysis 2018, 30, 2121–2130. [Google Scholar] [CrossRef]
- Shearer, G.C.; Chavan, S.; Bordiga, S.; Svelle, S.; Olsbye, U.; Lillerud, K.P. Defect engineering: Tuning the porosity and composition of the metal-organic framework UiO-66 via modulated synthesis. Chem. Mater. 2016, 28, 3749–3761. [Google Scholar] [CrossRef]
- Tang, Y.; Qiu, M.; Yang, J.; Shen, F.; Wang, X.; Qi, X. One-pot self-assembly synthesis of Ni-doped ordered mesoporous carbons for quantitative hydrogenation of furfural to furfuryl alcohol. Green Chem. 2021, 23, 1861–1870. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, F.; Ma, X.; Zhu, C.; Wang, Y.; Xie, Y.; Chou, S.-L.; Huang, Y.; Chen, Y. Regulation of morphology and electronic structure of FeCoNi layered double hydroxides for highly active and stable water oxidization catalysts. Adv. Energy Mater. 2021, 11, 2102141. [Google Scholar] [CrossRef]
- Li, W.; Li, M.; Liu, H.; Jia, W.; Yu, X.; Wang, S.; Zeng, X.; Sun, Y.; Wei, J.; Tang, X.; et al. Domino transformation of furfural to γ-valerolactone over SAPO-34 zeolite supported zirconium phosphate catalysts with tunable Lewis and Brønsted acid sites. Mol. Catal. 2021, 506, 111538. [Google Scholar] [CrossRef]
- Waal, J.C.V.D.; Tan, K.; Bekkum, H.V. Zeolite titanium beta: A selective and water resistant catalyst in Meerwein-Ponndorf-Verley, and Oppenauer reactions. Catal. Lett. 1996, 41, 63–67. [Google Scholar] [CrossRef]
- Cohen, R.; Graves, C.R.; Nguyen, S.T.; Martin, J.M.L.; Ratner, M.A. The mechanism of aluminum-catalyzed Meerwein-Schmidt-Ponndorf-Verley reduction of carbonyls to alcohols. J. Am. Chem. Soc. 2004, 126, 14796–14803. [Google Scholar] [CrossRef] [PubMed]
- Koehle, M.; Lobo, R.F. Lewis acidic zeolite Beta catalyst for the Meerwein-Ponndorf-Verley reduction of furfural. Catal. Sci. Technol. 2016, 6, 3018–3026. [Google Scholar] [CrossRef]
- Gao, Z.; Hong, Y.; Hu, Z.; Xu, B. Transfer hydrogenation of cinnamaldehyde with 2-propanol on Al2O3 and SiO2-Al2O3 catalysts: Role of Lewis and Brønsted acidic sites. Catal. Sci. Technol. 2017, 7, 4511–4519. [Google Scholar] [CrossRef]
- Komanoya, T.; Nakajima, K.; Kitano, M.; Hara, M. Synergistic catalysis by Lewis acid and Base sites on ZrO2 for Meerwein-Ponndorf-Verley reduction. J. Phys. Chem. C 2015, 119, 26540–26546. [Google Scholar] [CrossRef]
- Xue, K.; Mo, Y.; Long, B.; Wei, W.; Shan, C.; Guo, S.; Niu, L. Single-atom catalysts supported on ordered porous materials: Synthetic strategies and applications. InfoMat 2022, 4, e12296. [Google Scholar] [CrossRef]
- Peng, Q.; Li, X.; Wang, X.; Li, Y.; Xia, Y.; Liu, X.; Wang, H. New insights into the structure and catalytic performance of alizarin-zirconium hybrids for Meerwein-Ponndorf-Verley reductions: First-principles approach. Sustain. Energy Fuels 2021, 5, 4069–4079. [Google Scholar] [CrossRef]
- Cheng, Y.; Liu, Y.; Zhang, J.; Huang, R.; Wang, Y.; Cao, S.; He, L.; Peng, L. Acetic acid-regulated mesoporous zirconium-furandicarboxylate hybrid with high lewis acidity and lewis basicity for efficient conversion of furfural to furfuryl alcohol. Renew. Energy 2022, 184, 115–123. [Google Scholar] [CrossRef]
- Hu, L.; Li, N.; Dai, X.; Guo, Y.; Jiang, Y.; He, A.; Xu, J. Highly efficient production of 2,5-dihydroxymethylfuran from biomass-derived 5-hydroxymethylfurfural over an amorphous and mesoporous zirconium phosphonate catalyst. J. Energy Chem. 2019, 37, 82–92. [Google Scholar] [CrossRef] [Green Version]
- Cao, P.; Lin, L.; Qi, H.; Chen, R.; Wu, Z.; Li, N.; Zhang, T.; Luo, W. Zeolite-encapsulated Cu nanoparticles for the selective hydrogenation of furfural to furfuryl alcohol. ACS Catal. 2021, 11, 10246–10256. [Google Scholar] [CrossRef]
- Valekar, A.H.; Lee, M.; Yoon, J.W.; Kwak, J.; Hong, D.Y.; Oh, K.R.; Cha, G.Y.; Kwon, Y.U.; Jung, J.; Chang, J.S.; et al. Catalytic transfer hydrogenation of furfural to furfuryl alcohol under mild conditions over Zr-MOFs: Exploring the role of metal node coordination and modification. ACS Catal. 2020, 10, 3720–3732. [Google Scholar] [CrossRef]
- Zhou, S.; Dai, F.; Chen, Y.; Dang, C.; Zhang, C.; Liu, D.; Qi, H. Sustainable hydrothermal self-assembly of hafnium-lignosulfonate nanohybrids for highly efficient reductive upgrading of 5-hydroxymethylfurfural. Green Chem. 2019, 21, 1421–1431. [Google Scholar] [CrossRef]
- Karnjanakom, S.; Bayu, A.; Maneechakr, P.; Samart, C.; Kongparakul, S.; Guan, G. Rapid transformation of furfural to biofuel additive ethyl levulinate with in situ suppression of humins promoted by an acidic-oxygen environment. ACS Sustain. Chem. Eng. 2021, 9, 14170–14179. [Google Scholar] [CrossRef]
- Shi, N.; Liu, Q.; Cen, H.; Ju, R.; He, X.; Ma, L. Formation of humins during degradation of carbohydrates and furfural derivatives in various solvents. Biomass Convers. Biorefin. 2020, 10, 277–287. [Google Scholar] [CrossRef]
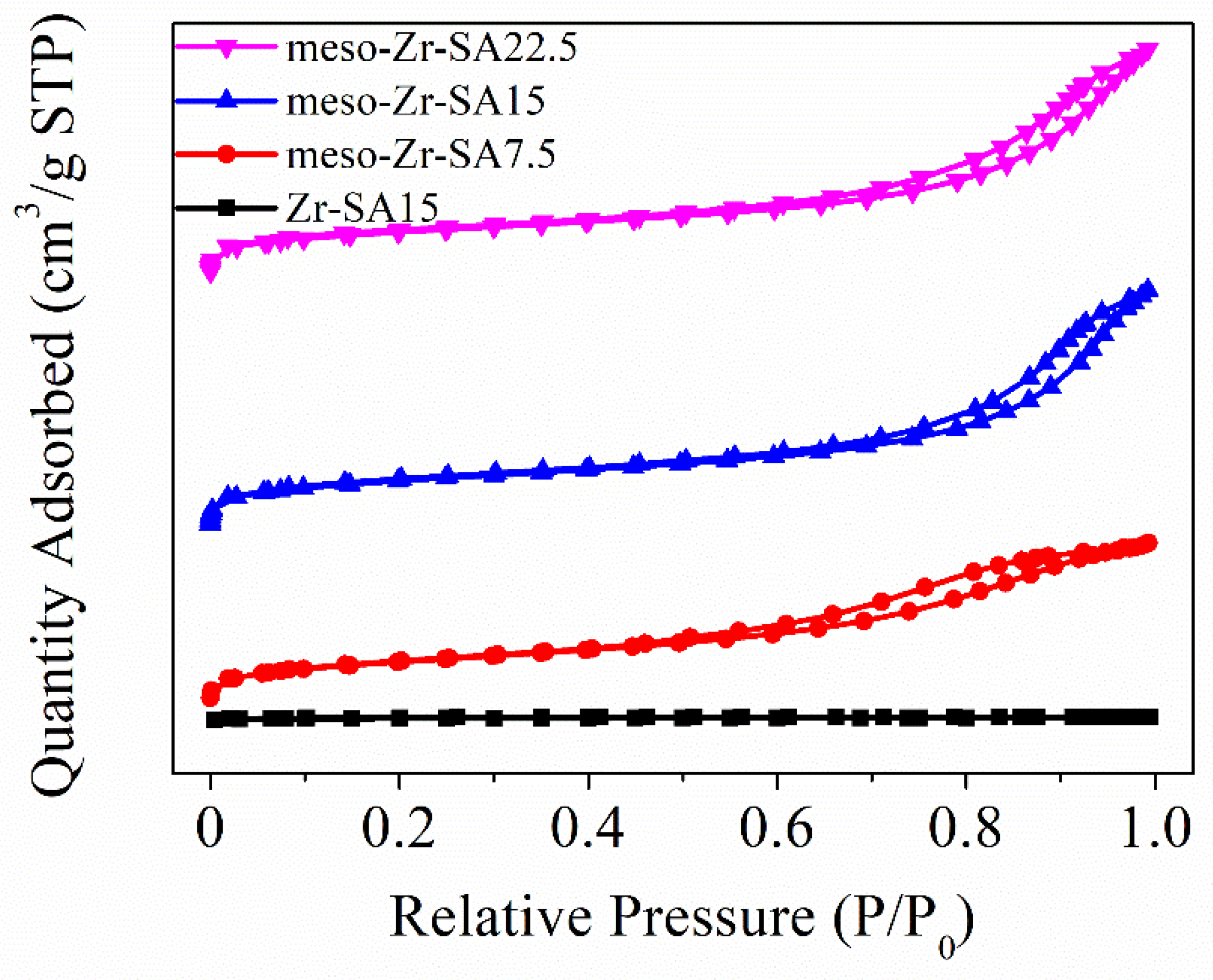

| Samples | Surface Area a (m2/g) | Pore Volume b (cm3/g) | Pore Width (nm) c | Zr Content (wt%) d | BA e Content (μmol/g) | LA f Content (μmol/g) | BA + LA (μmol/g) |
|---|---|---|---|---|---|---|---|
| meso-Zr-SA7.5 | 281.9 | 0.49 | 6.4 | 35.5 | 6.6 | 47.9 | 54.5 |
| meso-Zr-SA15 | 319.5 | 0.74 | 10.9 | 35.9 | 8.9 | 52.6 | 61.5 |
| meso-Zr-SA22.5 | 291.3 | 0.70 | 10.9 | 37.5 | 9.5 | 53.3 | 62.8 |
| Zr-SA15 | 28.1 | 0.02 | 3.7 | 36.3 | 5.6 | 44.9 | 50.5 |
| Entry | Catalyst | Time (h) | Furfural Conversion (%) | Furfuryl Alcohol Yield (%) | Furfuryl Alcohol Selectivity (%) |
|---|---|---|---|---|---|
| 1 | meso-Zr-SA15 a | 1 | 90.9 | 87.0 | 95.7 |
| 2 | meso-Zr-SA15 a | 2 | 98.9 | 97.8 | 98.9 |
| 3 | meso-Zr-SA15 a | 3 | 99.8 | 96.4 | 96.6 |
| 4 | nano-ZrO2 a | 2 | 27.2 | 4.9 | 17.7 |
| 5 | Zr-SA15 a | 2 | 35.9 | 1.1 | 3.1 |
| 6 | blank | 2 | 20.1 | 0 | 0 |
| Catalysts | Reductant | Reaction Conditions | Maximum Furfuryl Alcohol Yield | Reference |
|---|---|---|---|---|
| meso-Zr-SA15 | 2-propanol | 110 °C, 2 h | 97.8% | This work |
| MOF (UiO-66) | 2-propanol | 140 °C, 5 h | 97% | [13] |
| MOF (MOF-808) | 2-propanol | 140 °C, 5 h | 89% | [13] |
| Zirconium-alizarin hybrid | 2-propanol | 100 °C, 2 h | 88.3 | [46] |
| Zr-furandicarboxylate hybrid | 2-propanol | 140 °C, 4 h | 96% | [16] |
| Zr-furandicarboxylate hybrid | 2-propanol | 150 °C, 3 h | 99% | [47] |
| Zr-phosphonate hybrid | 2-butanol | 130 °C, 3 h | 98% | [48] |
| Zr-phytic acid hybrid | 2-propanol | 100 °C, 2 h | 99.3% | [14] |
| Zr-thiophenedicarboxylate hybrid | 2-propanol | 120 °C, 4 h | 92.2% | [23] |
| Zr-tannin hybrid | 2-propanol | 80 °C, 3 h | 91.2% | [18] |
| Ni0.5@OMC-600 | hydrogen | 3 MPa H2, 180 °C, 4 h | 98% | [37] |
| Ni@OMC | hydrogen | 3 MPa H2, 180 °C, 12 h | 91% | [31] |
| Zeolite-encapsulated Cu nanoparticles | hydrogen | 1 MPa H2, 110 °C, 2 h | 91.2% | [49] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Guo, H.; Shen, F. Highly Efficient Transfer Hydrogenation of Biomass-Derived Furfural to Furfuryl Alcohol over Mesoporous Zr-Containing Hybrids with 5-Sulfosalicylic Acid as a Ligand. Int. J. Environ. Res. Public Health 2022, 19, 9221. https://doi.org/10.3390/ijerph19159221
Yang J, Guo H, Shen F. Highly Efficient Transfer Hydrogenation of Biomass-Derived Furfural to Furfuryl Alcohol over Mesoporous Zr-Containing Hybrids with 5-Sulfosalicylic Acid as a Ligand. International Journal of Environmental Research and Public Health. 2022; 19(15):9221. https://doi.org/10.3390/ijerph19159221
Chicago/Turabian StyleYang, Jirui, Haixin Guo, and Feng Shen. 2022. "Highly Efficient Transfer Hydrogenation of Biomass-Derived Furfural to Furfuryl Alcohol over Mesoporous Zr-Containing Hybrids with 5-Sulfosalicylic Acid as a Ligand" International Journal of Environmental Research and Public Health 19, no. 15: 9221. https://doi.org/10.3390/ijerph19159221
APA StyleYang, J., Guo, H., & Shen, F. (2022). Highly Efficient Transfer Hydrogenation of Biomass-Derived Furfural to Furfuryl Alcohol over Mesoporous Zr-Containing Hybrids with 5-Sulfosalicylic Acid as a Ligand. International Journal of Environmental Research and Public Health, 19(15), 9221. https://doi.org/10.3390/ijerph19159221








