Effect of Cd on Pyrolysis Velocity and Deoxygenation Characteristics of Rice Straw: Analogized with Cd-Impregnated Representative Biomass Components
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Cd-Impregnated Biomass Components and Rice Straw
2.2. Thermogravimetric Analysis and Pyrolysis Experiments
2.3. TG-FTIR and TG-MS Experiments for Cd-Contaminated RS
2.4. Kinetic Analysis Based on Isoconversional Method
2.5. Characterization of Pyrolysis Residues
3. Results and Discussion
3.1. Pyrolysis Characteristics of Cd-Concentrated Cellulose, Hemicellulose, and Lignin
3.1.1. TG/DTG Analysis
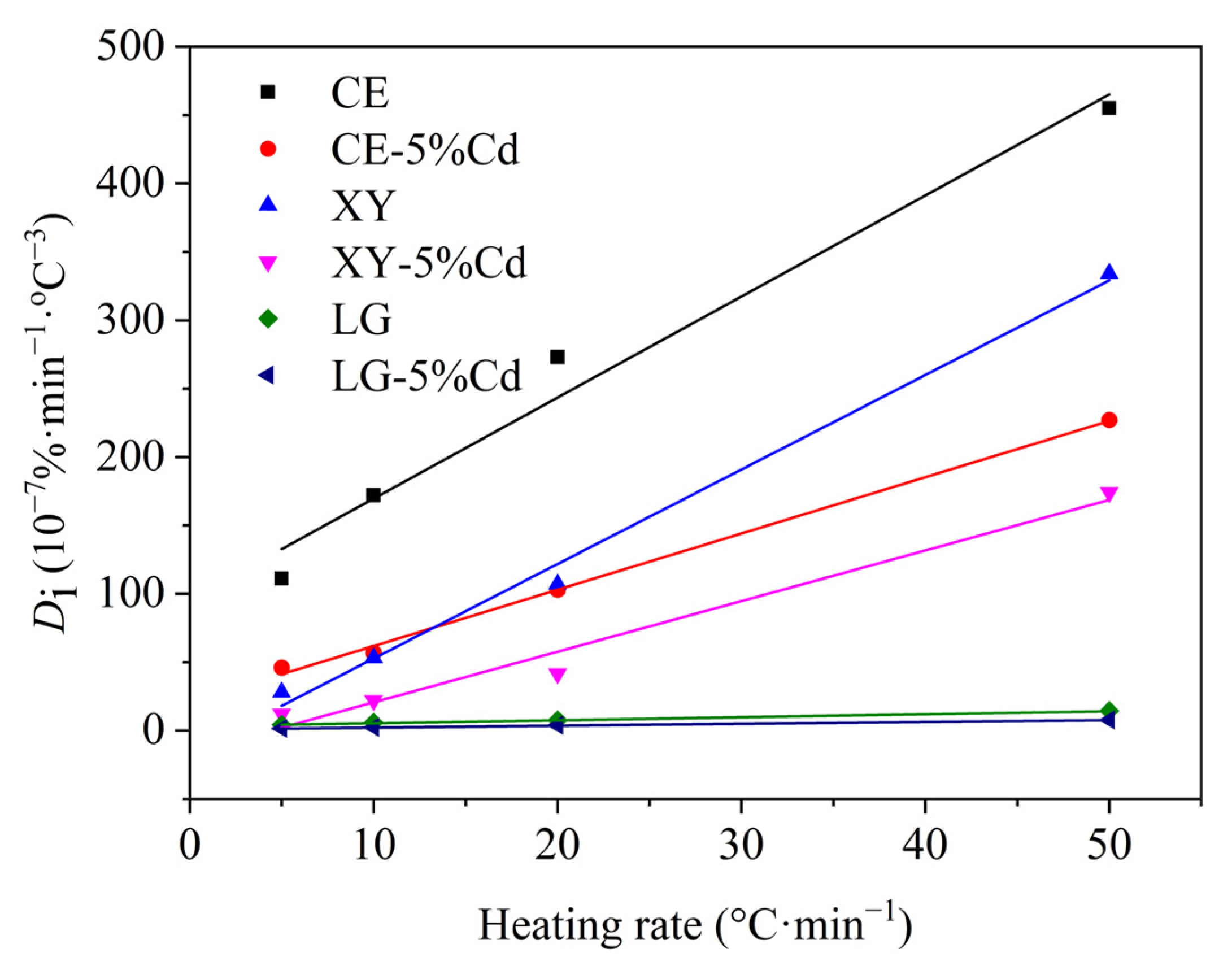
3.1.2. Devolatilization Performance
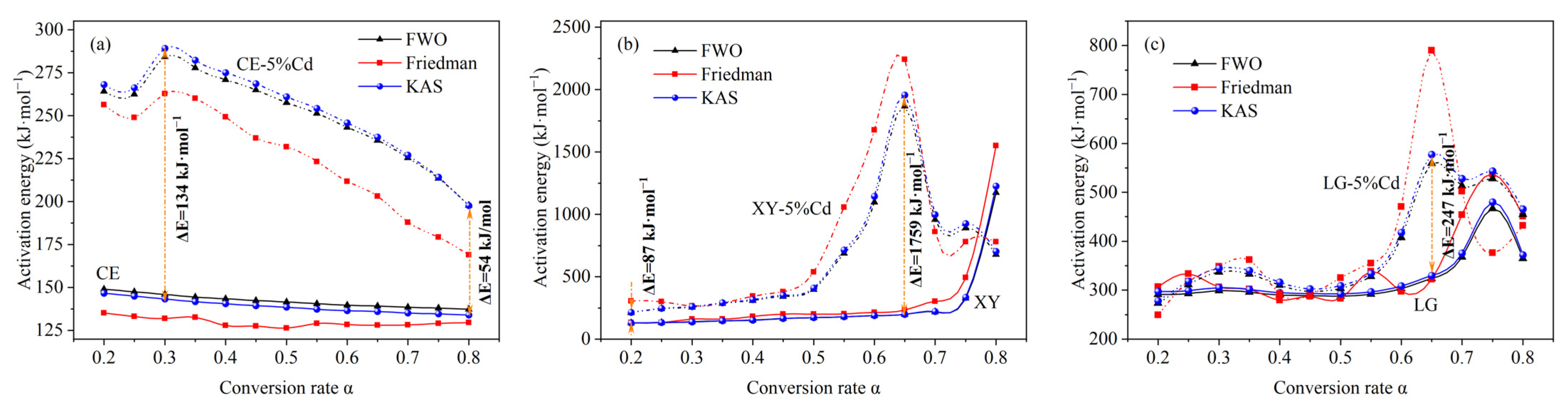
3.1.3. Pyrolysis Kinetics Analysis
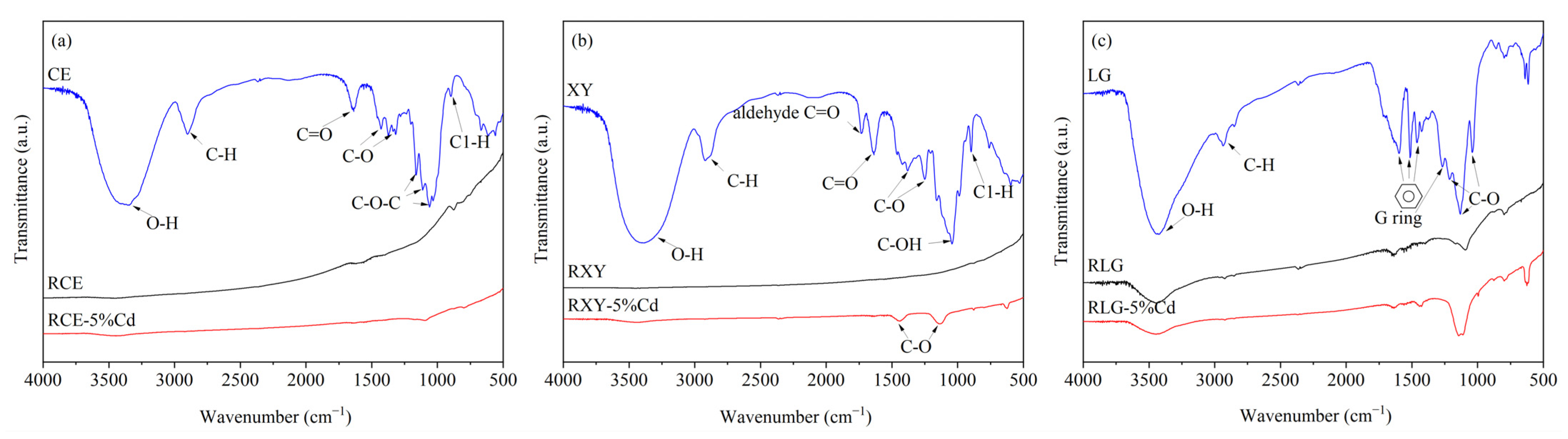
3.1.4. Characteristics of Pyrolysis Residues
3.2. Pyrolysis Characteristics of Cd-Contaminated Rice Straw
3.2.1. TG/DTG Analysis
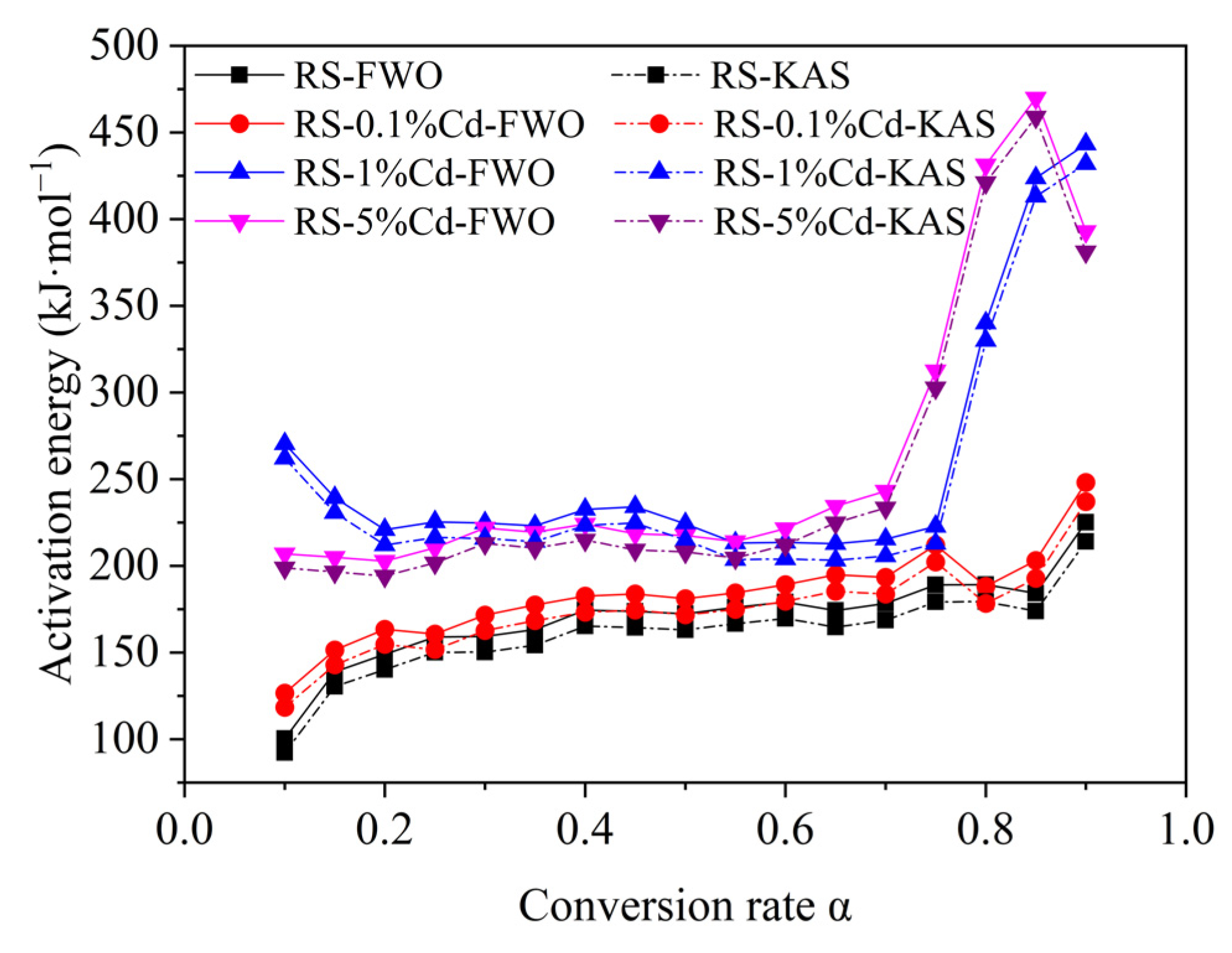
3.2.2. Pyrolysis Kinetics Analysis
3.3. Characteristics of Pyrolytic Volatiles from Cd-Contaminated Rice Straw
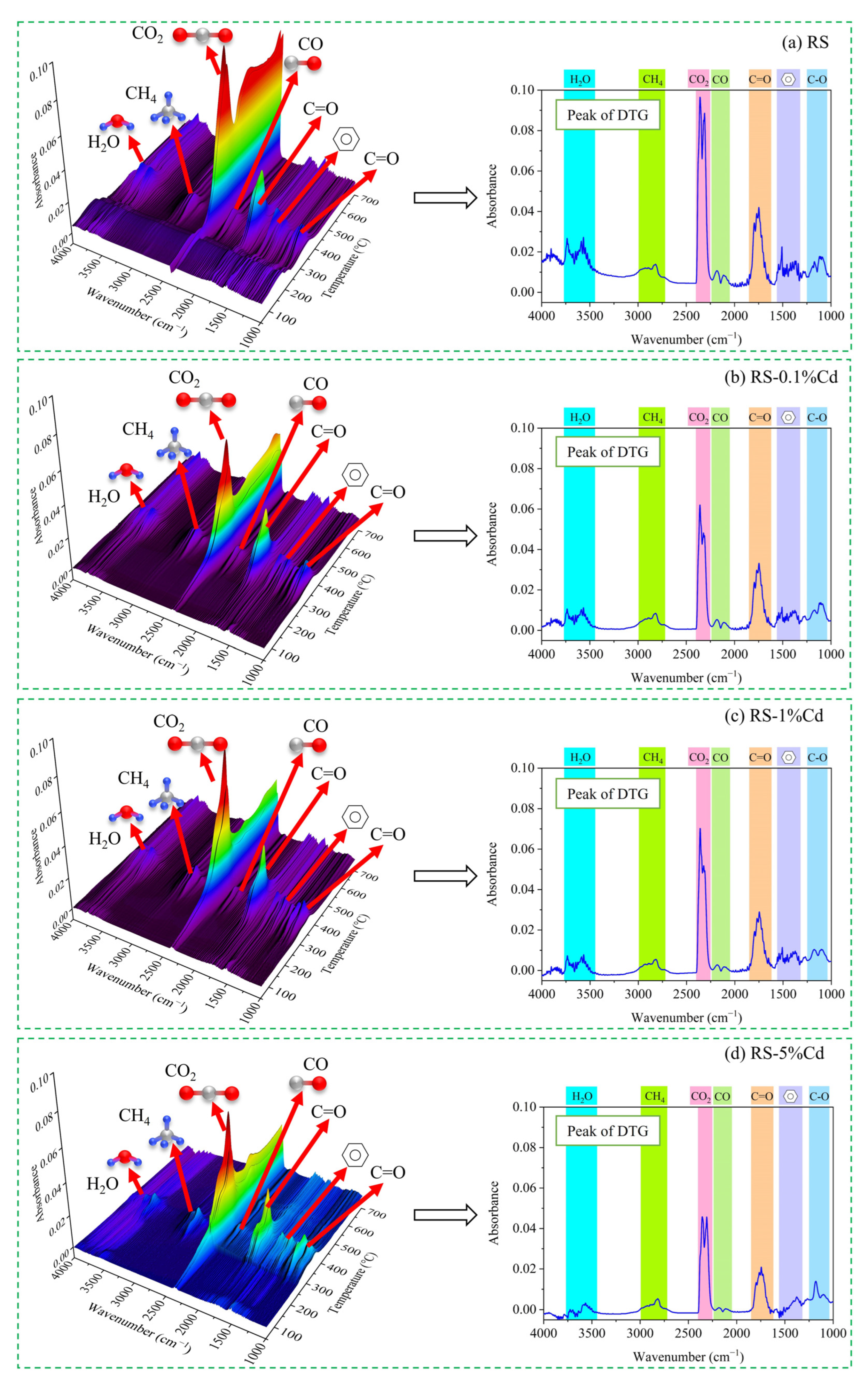
3.3.1. TG-FTIR Analysis
3.3.2. TG-GC/MS Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zeng, P.; Guo, Z.; Xiao, X.; Peng, C.; Liu, L.; Yan, D.; He, Y. Physiological stress responses, mineral element uptake and phytoremediation potential of Morus alba L. in cadmium-contaminated soil. Ecotoxicol. Environ. Saf. 2020, 189, 109973. [Google Scholar] [CrossRef] [PubMed]
- Dastyar, W.; Raheem, A.; He, J.; Zhao, M. Biofuel production using thermochemical conversion of heavy metal-contaminated biomass (HMCB) harvested from phytoextraction process. Chem. Eng. J. 2019, 358, 759–785. [Google Scholar] [CrossRef]
- Zeng, P.; Guo, Z.; Xiao, X.; Peng, C.; Feng, W.; Xin, L.; Xu, Z. Phytoextraction potential of Pteris vittata L. co-planted with woody species for As, Cd, Pb and Zn in contaminated soil. Sci. Total Environ. 2019, 650, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Guo, Z.; Zhang, Y.; Xiao, X.; Xu, Z.; Sun, Y. Adsorption-pyrolysis technology for recovering heavy metals in solution using contaminated biomass phytoremediation. Resour. Conserv. Recycl. 2018, 129, 20–26. [Google Scholar] [CrossRef]
- Zhao, F.-J.; Ma, Y.; Zhu, Y.-G.; Tang, Z.; McGrath, S.P. Soil contamination in China: Current status and mitigation strategies. Environ. Sci. Technol. 2015, 49, 750–759. [Google Scholar] [CrossRef]
- Xu, Z.; Guo, Z.; Xiao, X.; Zeng, P.; Xue, Q. Effect of inorganic potassium compounds on the hydrothermal carbonization of Cd-contaminated rice straw for experimental-scale hydrochar. Biomass Bioenergy 2019, 130, 105357. [Google Scholar] [CrossRef]
- Lu, S.; Du, Y.; Zhong, D.; Zhao, B.; Li, X.; Xu, M.; Li, Z.; Luo, Y.; Yan, J.; Wu, L. Comparison of trace element emissions from thermal treatments of heavy metal hyperaccumulators. Environ. Sci. Technol. 2012, 46, 5025–5031. [Google Scholar] [CrossRef]
- Li, C.; Ji, G.; Qu, Y.; Irfan, M.; Zhu, K.; Wang, X.; Li, A. Influencing mechanism of zinc mineral contamination on pyrolysis kinetic and product characteristics of corn biomass. J. Environ. Manag. 2021, 281, 111837. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, L.-A.; Xiao, H.; Guo, X.; Urbanovich, O.; Nagorskaya, L.; Li, X. A review on control factors of pyrolysis technology for plants containing heavy metals. Ecotoxicol. Environ. Saf. 2020, 191, 110181. [Google Scholar] [CrossRef]
- Kumar, R.; Strezov, V.; Weldekidan, H.; He, J.; Singh, S.; Kan, T.; Dastjerdi, B. Lignocellulose biomass pyrolysis for bio-oil production: A review of biomass pre-treatment methods for production of drop-in fuels. Renew. Sustain. Energy Rev. 2020, 123, 109763. [Google Scholar] [CrossRef]
- He, J.; Strezov, V.; Zhou, X.; Kumar, R.; Kan, T. Pyrolysis of heavy metal contaminated biomass pre-treated with ferric salts: Product characterisation and heavy metal deportment. Bioresour. Technol. 2020, 313, 123641. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Strezov, V.; Kumar, R.; Weldekidan, H.; Jahan, S.; Dastjerdi, B.H.; Zhou, X.; Kan, T. Pyrolysis of heavy metal contaminated Avicennia marina biomass from phytoremediation: Characterisation of biomass and pyrolysis products. J. Clean. Prod. 2019, 234, 1235–1245. [Google Scholar] [CrossRef]
- Gong, X.; Huang, D.; Liu, Y.; Zeng, G.; Wang, R.; Wei, J.; Huang, C.; Xu, P.; Wan, J.; Zhang, C. Pyrolysis and reutilization of plant residues after phytoremediation of heavy metals contaminated sediments: For heavy metals stabilization and dye adsorption. Bioresour. Technol. 2018, 253, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Yao, W.; Li, R.; Ali, A.; Du, J.; Guo, D.; Xiao, R.; Guo, Z.; Zhang, Z.; Awasthi, M.K. Effect of pyrolysis temperature on chemical form, behavior and environmental risk of Zn, Pb and Cd in biochar produced from phytoremediation residue. Bioresour. Technol. 2018, 249, 487–493. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, X.; Li, Y.; Han, L. Influence of pyrolysis temperature on chemical speciation, leaching ability, and environmental risk of heavy metals in biochar derived from cow manure. Bioresour. Technol. 2020, 302, 122850. [Google Scholar] [CrossRef]
- Han, Z.; Guo, Z.; Zhang, Y.; Xiao, X.; Xu, Z.; Sun, Y. Pyrolysis characteristics of biomass impregnated with cadmium, copper and lead: Influence and distribution. Waste Biomass Valorization 2018, 9, 1223–1230. [Google Scholar] [CrossRef]
- Martín-Lara, M.A.; Blázquez, G.; Ronda, A.; Calero, M. Kinetic study of the pyrolysis of pine cone shell through non-isothermal thermogravimetry: Effect of heavy metals incorporated by biosorption. Renew. Energy 2016, 96, 613–624. [Google Scholar] [CrossRef]
- Liu, W.-J.; Tian, K.; Jiang, H.; Zhang, X.-S.; Ding, H.-S.; Yu, H.-Q. Selectively improving the bio-oil quality by catalytic fast pyrolysis of heavy-metal-polluted biomass: Take copper (Cu) as an example. Environ. Sci. Technol. 2012, 46, 7849–7856. [Google Scholar] [CrossRef]
- Jiu, B.; Li, B.; Yu, Q. Effects of Pb on pyrolysis behavior of water hyacinth. J. Anal. Appl. Pyrolysis 2015, 112, 270–275. [Google Scholar] [CrossRef]
- Mayer, Z.A.; Apfelbacher, A.; Hornung, A. A comparative study on the pyrolysis of metal- and ash-enriched wood and the combustion properties of the gained char. J. Anal. Appl. Pyrolysis 2012, 96, 196–202. [Google Scholar] [CrossRef] [Green Version]
- Zhong, D.; Zhong, Z.; Wu, L.; Ding, K.; Luo, Y.; Christie, P. Pyrolysis of Sedum plumbizincicola, a zinc and cadmium hyperaccumulator: Pyrolysis kinetics, heavy metal behaviour and bio-oil production. Clean Technol. Environ. Policy 2016, 18, 2315–2323. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, H.; Wu, H.; Wang, M.; Cheng, D. Catalytic pyrolysis of rice husk by mixing with zinc oxide: Characterization of bio-oil and its rheological behavior. Fuel Process. Technol. 2013, 106, 385–391. [Google Scholar] [CrossRef]
- Lu, Q.; Wang, Z.; Dong, C.-Q.; Zhang, Z.-F.; Zhang, Y.; Yang, Y.-P.; Zhu, X.-F. Selective fast pyrolysis of biomass impregnated with ZnCl2: Furfural production together with acetic acid and activated carbon as by-products. J. Anal. Appl. Pyrolysis 2011, 91, 273–279. [Google Scholar] [CrossRef]
- Lu, Q.; Dong, C.-Q.; Zhang, X.-M.; Tian, H.-Y.; Yang, Y.-P.; Zhu, X.-F. Selective fast pyrolysis of biomass impregnated with ZnCl2 to produce furfural: Analytical Py-GC/MS study. J. Anal. Appl. Pyrolysis 2011, 90, 204–212. [Google Scholar] [CrossRef]
- Nzihou, A.; Stanmore, B.; Lyczko, N.; Minh, D.P. The catalytic effect of inherent and adsorbed metals on the fast/flash pyrolysis of biomass: A review. Energy 2019, 170, 326–337. [Google Scholar] [CrossRef] [Green Version]
- Fan, H.; Gu, J.; Wang, Y.; Yuan, H.; Chen, Y.; Luo, B. Effect of potassium on the pyrolysis of biomass components: Pyrolysis behaviors, product distribution and kinetic characteristics. Waste Manag. 2021, 121, 255–264. [Google Scholar] [CrossRef]
- Gan, D.K.W.; Loy, A.C.M.; Chin, B.L.F.; Yusup, S.; Unrean, P.; Rianawati, E.; Acda, M.N. Kinetics and thermodynamic analysis in one-pot pyrolysis of rice hull using renewable calcium oxide based catalysts. Bioresour. Technol. 2018, 265, 180–190. [Google Scholar] [CrossRef]
- Açıkalın, K.; Gözke, G. Thermogravimetric pyrolysis of onion skins: Determination of kinetic and thermodynamic parameters for devolatilization stages using the combinations of isoconversional and master plot methods. Bioresour. Technol. 2021, 342, 125936. [Google Scholar] [CrossRef]
- Fakayode, O.A.; Wang, Z.; Wahia, H.; Mustapha, A.T.; Zhou, C.; Ma, H. Higher heating value, exergy, pyrolysis kinetics and thermodynamic analysis of ultrasound-assisted deep eutectic solvent pretreated watermelon rind biomass. Bioresour. Technol. 2021, 332, 125040. [Google Scholar] [CrossRef]
- Sahoo, A.; Kumar, S.; Kumar, J.; Bhaskar, T. A detailed assessment of pyrolysis kinetics of invasive lignocellulosic biomasses (Prosopis juliflora and Lantana camara) by thermogravimetric analysis. Bioresour. Technol. 2021, 319, 124060. [Google Scholar] [CrossRef]
- Varhegyi, G.; Jakab, E.; Antal, M.J. Is the Broido-Shafizadeh Model for Cellulose Pyrolysis True? Energy Fuels 1994, 8, 1345–1352. [Google Scholar] [CrossRef] [Green Version]
- Bradbury, A.G.W.; Sakai, Y.; Shafizadeh, F. A kinetic model for pyrolysis of cellulose. J. Appl. Polym. Sci. 1979, 23, 3271–3280. [Google Scholar] [CrossRef]
- Shen, D.K.; Gu, S.; Bridgwater, A.V. Study on the pyrolytic behaviour of xylan-based hemicellulose using TG-FTIR and Py-GC-FTIR. J. Anal. Appl. Pyrolysis 2010, 87, 199–206. [Google Scholar] [CrossRef] [Green Version]
- Hu, B.; Xie, W.-L.; Li, H.; Li, K.; Lu, Q.; Yang, Y.-P. On the mechanism of xylan pyrolysis by combined experimental and computational approaches. Proc. Combust. Inst. 2021, 38, 4215–4223. [Google Scholar] [CrossRef]
- Giudicianni, P.; Gargiulo, V.; Alfè, M.; Ragucci, R.; Ferreiro, A.; Rabaçal, M.; Costa, M. Slow pyrolysis of xylan as pentose model compound for hardwood hemicellulose: A study of the catalytic effect of Na ions. J. Anal. Appl. Pyrolysis 2019, 137, 266–275. [Google Scholar] [CrossRef]
- Giudicianni, P.; Gargiulo, V.; Grottola, C.M.; Alfè, M.; Ragucci, R. Effect of alkali metal ions presence on the products of xylan steam assisted slow pyrolysis. Fuel 2018, 216, 36–43. [Google Scholar] [CrossRef]
- Li, J.; Bai, X.; Fang, Y.; Chen, Y.; Wang, X.; Chen, H.; Yang, H. Comprehensive mechanism of initial stage for lignin pyrolysis. Combust. Flame 2020, 215, 1–9. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, J.; Zhang, B.; Guo, W.; Yang, G.; Yang, B. Synergistic effects from co-pyrolysis of lignocellulosic biomass main component with low-rank coal: Online and offline analysis on products distribution and kinetic characteristics. Appl. Energy 2020, 276, 115461. [Google Scholar] [CrossRef]
- Usino, D.O.; Supriyanto; Ylitervo, P.; Pettersson, A.; Richards, T. Influence of temperature and time on initial pyrolysis of cellulose and xylan. J. Anal. Appl. Pyrolysis 2020, 147, 104782. [Google Scholar] [CrossRef]
- Zong, P.; Jiang, Y.; Tian, Y.; Li, J.; Yuan, M.; Ji, Y.; Chen, M.; Li, D.; Qiao, Y. Pyrolysis behavior and product distributions of biomass six group components: Starch, cellulose, hemicellulose, lignin, protein and oil. Energy Convers. Manag. 2020, 216, 112777. [Google Scholar] [CrossRef]
- Luo, L.; Zhang, Z.; Li, C.; Nishu; He, F.; Zhang, X.; Cai, J. Insight into master plots method for kinetic analysis of lignocellulosic biomass pyrolysis. Energy 2021, 233, 121194. [Google Scholar] [CrossRef]
- Hong, Z.; Niu, W.; Zhang, K.; Su, J.; Liu, J.; Li, L.; Wu, F. Effects of temperature and particle size on the compositions, energy conversions and structural characteristics of pyrolysis products from different crop residues. Energy 2020, 190, 116413. [Google Scholar] [CrossRef]
- Zhang, J.; Choi, Y.S.; Yoo, C.G.; Millan, M. Cellulose, xylan and lignin interactions during pyrolysis of lignocellulosic biomass. ACS Sustain. Chem. Eng. 2015, 3, 293–301. [Google Scholar] [CrossRef]
- Wang, G.; Li, W.; Li, B.; Chen, H. TG study on pyrolysis of biomass and its three components under syngas. Fuel 2008, 87, 552–558. [Google Scholar] [CrossRef]
- Chen, D.; Cen, K.; Zhuang, X.; Gan, Z.; Zhou, J.; Zhang, Y.; Zhang, H. Insight into biomass pyrolysis mechanism based on cellulose, hemicellulose, and lignin: Evolution of volatiles and kinetics, elucidation of reaction pathways, and characterization of gas, biochar and bio-oil. Combust. Flame 2022, 242, 112142. [Google Scholar] [CrossRef]
- Zeng, P.; Guo, Z.; Cao, X.; Xiao, X.; Liu, Y.; Shi, L. Phytostabilization potential of ornamental plants grown in soil contaminated with cadmium. Int. J. Phytoremediat. 2018, 20, 311–320. [Google Scholar] [CrossRef]





| Sample | Proximate Analysis (%) | Ultimate Analysis (%) | Cd (mg·kg−1) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M 1 | A 2 | V 3 | FC 4 | C | H | O | H/C | O/C | ||
| CE | 3.88 | 0.02 | 91.43 | 4.67 | 42.4 | 6.3 | 51.2 | 1.79 | 0.90 | - |
| XY | 1.68 | 0.02 | 91.36 | 6.94 | 41.1 | 6.6 | 52.3 | 1.93 | 0.95 | - |
| LG | 12.26 | 15.59 | 47.14 | 25.01 | 47.5 | 4.6 | 28.3 | 1.15 | 0.45 | - |
| RS | 8.71 | 12.00 | 66.31 | 12.98 | 38.4 | 6.6 | 40.5 | 2.06 | 0.79 | 9.0 |
| Sample | C (%) | H (%) | O (%) |
|---|---|---|---|
| CE | 42.44 | 6.32 | 51.20 |
| RCE | 89.55 | 2.26 | 7.40 |
| RCE-5%Cd | 88.67 | 2.04 | 8.64 |
| XY | 41.10 | 6.60 | 52.27 |
| RXY | 85.85 | 2.46 | 11.10 |
| RXY-5%Cd | 83.96 | 2.57 | 12.93 |
| LG | 47.51 | 4.55 | 43.87 |
| RLG | 60.95 | 1.98 | 33.25 |
| RLG-5%Cd | 60.91 | 1.84 | 33.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Guo, Z.; Xie, H.; Hu, Y. Effect of Cd on Pyrolysis Velocity and Deoxygenation Characteristics of Rice Straw: Analogized with Cd-Impregnated Representative Biomass Components. Int. J. Environ. Res. Public Health 2022, 19, 8953. https://doi.org/10.3390/ijerph19158953
Xu Z, Guo Z, Xie H, Hu Y. Effect of Cd on Pyrolysis Velocity and Deoxygenation Characteristics of Rice Straw: Analogized with Cd-Impregnated Representative Biomass Components. International Journal of Environmental Research and Public Health. 2022; 19(15):8953. https://doi.org/10.3390/ijerph19158953
Chicago/Turabian StyleXu, Zhi, Zhaohui Guo, Huimin Xie, and Yulian Hu. 2022. "Effect of Cd on Pyrolysis Velocity and Deoxygenation Characteristics of Rice Straw: Analogized with Cd-Impregnated Representative Biomass Components" International Journal of Environmental Research and Public Health 19, no. 15: 8953. https://doi.org/10.3390/ijerph19158953
APA StyleXu, Z., Guo, Z., Xie, H., & Hu, Y. (2022). Effect of Cd on Pyrolysis Velocity and Deoxygenation Characteristics of Rice Straw: Analogized with Cd-Impregnated Representative Biomass Components. International Journal of Environmental Research and Public Health, 19(15), 8953. https://doi.org/10.3390/ijerph19158953





