Spatial-Temporal Distribution, Morphological Transformation, and Potential Risk of Dissolved Inorganic Nitrogen in the Contaminated Unconfined Aquifer from a Retired Nitrogenous Fertilizer Plant
Abstract
:1. Introduction
2. Materials and Methods
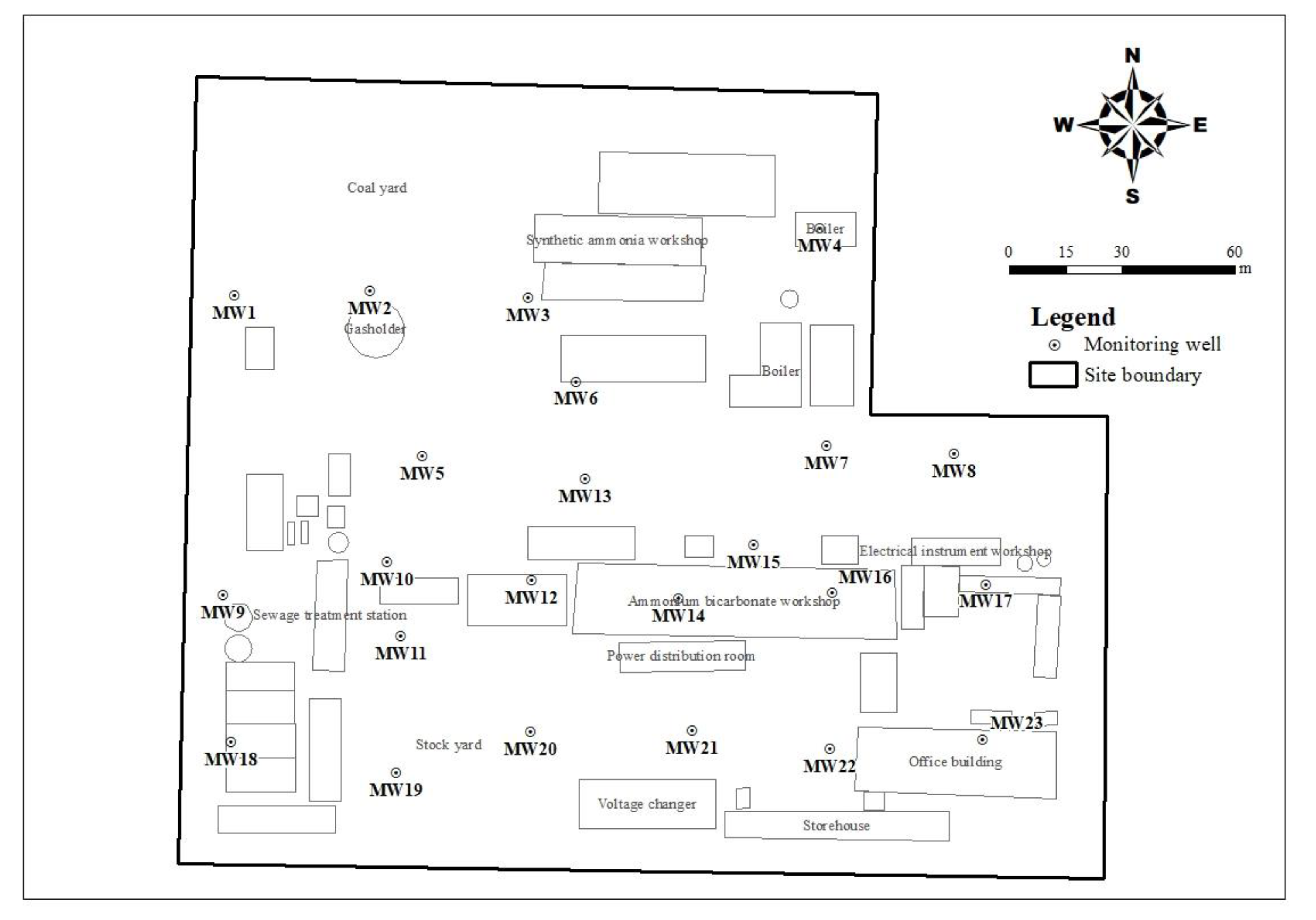
2.1. Overview of the Study Area
2.2. Sampling and Analysis
3. Results and Discussion
3.1. Concentration and Distribution of Dissolved Inorganic Nitrogen (DIN)
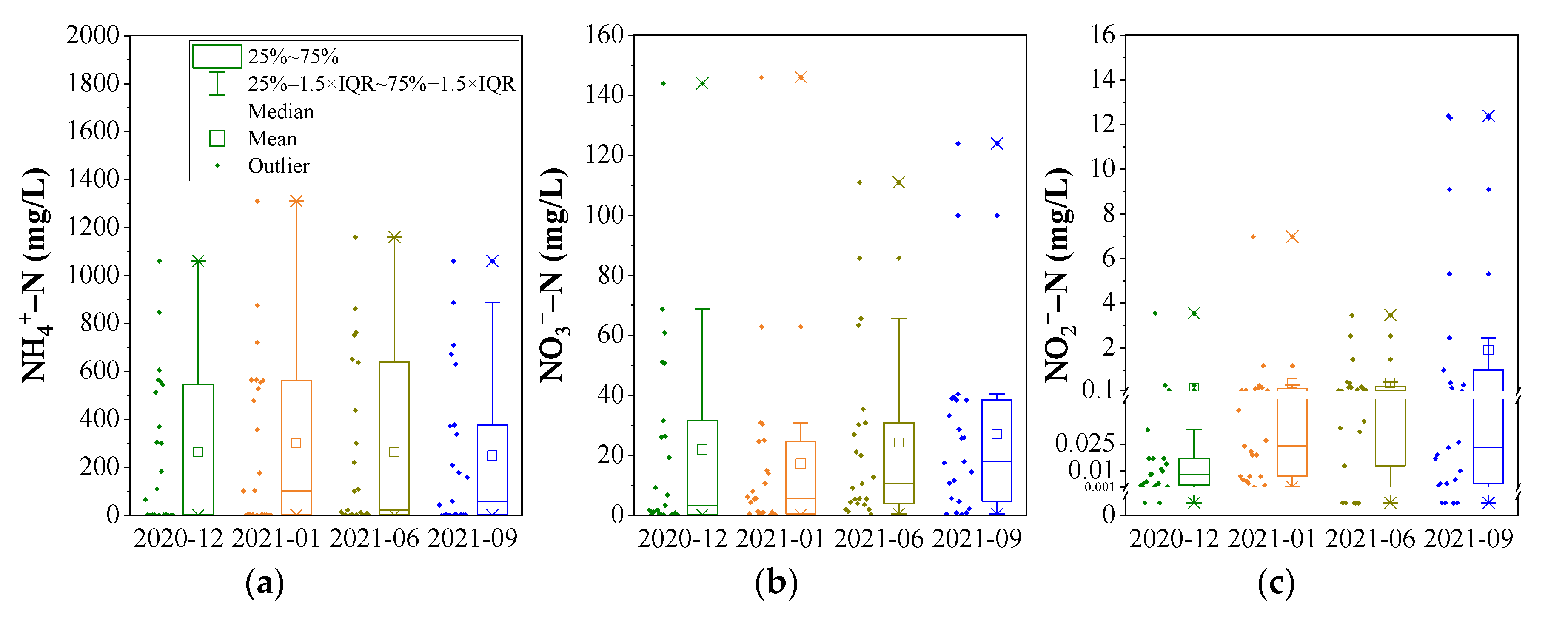
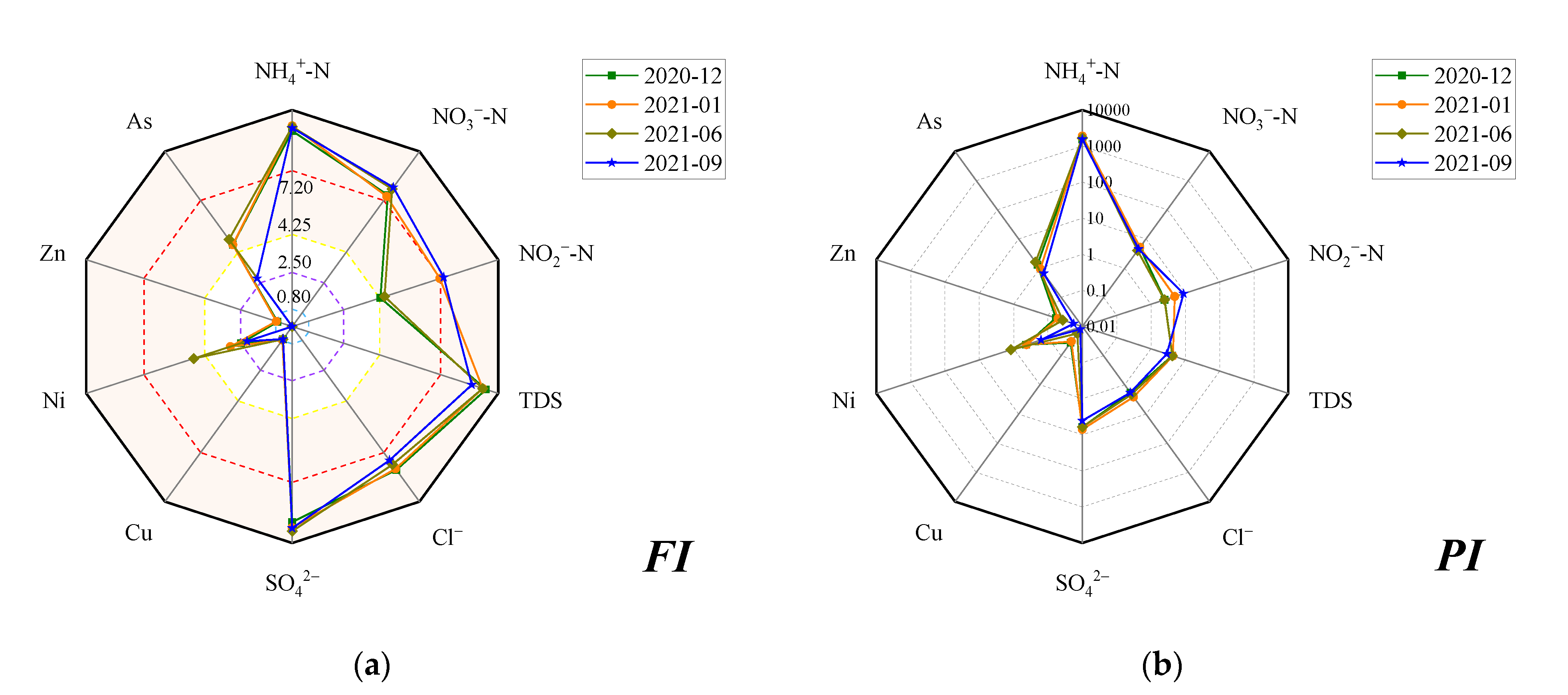
3.1.1. Concentration of DIN Species and Groundwater Quality Assessment
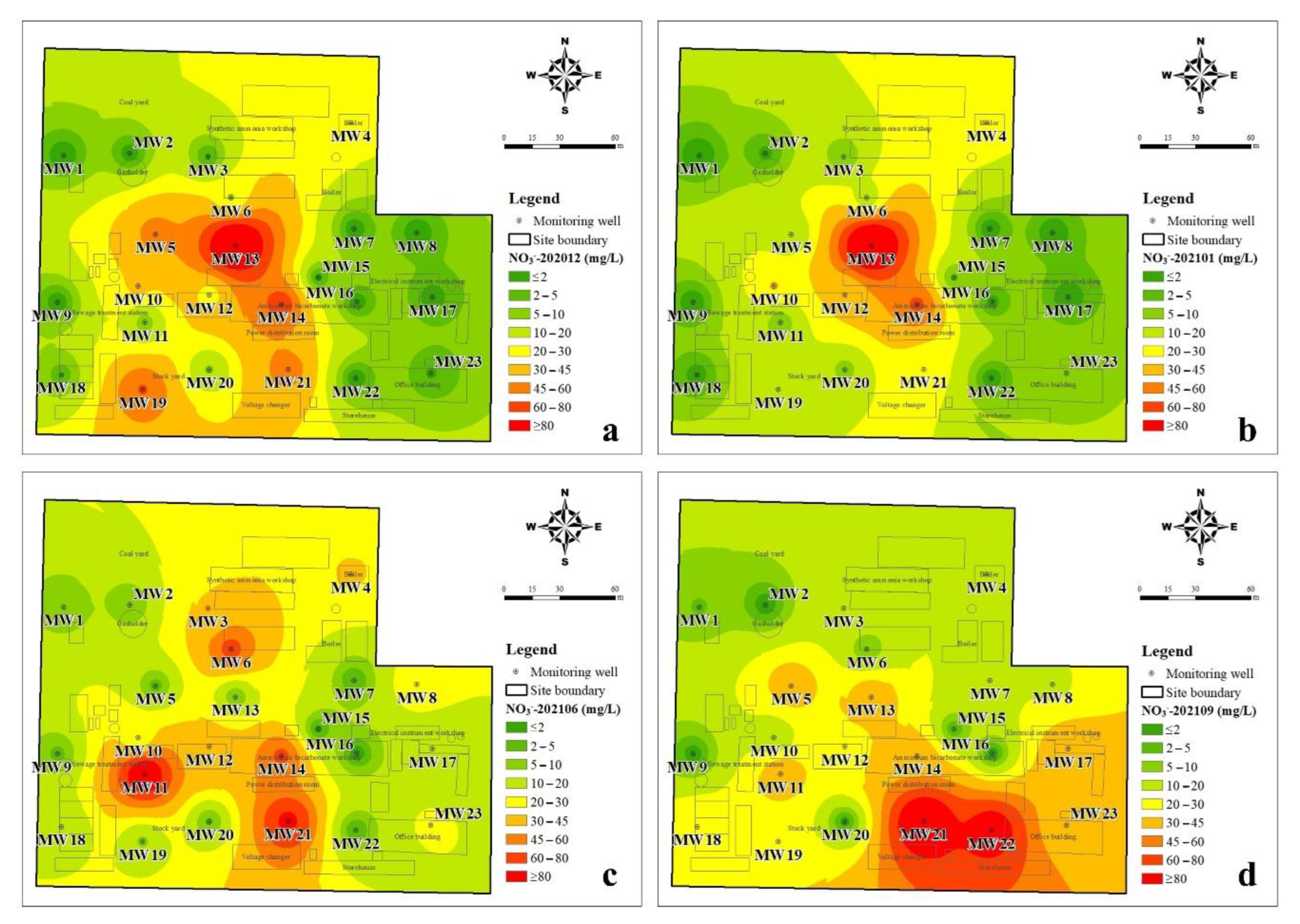
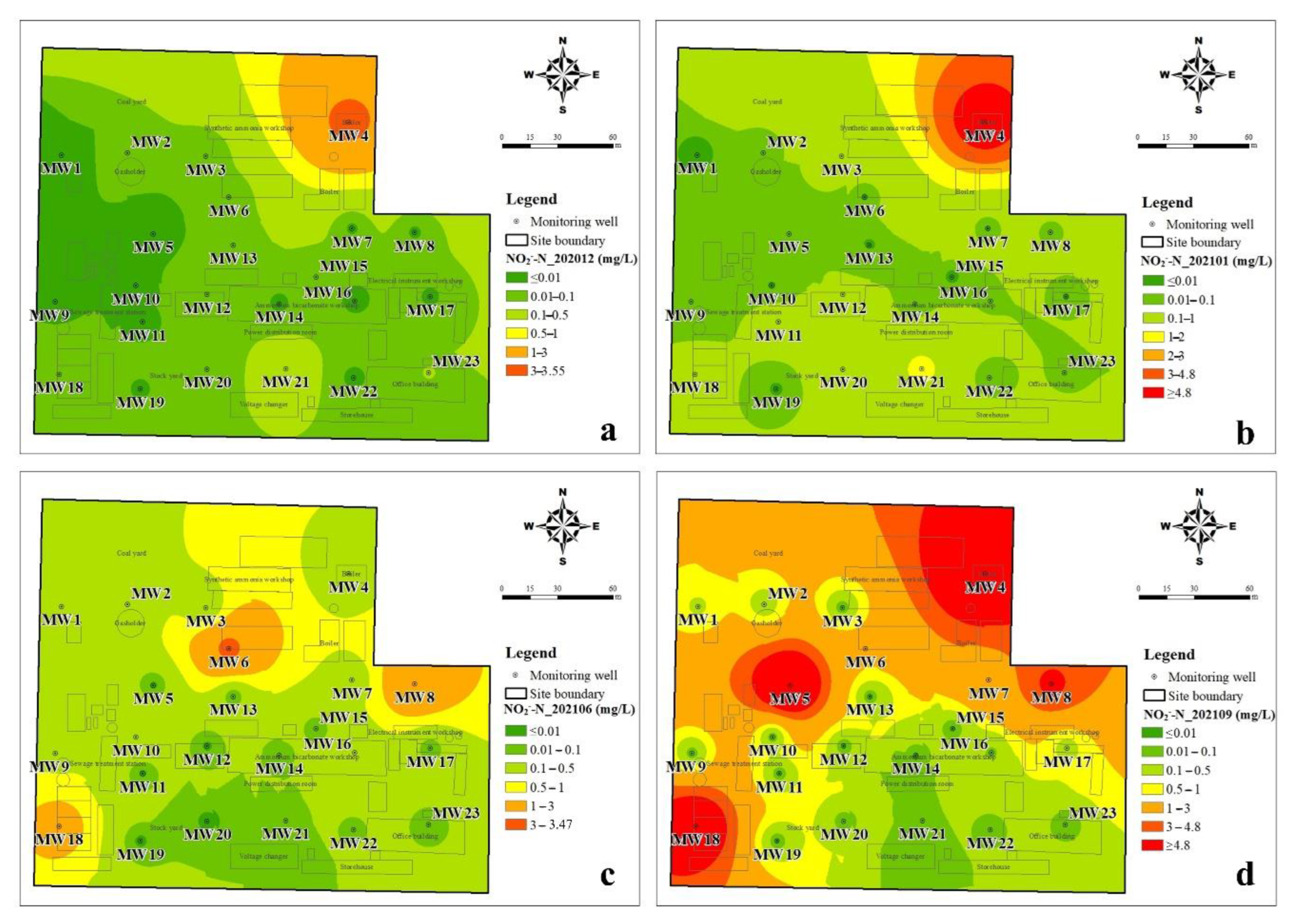
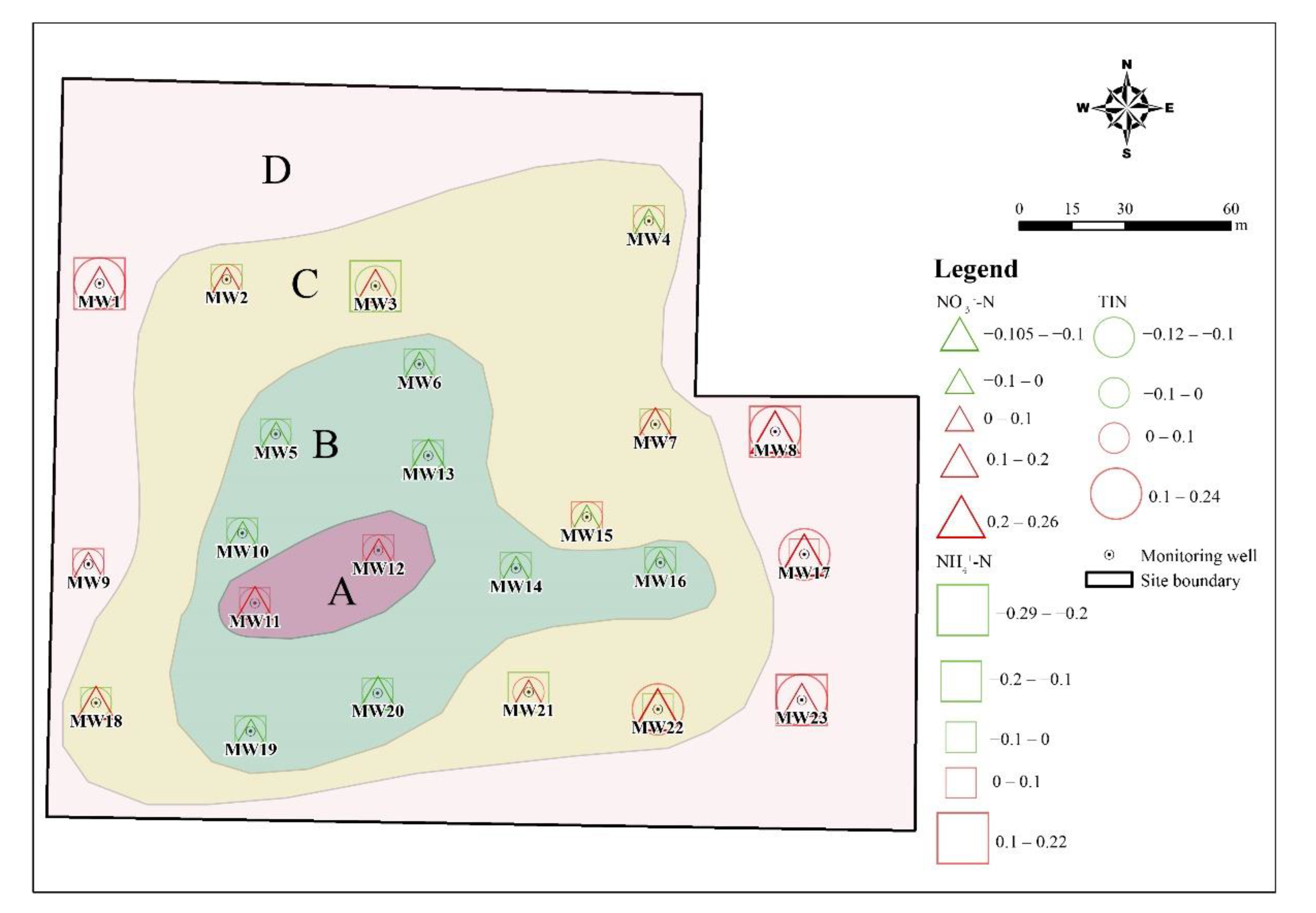
3.1.2. The Spatial Distribution Characteristics of DIN
3.2. Indication of pH and ORP Condition
3.3. Impact Factors of the Transfer and Transformation of DIN
3.3.1. The Transformation of DIN and Correlation Analysis
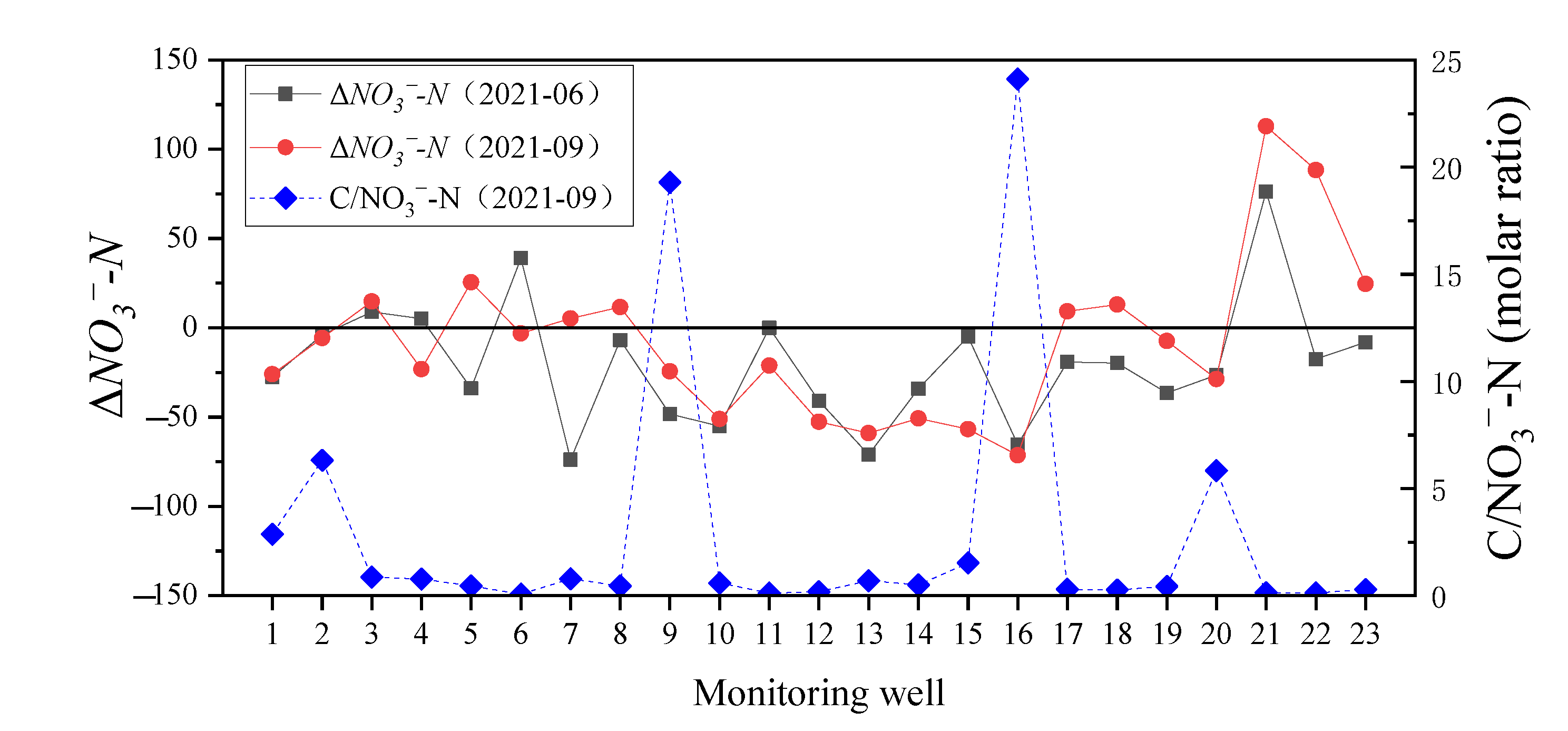
3.3.2. Effect of Rainfall on the Transfer of Nitrate-Nitrogen
3.4. The Temporal Tend of DIN in the Monitoring Wells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Steffen, W.; Richardson, K.; Rockström, J.; Cornell, S.E.; Fetzer, I.; Bennett, E.M.; Biggs, R.; Carpenter, S.R.; de Vries, W.; de Wit, C.A.; et al. Planetary boundaries: Guiding human development on a changing planet. Science 2015, 347, 1259855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xin, J.; Liu, Y.; Chen, F.; Duan, Y.; Wei, G.; Zheng, X.; Li, M. The missing nitrogen pieces: A critical review on the distribution, transformation, and budget of nitrogen in the vadose zone-groundwater system. Water Res. 2019, 165, 114977. [Google Scholar] [CrossRef]
- Xin, J.; Wang, Y.; Shen, Z.; Liu, Y.; Wang, H.; Zheng, X. Critical review of measures and decision support tools for groundwater nitrate management: A surface-to-groundwater profile perspective. J. Hydrol. 2021, 598, 126386. [Google Scholar] [CrossRef]
- Welch, A. Exposing the Dangers of Anhydrous Ammonia. Nurse Pract. 2006, 31, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Deurer, M.; von der Heide, C.; Boettcher, J.; Duijnisveld, W.H.M.; Weymann, D.; Well, R. The dynamics of N2O near the groundwater table and the transfer of N2O into the unsaturated zone: A case study from a sandy aquifer in Germany. Catena 2008, 72, 362–373. [Google Scholar] [CrossRef]
- Ma, E.; Liang, X.; Zhang, J.; Zhang, Y.-K. Dynamics in Diffusive Emissions of Dissolved Gases from Groundwater Induced by Fluctuated Ground Surface Temperature. Environ. Sci. Technol. 2022, 56, 2355–2365. [Google Scholar] [CrossRef]
- Xie, Y.; Tan, C.; Zhang, C.; Wang, J.; Wang, M.; Yang, M.; Guo, G. Characteristics of Ammonia-Nitrogen Distribution and Determination of Risk Control Targets at an Abandoned Fertilizer Production Site. Res. Environ. Sci. 2019, 32, 465–474. [Google Scholar]
- GB/T14848-2017; General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China (AQSIQ). Quality Standard for Ground Water. General Administration of Quality Supervision: Beijing, China, 1993.
- Abiriga, D.; Vestgarden, L.S.; Klempe, H. Long-term redox conditions in a landfill-leachate-contaminated groundwater. Sci. Total Environ. 2021, 755, 143725. [Google Scholar] [CrossRef]
- Brooks, T.W.; Kroeger, K.D.; Michael, H.A.; York, J.K. Oxygen-controlled recirculating seepage meter reveals extent of nitrogen transformation in discharging coastal groundwater at the aquifer–estuary interface. Limnol. Oceanogr. 2021, 66, 3055–3069. [Google Scholar] [CrossRef]
- Böhlke, J.K.; Smith, R.L.; Miller, D.N. Ammonium transport and reaction in contaminated groundwater: Application of isotope tracers and isotope fractionation studies. Water Resour. Res. 2006, 42. [Google Scholar] [CrossRef]
- Smith, R.L.; Böhlke, J.K.; Song, B.; Tobias, C.R. Role of Anaerobic Ammonium Oxidation (Anammox) in Nitrogen Removal from a Freshwater Aquifer. Environ. Sci. Technol. 2015, 49, 12169–12177. [Google Scholar] [CrossRef] [PubMed]
- Burgin, A.J.; Hamilton, S.K. Have we overemphasized the role of denitrification in aquatic ecosystems? A review of nitrate removal pathways. Front. Ecol. Environ. 2007, 5, 89–96. [Google Scholar] [CrossRef] [Green Version]
- Dalsgaard, T.; Canfield, D.E.; Petersen, J.; Thamdrup, B.; Acuña-González, J. N2 production by the anammox reaction in the anoxic water column of Golfo Dulce, Costa Rica. Nature 2003, 422, 606–608. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Radny, D.; Huang, S.; Zhuang, L.; Zhao, S.; Berg, M.; Jetten, M.S.M.; Zhu, G. Nitrogen loss by anaerobic ammonium oxidation in unconfined aquifer soils. Sci. Rep. 2017, 7, 40173. [Google Scholar] [CrossRef]
- Hancock, J.T. Considerations of the importance of redox state for reactive nitrogen species action. J. Exp. Bot. 2019, 70, 4323–4331. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, B.; Zhou, N. Effects of Redox Potential on the Environmental Behavior of Nitrogen in Riparian Zones of West Dongting Lake Wetlands, China. Wetlands 2020, 40, 1307–1316. [Google Scholar] [CrossRef]
- Chen, A.; Lei, B.; Hu, W.; Wang, H.; Zhai, L.; Mao, Y.; Fu, B.; Zhang, D. Temporal-spatial variations and influencing factors of nitrogen in the shallow groundwater of the nearshore vegetable field of Erhai Lake, China. Environ. Sci. Pollut. Res. 2018, 25, 4858–4870. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, P.; Cui, R.; Yang, H.; Li, G.; Chen, A.; Wang, H. Electrical conductivity and dissolved oxygen as predictors of nitrate concentrations in shallow groundwater in Erhai Lake region. Sci. Total Environ. 2022, 802, 149879. [Google Scholar] [CrossRef]
- Lv, X.; Han, Z.; Zhang, H.; Kong, X.; Song, L.; Li, H. Contamination characteristics and source of ammonia nitrogen in the groundwater of a chemical fertilizer contaminated site. J. Arid. Land Resour. Environ. 2018, 32, 176–182. [Google Scholar]
- Xu, T.; Liu, S.; Yan, X.; Li, M. Distribution characteristics and non-carcinogenic risk assessment of ammonia nitrogen in the soil and groundwater polluted by a chemical fertilizer contaminated site. Environ. Pollut. Control. 2021, 43, 211–216. [Google Scholar]
- Han, Z.; Ma, H.; Shi, G.; He, L.; Wei, L.; Shi, Q. A review of groundwater contamination near municipal solid waste landfill sites in China. Sci. Total Environ. 2016, 569, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zheng, X.; Zheng, T.; Luan, Y.; Xin, J. Influencing factors and performance of enhanced denitrification layer in the vadose zone. Earth Sci. Front. 2021, 28, 136–145. [Google Scholar]
- Kuypers, M.M.M.; Marchant, H.K.; Kartal, B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Frindte, K.; Allgaier, M.; Grossart, H.-P.; Eckert, W. Redox stability regulates community structure of active microbes at the sediment-water interface. Environ. Microbiol. Rep. 2016, 8, 798–804. [Google Scholar] [CrossRef]
- Stenstrom, M.K.; Poduska, R.A. The effect of dissolved oxygen concentration on nitrification. Water Res. 1980, 14, 643–649. [Google Scholar] [CrossRef]
- Jahangir, M.M.R.; Johnston, P.; Barrett, M.; Khalil, M.I.; Groffman, P.M.; Boeckx, P.; Fenton, O.; Murphy, J.; Richards, K.G. Denitrification and indirect N2O emissions in groundwater: Hydrologic and biogeochemical influences. J. Contam. Hydrol. 2013, 152, 70–81. [Google Scholar] [CrossRef]
- Ravishankara, A.R.; Daniel, J.S.; Portmann, R.W. Nitrous Oxide (N2O): The Dominant Ozone-Depleting Substance Emitted in the 21st Century. Science 2009, 326, 123–125. [Google Scholar] [CrossRef] [Green Version]
- Appelo, C.A.J.; Postma, D. Geochemistry, Groundwater and Pollution, 2nd ed.; Appelo, C.A.J., Postma, D., Eds.; CRC Press: London, UK, 2005. [Google Scholar] [CrossRef]
- Jampani, M.; Liedl, R.; Hülsmann, S.; Sonkamble, S.; Amerasinghe, P. Hydrogeochemical and mixing processes controlling groundwater chemistry in a wastewater irrigated agricultural system of India. Chemosphere 2020, 239, 124741. [Google Scholar] [CrossRef]
- Kurunc, A.; Ersahin, S.; Uz, B.Y.; Sonmez, N.K.; Uz, I.; Kaman, H.; Bacalan, G.E.; Emekli, Y. Identification of nitrate leaching hot spots in a large area with contrasting soil texture and management. Agric. Water Manag. 2011, 98, 1013–1019. [Google Scholar] [CrossRef]
- Yang, S.; Wu, H.; Dong, Y.; Zhao, X.; Song, X.; Yang, J.; Hallett, P.D.; Zhang, G.-L. Deep Nitrate Accumulation in a Highly Weathered Subtropical Critical Zone Depends on the Regolith Structure and Planting Year. Environ. Sci. Technol. 2020, 54, 13739–13747. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, C.; Nelson, W.C.; Shi, L.; Xu, F.; Liu, Y.; Yan, A.; Zhong, L.; Thompson, C.; Fredrickson, J.K.; et al. Effect of Water Chemistry and Hydrodynamics on Nitrogen Transformation Activity and Microbial Community Functional Potential in Hyporheic Zone Sediment Columns. Environ. Sci. Technol. 2017, 51, 4877–4886. [Google Scholar] [CrossRef] [PubMed]
- Rivett, M.O.; Buss, S.R.; Morgan, P.; Smith, J.W.N.; Bemment, C.D. Nitrate attenuation in groundwater: A review of biogeochemical controlling processes. Water Res. 2008, 42, 4215–4232. [Google Scholar] [CrossRef] [PubMed]
- Rust, C.M.; Aelion, C.M.; Flora, J.R.V. Control of pH during denitrification in subsurface sediment microcosms using encapsulated phosphate buffer. Water Res. 2000, 34, 1447–1454. [Google Scholar] [CrossRef]
- Jahangir, M.M.R.; Fenton, O.; Mueller, C.; Harrington, R.; Johnston, P.; Richards, K.G. In Situ denitrification and DNRA rates in groundwater beneath an integrated constructed wetland. Water Res. 2017, 111, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Putz, M.; Schleusner, P.; Rutting, T.; Hallin, S. Relative abundance of denitrifying and DNRA bacteria and their activity determine nitrogen retention or loss in agricultural soil. Soil Biol. Biochem. 2018, 123, 97–104. [Google Scholar] [CrossRef]
- Renwick, W.H.; Vanni, M.J.; Zhang, Q.; Patton, J. Water Quality Trends and Changing Agricultural Practices in a Midwest U.S. Watershed, 1994–2006. J. Environ. Qual. 2008, 37, 1862–1874. [Google Scholar] [CrossRef] [Green Version]
- Kaown, D.; Hyun, Y.; Bae, G.-O.; Oh, C.W.; Lee, K.-K. Evaluation of spatio-temporal trends of groundwater quality in different land uses using Kendall test. Geosci. J. 2012, 16, 65–75. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, K.; Jiang, D.; Chen, Y.; Wei, J.; Xia, F.; Xie, W.; Zhou, Y.; Li, X.; Deng, S. Spatial-Temporal Distribution, Morphological Transformation, and Potential Risk of Dissolved Inorganic Nitrogen in the Contaminated Unconfined Aquifer from a Retired Nitrogenous Fertilizer Plant. Int. J. Environ. Res. Public Health 2022, 19, 8022. https://doi.org/10.3390/ijerph19138022
Yang K, Jiang D, Chen Y, Wei J, Xia F, Xie W, Zhou Y, Li X, Deng S. Spatial-Temporal Distribution, Morphological Transformation, and Potential Risk of Dissolved Inorganic Nitrogen in the Contaminated Unconfined Aquifer from a Retired Nitrogenous Fertilizer Plant. International Journal of Environmental Research and Public Health. 2022; 19(13):8022. https://doi.org/10.3390/ijerph19138022
Chicago/Turabian StyleYang, Kunhua, Dengdeng Jiang, Yun Chen, Jing Wei, Feiyang Xia, Wenyi Xie, Yan Zhou, Xuwei Li, and Shaopo Deng. 2022. "Spatial-Temporal Distribution, Morphological Transformation, and Potential Risk of Dissolved Inorganic Nitrogen in the Contaminated Unconfined Aquifer from a Retired Nitrogenous Fertilizer Plant" International Journal of Environmental Research and Public Health 19, no. 13: 8022. https://doi.org/10.3390/ijerph19138022
APA StyleYang, K., Jiang, D., Chen, Y., Wei, J., Xia, F., Xie, W., Zhou, Y., Li, X., & Deng, S. (2022). Spatial-Temporal Distribution, Morphological Transformation, and Potential Risk of Dissolved Inorganic Nitrogen in the Contaminated Unconfined Aquifer from a Retired Nitrogenous Fertilizer Plant. International Journal of Environmental Research and Public Health, 19(13), 8022. https://doi.org/10.3390/ijerph19138022






