Derivation of Soil Criteria of Cadmium for Safe Rice Production Applying Soil–Plant Transfer Model and Species Sensitivity Distribution
Abstract
:1. Introduction
2. Materials and Methods
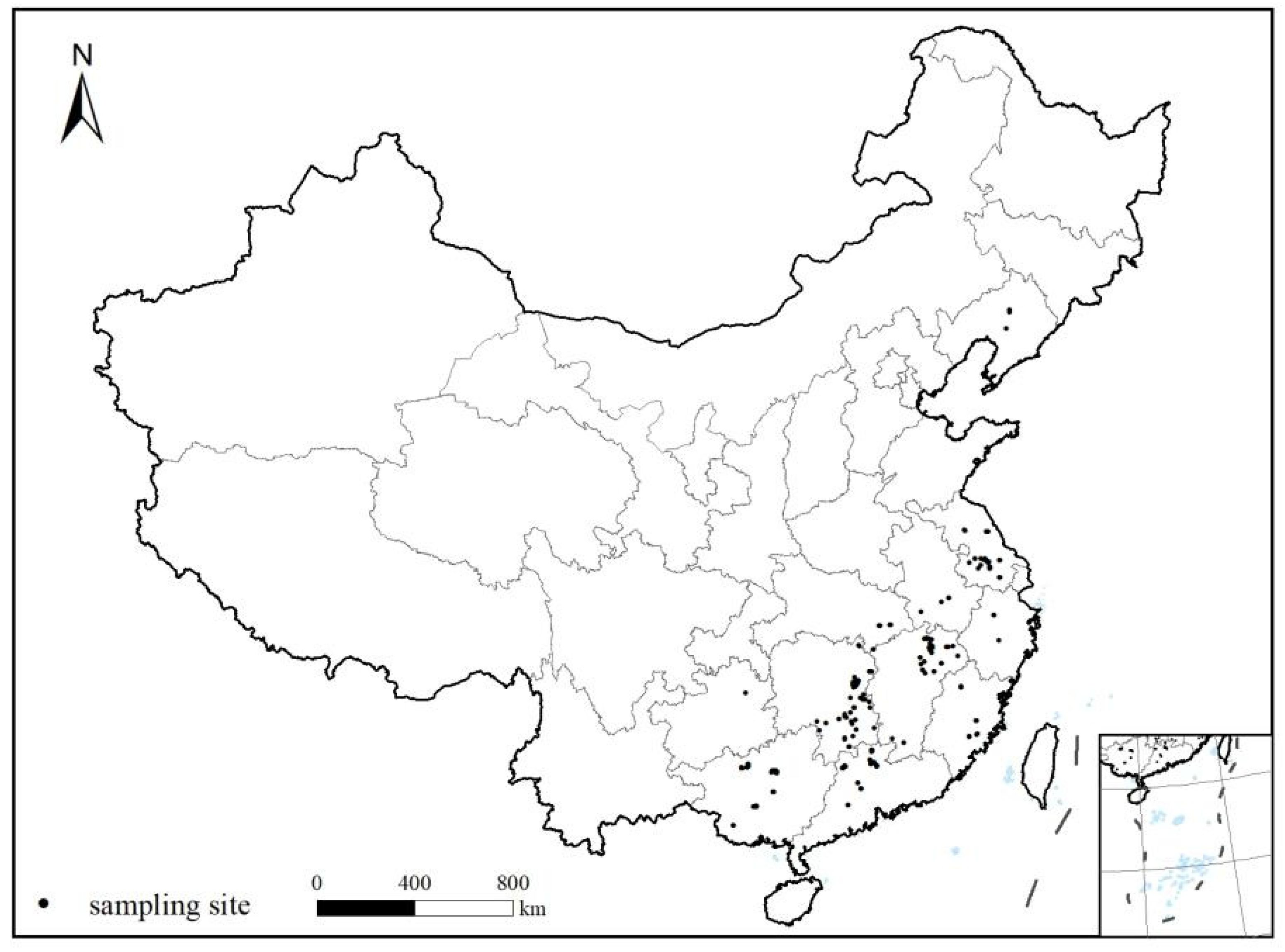
2.1. Study Sites and Sampling
2.2. Chemical Analyses
2.3. Data Analysis
2.3.1. Constructing the Soil–Plant Transfer Model
2.3.2. Constructing the Species Sensitivity Distribution
2.3.3. Deriving and Validating the Soil Criteria
2.3.4. Statistics Analysis
3. Results
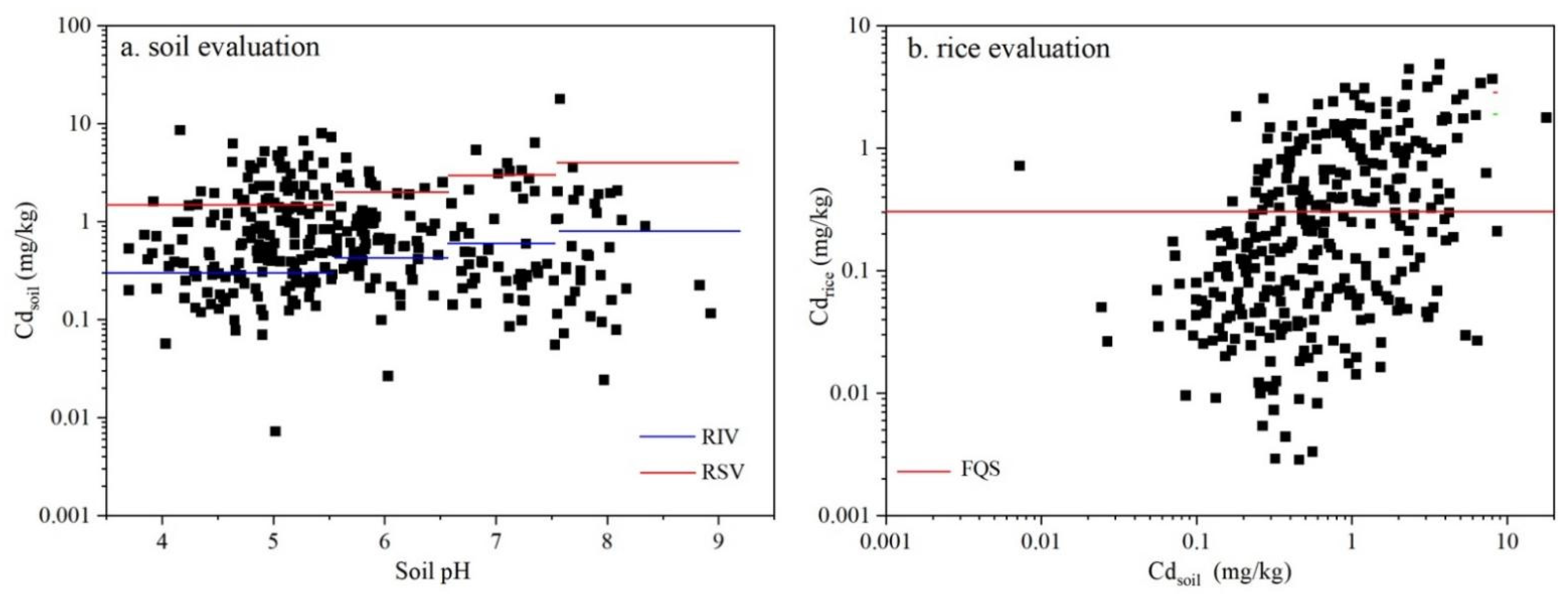
3.1. Cd Concentrations in Soils and Crops
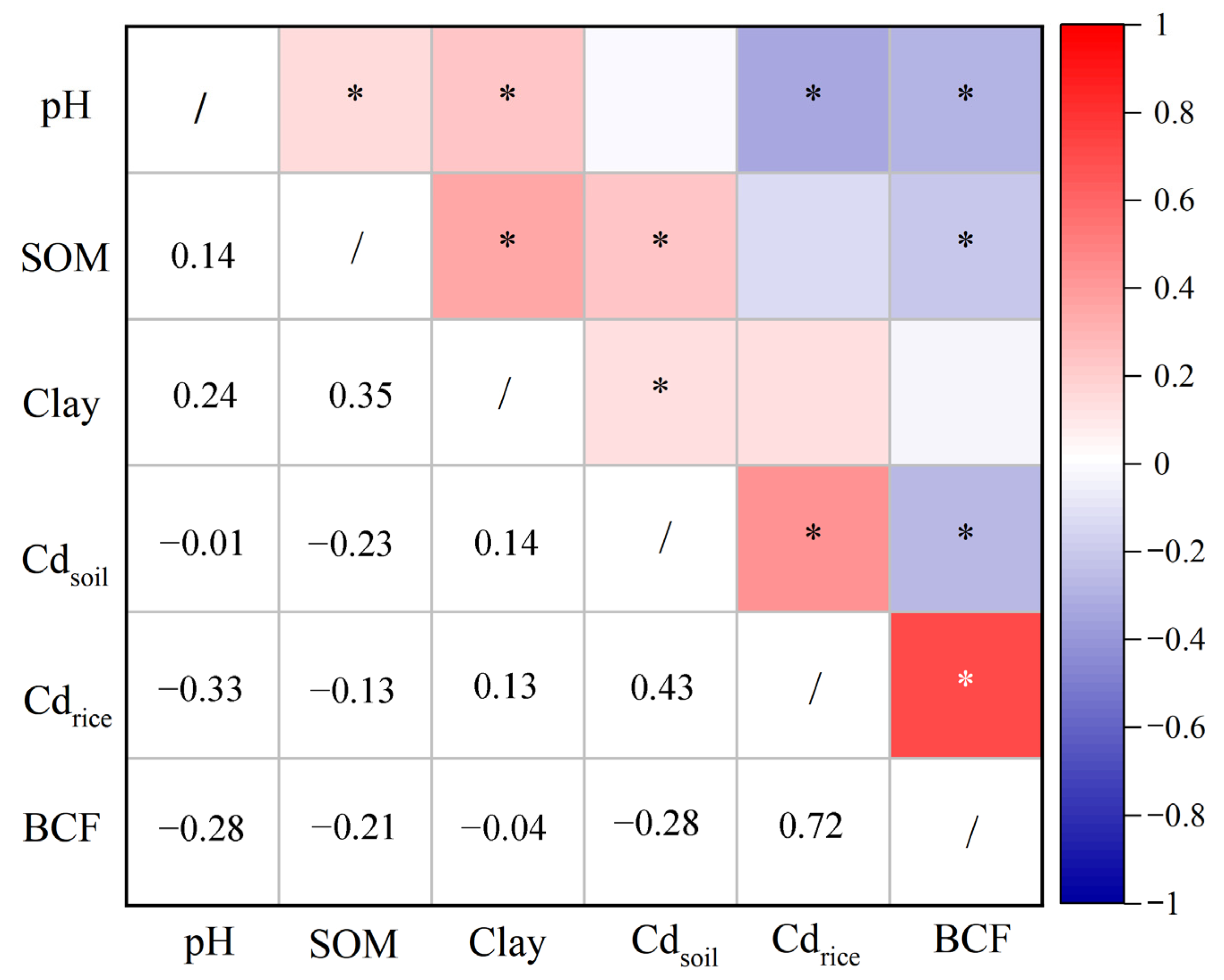
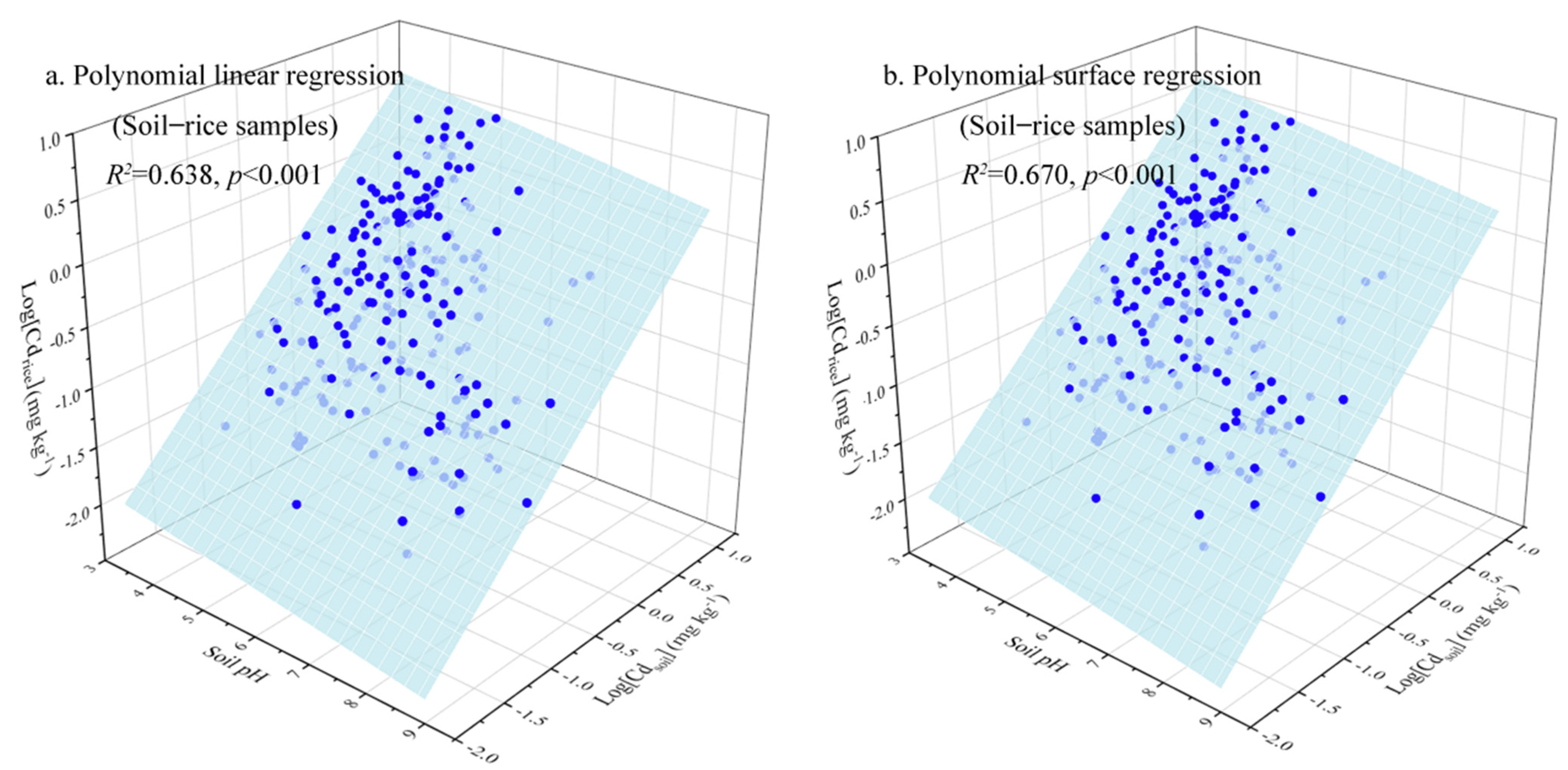
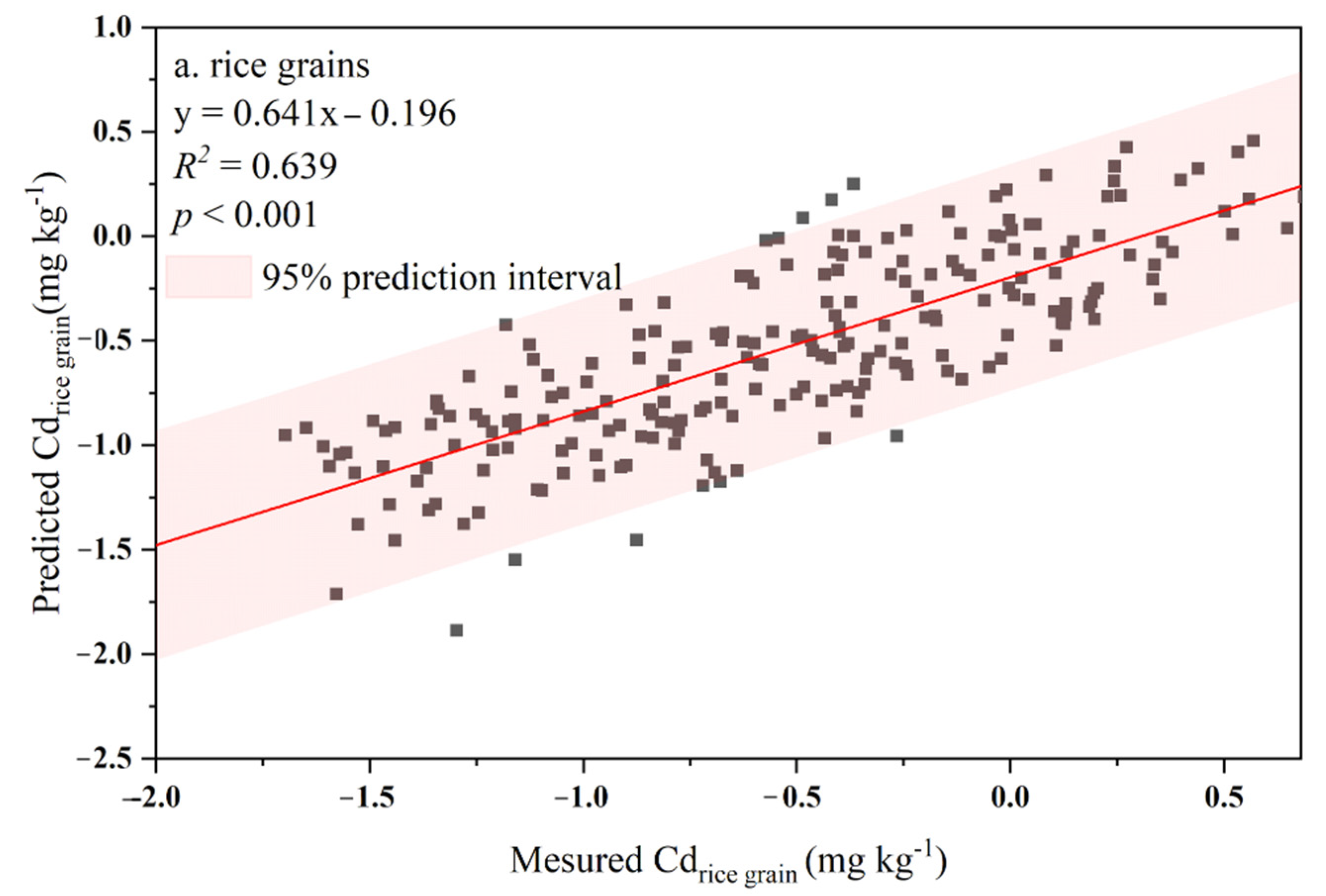
3.2. Soil–Plant Transfer Models for Cd Accumulation
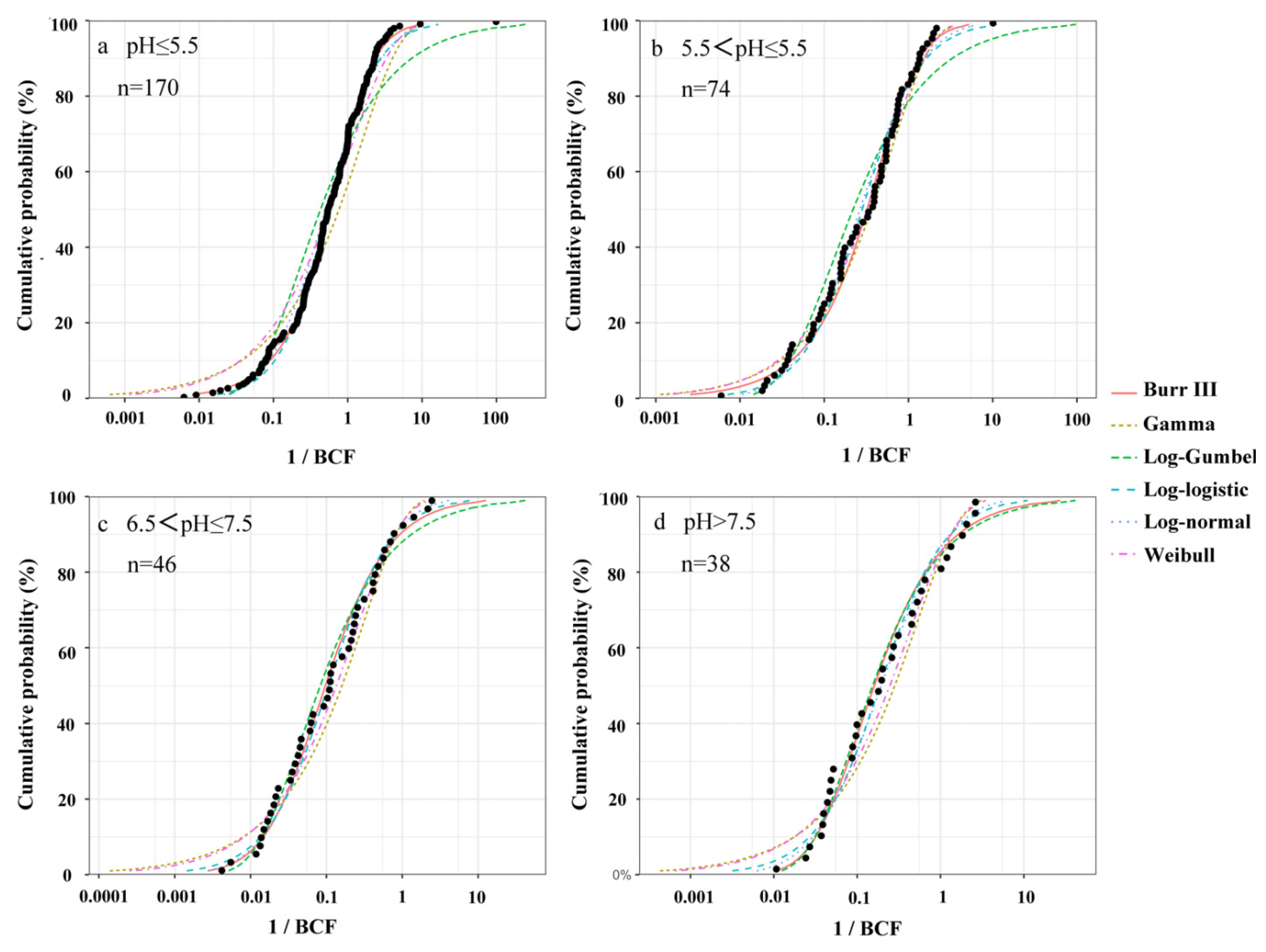
3.3. Species Sensitivity Distribution for Cd Accumulation
3.4. Derivation and Validation of Soil Criteria
4. Discussion
5. Conclusions
- The field data with a proper Cd concentration gradient could increase the model accuracy in the soil–plant transfer system and decrease inaccuracy in the derivation of soil criteria.
- The probabilistic soil criteria (SC5, SC50 and SC95) for protecting 95%, 50% and 5% of the rice safety were 0.06–0.11, 0.33–0.59, and 1.51–2.82 mg kg−1 according to soil pH ranges, respectively.
- The proposed soil criteria were comparable to the Chinese SQS in highly acidic and acidic soils, while more strict soil criteria were observed in neutral and alkaline groups.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perrodin, Y.; Boillot, C.; Angerville, R.; Donguy, G.; Emmanuel, E. Ecological risk assessment of urban and industrial systems: A review. Sci. Total Environ. 2011, 409, 5162–5176. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Li, H.; Guo, G.; Semple, K.T.; Jones, K.C. Soil contamination in China: Current priorities, defining background levels and standards for heavy metals. J. Environ. Manag. 2019, 251, 109512. [Google Scholar] [CrossRef]
- Chen, N.; Zheng, Y.; He, X.; Li, X.; Zhang, X. Analysis of the bulletin of national soil pollution survey. J. Agro-Environ. Sci. 2017, 36, 1689–1692. (In Chinese) [Google Scholar]
- Zhang, L.; Gao, J.; Li, X. Chinese total diet study in 2000. Cadmium intakes by different age-sex population groups. J. Hyg. Res. 2008, 37, 338–342. (In Chinese) [Google Scholar]
- Chen, H.; Yang, X.; Wang, P.; Wang, Z.; Li, M.; Zhao, F. Dietary cadmium intake from rice and vegetables and potential health risk: A case study in Xiangtan, southern China. Sci. Total Environ. 2018, 639, 271–277. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Cadmium in food-Scientific opinion of the panel on contaminants in the food Chain. EFSA J. 2009, 7, 980. [Google Scholar]
- BBodSchV. Federal Soil Protection and Contaminated Sites Ordinance; German Federal Council: Berlin, Germany, 1999. [Google Scholar]
- EA. Updated Technical Background to the CLEA Model; Environment Agency: Bristol, UK, 2009. [Google Scholar]
- Swartjes, F.A.; Rutgers, M.; Lijzen, J.P.A.; Janssen, P.J.C.M.; Otte, P.M.; Wintersen, A.; Brand, E.; Posthuma, L. State of the art of contaminated site management in The Netherlands: Policy framework and risk assessment tools. Sci. Total Environ. 2012, 427–428, 1–10. [Google Scholar] [CrossRef]
- Carlon, C.; D’Alessandro, M.; Swartjes, F. Derivation Methods of Soil Screening Values in Europe. A Review and Evaluation of National Procedures towards Harmonization; ISPA: European Commission, Joint Research Centre: Ispra, Italy, 2007. [Google Scholar]
- Chen, S.; Wang, M.; Li, S.; Zhao, Z.; Wen-di, E. Overview on current criteria for heavy metals and its hint for the revision of soil environmental quality standards in China. J. Integr. Agric. 2018, 17, 765–774. [Google Scholar] [CrossRef] [Green Version]
- Provoost, J.; Cornelis, C.; Swartjes, F. Comparison of soil clean-up standards for trace elements between countries: Why do they differ? J. Soil. Sediment. 2006, 6, 173–181. [Google Scholar] [CrossRef]
- Suter, G. Soil quality standards for trace elements: Derivation, implementation, and interpretation. Integr. Environ. Asses. 2011, 7, 510–511. [Google Scholar]
- GB15618-2018. Soil Environmental Quality: Risk Control Standard for Soil Contamination of Agricultural Land (Trial); Ministry of Ecology and Environment of the People’s Republic of China, State Administration for Market Regulation: Beijing, China, 2018.
- Jia, C.; Jukes, D. The national food safety control system of China—A systematic review. Food Control 2013, 32, 236–245. [Google Scholar] [CrossRef]
- Ding, C.; Ma, Y.; Li, X.; Zhang, T.; Wang, X. Determination and validation of soil thresholds for cadmium based on food quality standard and health risk assessment. Sci. Total Environ. 2018, 619, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Ma, Y.; Li, X.; Zhang, T.; Wang, X. Derivation of soil thresholds for lead applying species sensitivity distribution: A case study for root vegetables. J. Hazard. Mater. 2016, 303, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Li, X.; Zhang, T.; Ma, Y.; Wang, X. Phytotoxicity and accumulation of chromium in carrot plants and the derivation of soil thresholds for Chinese soils. Ecotoxicol. Environ. Saf. 2014, 108, 179–186. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, W.; Wang, M.; Peng, C. Regional accumulation characteristics of cadmium in vegetables: Influencing factors, transfer model and indication of soil threshold content. Environ. Pollut. 2016, 219, 1036–1043. [Google Scholar] [CrossRef]
- De Vries, W.; McLaughlin, M.J. Modeling the cadmium balance in Australian agricultural systems in view of potential impacts on food and water quality. Sci. Total Environ. 2013, 461–462, 240–257. [Google Scholar] [CrossRef]
- Zhang, S.; Song, J.; Cheng, Y.; McBride, M.B. Derivation of regional risk screening values and intervention values for cadmium-contaminated agricultural land in the Guizhou Plateau. Land Degrad. Dev. 2018, 29, 2366–2377. [Google Scholar] [CrossRef]
- Zhang, S.; Song, J.; Cheng, Y.; Lv, M. Proper management of lead-contaminated agricultural lands against the exceedance of lead in agricultural produce: Derivation of local soil criteria. Sci. Total Environ. 2018, 634, 321–330. [Google Scholar] [CrossRef]
- Romero-Freire, A.; Peinado, F.M.; Van Gestel, C. Effect of soil properties on the toxicity of Pb: Assessment of the appropriateness of guideline values. J. Hazard. Mater. 2015, 289, 46–53. [Google Scholar] [CrossRef]
- Krauss, M.; Wilcke, W.; Kobza, J.; Zech, W. Predicting heavy metal transfer from soil to plant: Potential use of Freundlich-type functions. J. Plant Nutr. Soil Sci. 2002, 165, 3–8. [Google Scholar] [CrossRef]
- Efroymson, R.A.; Sample, B.E.; Suter, G.W. Uptake of inorganic chemicals from soil by plant leaves: Regressions of field data. Environ. Toxicol. Chem. 2001, 20, 2561–2571. [Google Scholar] [CrossRef] [PubMed]
- Römkens, P.; Guo, H.; Chu, C.; Liu, T.; Chiang, C.; Koopmans, G. Prediction of cadmium uptake by brown rice and derivation of soil–plant transfer models to improve soil protection guidelines. Environ. Pollut. 2009, 157, 2435–2444. [Google Scholar] [CrossRef] [PubMed]
- Römkens, P.; Brus, D.; Guo, H.; Chu, C.; Chiang, C.; Koopmans, G. Impact of model uncertainty on soil quality standards for cadmium in rice paddy fields. Sci. Total Environ. 2011, 409, 3098–3105. [Google Scholar] [CrossRef] [PubMed]
- Brus, D.; De Gruijter, J.; Römkens, P. Probabilistic quality standards for heavy metals in soil derived from quality standards in crops. Geoderma 2005, 128, 301–311. [Google Scholar] [CrossRef]
- Posthuma, L.; Suter, G., II; Traas, T. Species Sensitivity Distributions in Ecotoxicology; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Wu, X.; Cai, Q.; Xu, Q.; Zhou, Z.; Shi, J.Y. Wheat (Triticum aestivum L.) grains uptake of lead (Pb), transfer factors and prediction models for various types of soils from China. Ecotoxicol. Environ. Saf. 2020, 206, 111387. [Google Scholar] [CrossRef]
- Lu, R. Soil Agrochemical Analyses; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Wang, M.; Faber, J.H.; Chen, W.; Li, X.; Markert, B. Effects of land use intensity on the natural attenuation capacity of urban soils in Beijing, China. Ecotoxicol. Environ. Saf. 2015, 117, 89–95. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Chen, W.; Peng, C. Risk assessment of Cd polluted paddy soils in the industrial and township areas in Hunan, Southern China. Chemosphere 2016, 144, 346–351. [Google Scholar] [CrossRef]
- Wang, M.; Liu, R.; Chen, W.; Peng, C.; Markert, B. Effects of urbanization on heavy metal accumulation in surface soils, Beijing. J. Environ. Sci. 2017, 64, 328–334. [Google Scholar] [CrossRef]
- Schwarz, C.; Tillmanns, A. Improving Statistical Methods to Derive Species Sensitivity Distributions; Water Science Series, WSS2019-07; Province of British Columbia: Victoria, BC, Canada, 2019. [Google Scholar]
- CCME. Protocol for the Derivation of Water Quality Guidelines for the Protection of Aquatic Life; Canadian Council of Ministers of the Environment: Ottawa, ON, Canada, 2007. [Google Scholar]
- Xia, J. Detailed Notes for Soil Environmental Quality Standard; China Environmental Science Press: Beijing, China, 1996. [Google Scholar]
- Zhang, H.; Luo, Y.; Song, J.; Zhang, H.; Xia, J.; Zhao, Q. Predicting As, Cd and Pb uptake by rice and vegetables using field data from China. J. Environ. Sci. 2011, 23, 70–78. [Google Scholar] [CrossRef]
- CDFA. Development of Risk Based Concentrations for Arsenic, Cadmium, and Lead in Inorganic Fertilizers; The Report of the Heavy Metal Task Force, Agricultural Commodities and Regulatory Services; California Department of Food and Agriculture: Sacramento, CA, USA, 1998. [Google Scholar]
- Chen, W.; Li, L.; Chang, A.C.; Wu, L.; Chaney, R.L.; Smith, R.; Ajwa, H.A. Characterizing the solid-solution partitioning coefficient and plant uptake factor of As, Cd, and Pb in California croplands. Agric. Ecosyst. Environ. 2009, 129, 212–220. [Google Scholar] [CrossRef]
- Thakali, S.; Allen, H.E.; Di Toro, D.M.; Ponizovsky, A.A.; Rooney, C.P.; Zhao, F.; McGrath, S.P.; Criel, P.; Van Eeckhout, H.; Janssen, C.R. Terrestrial biotic ligand model. 2. Application to Ni and Cu toxicities to plants, invertebrates, and microbes in soil. Environ. Sci. Technol. 2006, 40, 7094–7100. [Google Scholar] [CrossRef] [PubMed]
- Thakali, S.; Allen, H.E.; Di Toro, D.M.; Ponizovsky, A.A.; Rooney, C.P.; Zhao, F.; McGrath, S.P. A terrestrial biotic ligand model. 1. Development and application to Cu and Ni toxicities to barley root elongation in soils. Environ. Sci. Technol. 2006, 40, 7085–7093. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, X.; Yang, J.; Liu, Y.; Naidu, R. Predicting the thresholds of metals with limited toxicity data with invertebrates in standard soils using quantitative ion character-activity relationships (QICAR). J. Hazard. Mater. 2021, 423, 126982. [Google Scholar] [CrossRef] [PubMed]
- EA. Derivation and Use of Soil Screening Values for Assessing Ecological Risks; ShARE id26; Environment Agency: Bristol, UK, 2017. [Google Scholar]
- Hue, N.; Uchida, R.; Ho, M. Empirical Models for the Uptake of Inorganic Chemicals from Soil by Plants; US Department of Energy Office of Environmental Management: Washington, DC, USA, 1998. [Google Scholar]
- Zhao, F.; Adams, M.; Dumont, C.; McGrath, S.P.; Chaudri, A.M.; Nicholson, F.A.; Chambers, B.J.; Sinclair, A.H. Factors affecting the concentrations of lead in British wheat and barley grain. Environ. Pollut. 2004, 131, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Bacigalupo, C.; Hale, B. Soil–plant transfer factors for garden produce from contaminated soils: Site specific versus generic estimates for As and Pb. Hum. Ecol. Risk Assess. 2011, 17, 394–413. [Google Scholar] [CrossRef]
- Mason, C.H.; Perreault Jr, W.D. Collinearity, power, and interpretation of multiple regression analysis. J. Market. Res. 1991, 28, 268–280. [Google Scholar] [CrossRef]
- Li, X.; Jiang, R.; Wang, G.; Chen, Y.; Long, T.; Lin, Y. A comparative study of soil environmental standards for agricultural land among different countries and its implication for China. Environ. Sci. 2022, 43, 577–585. (In Chinese) [Google Scholar]
- Yang, Y.; Chang, A.C.; Wang, M.; Chen, W.; Peng, C. Assessing cadmium exposure risks of vegetables with plant uptake factor and soil property. Environ. Pollut. 2018, 238, 263–269. [Google Scholar] [CrossRef]
- Terytze, K.; Kördel, W.; Müller, J.; Herrchen, M.; Nester, A. Soil-plant transfer of organic chemicals and derivation of trigger values. In Environmentally Sound Management (ESM). Practices on Cleaning Up Obsolete Stokpiles of Pesticides for Central European and EECCA Countries. Proceedings of the 8th International HCH and Pesticides Forum, Sofia, Bulgaria, 26–28 May 2005; Schulz, N., Atanassov, I., Vijgen, J., Eds.; GorexPress: Sofia, Bulgaria, 2006; pp. 171–174. [Google Scholar]
- Li, K.; Cao, C.L.; Ma, Y.; Su, D.; Li, J. Identification of cadmium bioaccumulation in rice (Oryza sativa L.) by the soil-plant transfer model and species sensitivity distribution. Sci. Total Environ. 2019, 692, 1022–1028. [Google Scholar] [CrossRef]
- Wheeler, J.; Grist, E.; Leung, K.; Morritt, D.; Crane, M. Species sensitivity distributions: Data and model choice. Mar. Pollut. Bull. 2002, 45, 192–202. [Google Scholar] [CrossRef]
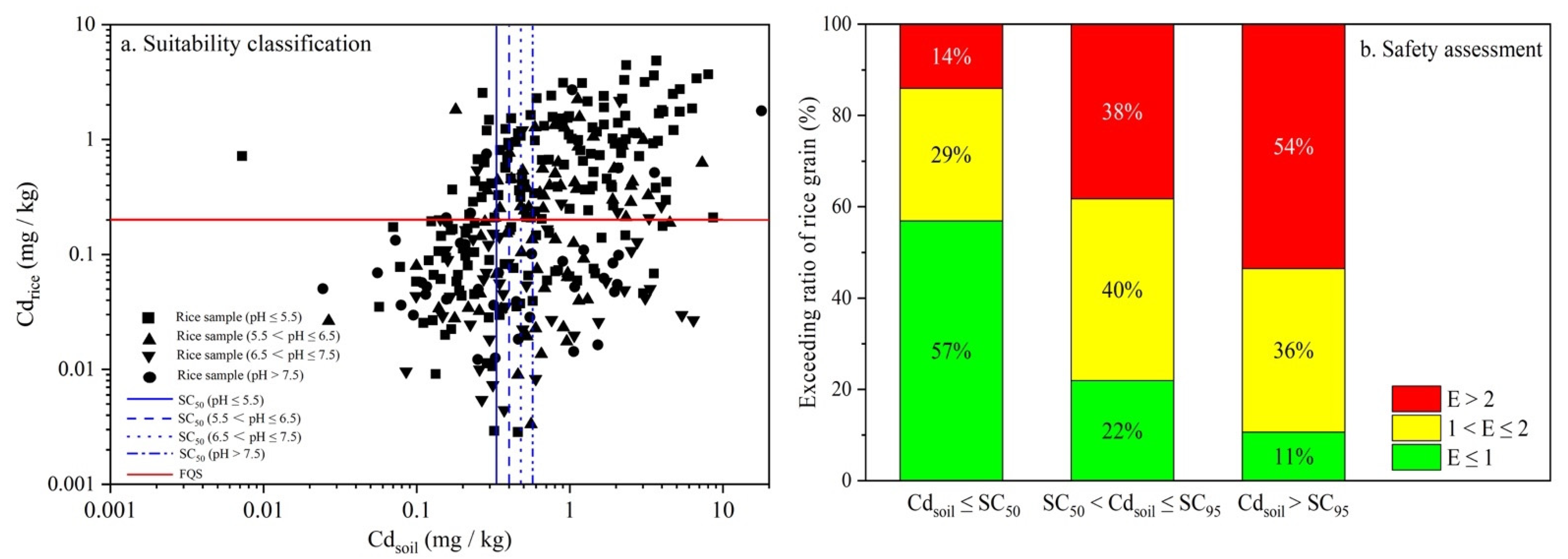
| Suitability Classification | Soil | Rice Grain |
|---|---|---|
| A | Cdsoil > SCx | Cdrice > FQS |
| B | Cdsoil ≤ SCx | Cdrice > FQS |
| C | Cdsoil ≤ SCx | Cdrice ≤ FQS |
| D | Cdsoil > SCx | Cdrice ≤ FQS |
| Variables | Min | Max | Mean | Median | SD |
|---|---|---|---|---|---|
| Cdsoil (mg kg−1) | 0.007 | 17.9 | 1.19 | 0.563 | 1.65 |
| Cdrice (mg kg−1) | 0.003 | 4.87 | 0.550 | 0.202 | 0.783 |
| BCF | 0.004 | 10.1 | 0.730 | 0.393 | 5.48 |
| pH | 3.70 | 8.93 | 5.74 | 5.45 | 1.14 |
| SOM (g kg−1) | 1.24 | 8.96 | 3.63 | 3.58 | 19.8 |
| Clay (%) | 6.60 | 61.8 | 28.2 | 28.2 | 10.7 |
| pH | Models | AD | KS | AIC | BIC | AICc (Delta) |
|---|---|---|---|---|---|---|
| pH ≤ 5.5 | Burr III | 0.290 | 0.037 | 346 | 356 | 0 |
| Gamma | / | 0.150 | 437 | 444 | 90.9 | |
| Log-Gumbel | 5.87 | 0.129 | 406 | 412 | 59.5 | |
| Log-logistic | 0.816 | 0.053 | 350 | 356 | 3.25 | |
| Log-normal | 1.23 | 0.069 | 355 | 362 | 8.92 | |
| Weibull | 3.49 | 0.098 | 392 | 398 | 45.1 | |
| 5.5 < pH ≤ 6.5 | Burr III | 0.424 | 0.080 | 67.8 | 74.7 | 2.24 |
| Gamma | 0.770 | 0.080 | 75.1 | 79.7 | 9.36 | |
| Log-Gumbel | 1.83 | 0.120 | 82.5 | 87.1 | 16.7 | |
| Log-logistic | 0.652 | 0.088 | 67.9 | 72.4 | 2.15 | |
| Log-normal | 0.602 | 0.100 | 65.7 | 70.3 | 0 | |
| Weibull | 0.477 | 0.060 | 70.4 | 74.9 | 4.63 | |
| 6.5 < pH ≤ 7.5 | Burr III | 0.356 | 0.086 | 25.7 | 20.1 | 4.87 |
| Gamma | 0.956 | 0.125 | 22.2 | 18.5 | 8.02 | |
| Log-Gumbel | 0.509 | 0.094 | 26.0 | 22.3 | 4.23 | |
| Log-logistic | 0.345 | 0.076 | 7.31 | 23.6 | 2.94 | |
| Log-normal | 0.260 | 0.072 | 30.3 | 26.5 | 0 | |
| Weibull | 0.514 | 0.087 | 25.5 | 21.8 | 4.69 | |
| pH > 7.5 | Burr III | 0.350 | 0.098 | 18.8 | 23.4 | 3.41 |
| Gamma | 1.02 | 0.139 | 21.7 | 24.7 | 5.86 | |
| Log-Gumbel | 0.347 | 0.089 | 17.1 | 20.1 | 1.26 | |
| Log-logistic | 0.427 | 0.104 | 18.4 | 21.4 | 2.56 | |
| Log-normal | 0.396 | 0.108 | 15.8 | 18.9 | 0 | |
| Weibull | 0.712 | 0.112 | 19.9 | 22.9 | 4.09 |
| Soil Criteria (mg kg−1) | pH ≤ 5.5 | 5.5 < pH ≤ 6.5 | 6.5 < pH ≤ 7.5 | pH > 7.5 |
|---|---|---|---|---|
| SC5 | 0.06 | 0.08 | 0.11 | 0.09 |
| SC50 | 0.33 | 0.40 | 0.49 | 0.59 |
| SC95 | 1.51 | 1.85 | 2.27 | 2.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Du, J.; Sun, L.; Zhang, Y.; Feng, Y.; Zheng, L.; Wang, G.; Huang, X. Derivation of Soil Criteria of Cadmium for Safe Rice Production Applying Soil–Plant Transfer Model and Species Sensitivity Distribution. Int. J. Environ. Res. Public Health 2022, 19, 8854. https://doi.org/10.3390/ijerph19148854
Li X, Du J, Sun L, Zhang Y, Feng Y, Zheng L, Wang G, Huang X. Derivation of Soil Criteria of Cadmium for Safe Rice Production Applying Soil–Plant Transfer Model and Species Sensitivity Distribution. International Journal of Environmental Research and Public Health. 2022; 19(14):8854. https://doi.org/10.3390/ijerph19148854
Chicago/Turabian StyleLi, Xuzhi, Junyang Du, Li Sun, Ya Zhang, Yanhong Feng, Liping Zheng, Guoqing Wang, and Xinghua Huang. 2022. "Derivation of Soil Criteria of Cadmium for Safe Rice Production Applying Soil–Plant Transfer Model and Species Sensitivity Distribution" International Journal of Environmental Research and Public Health 19, no. 14: 8854. https://doi.org/10.3390/ijerph19148854
APA StyleLi, X., Du, J., Sun, L., Zhang, Y., Feng, Y., Zheng, L., Wang, G., & Huang, X. (2022). Derivation of Soil Criteria of Cadmium for Safe Rice Production Applying Soil–Plant Transfer Model and Species Sensitivity Distribution. International Journal of Environmental Research and Public Health, 19(14), 8854. https://doi.org/10.3390/ijerph19148854





