Adhesion and Removal of Thirdhand Smoke from Indoor Fabrics: A Method for Rapid Assessment and Identification of Chemical Repositories
Abstract
1. Introduction
2. Materials and Methods
2.1. Exposure of Household Fabrics to Firsthand and Secondhand Cigarette Smoke
2.2. Preparation of THS Aqueous Extracts for Fluorescence Intensity and LC-MS/MS Analysis
2.3. Fluorescence Intensity
2.4. LC-MS/MS Analysis of Nicotine, Tobacco Alkaloids, and TSNAs
2.5. Exposure to Pure Nicotine
2.6. Preparation of Aqueous Extracts from Nicotine-Treated Fabrics for HPLC Analysis
2.7. HPLC Analysis of Nicotine
3. Results
3.1. THS Fabric Exposure
3.2. Autofluorescence of THS Extracts Is Directly Related to the Length of Exposure
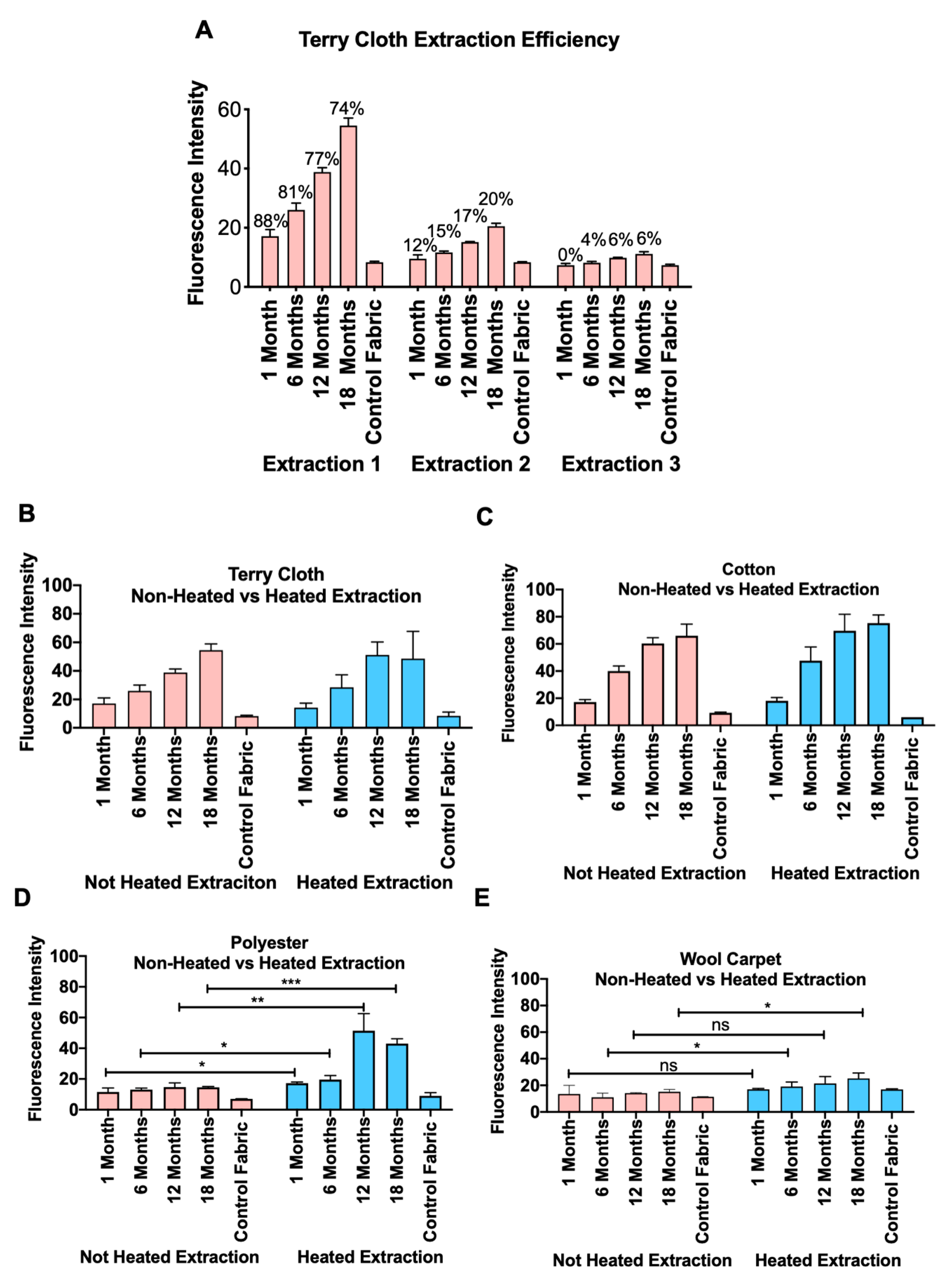
3.3. Heating Increased the Release of THS from Polyester and Wool Carpet
3.4. Analysis of Chemicals Released into PBS from THS-Exposed Fabrics as Measured Using HPLC
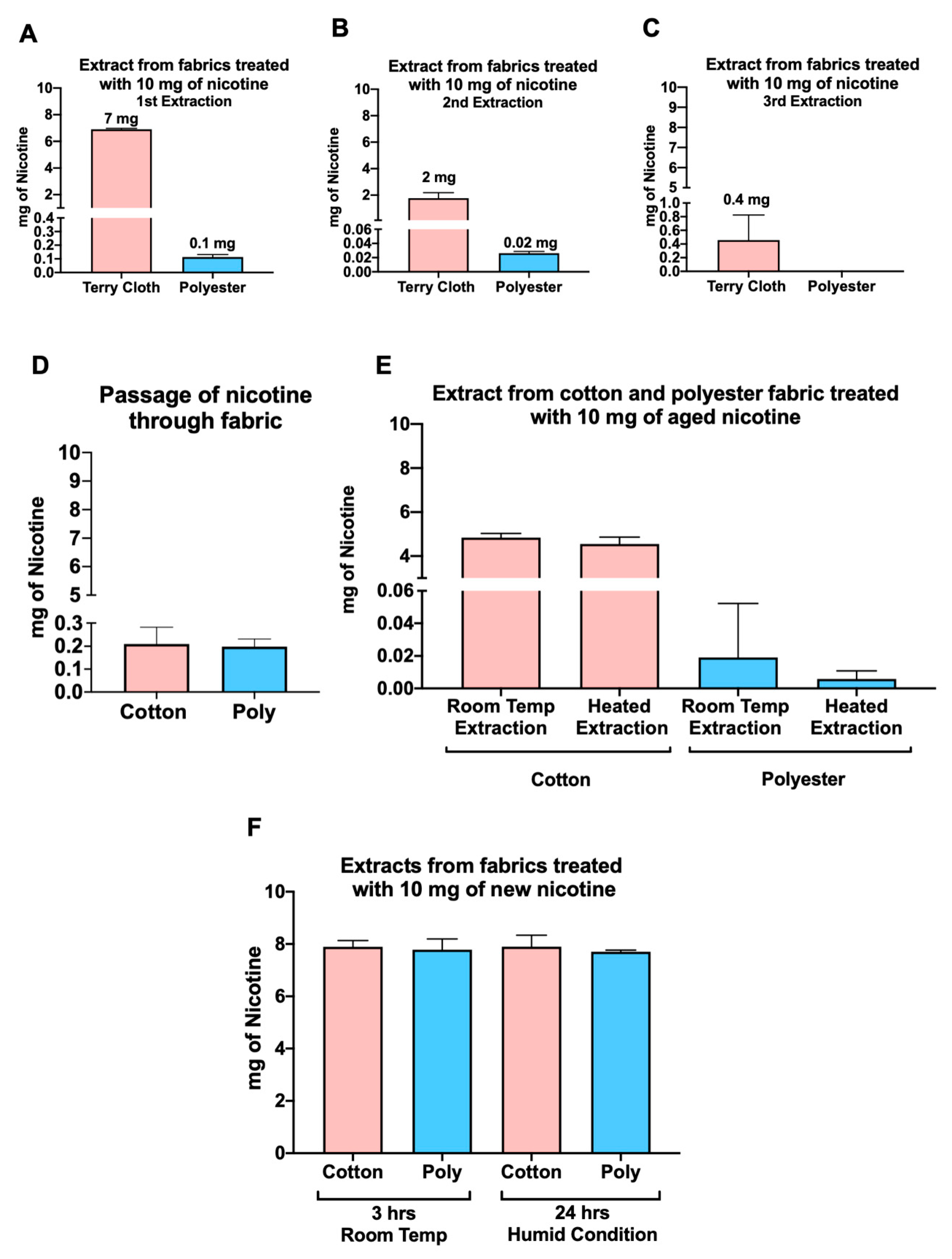
3.5. Factors Affecting the Efficiency of Nicotine Extraction from Fabrics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matt, G.E.; Quintana, P.J.E.; Destaillats, H.; Gundel, L.A.; Sleiman, M.; Singer, B.C.; Jacob, P.; Benowitz, N.; Winickoff, J.P.; Rehan, V.; et al. Thirdhand Tobacco Smoke: Emerging Evidence and Arguments for a Multidisciplinary Research Agenda. Environ. Health Perspect. 2011, 119, 1218–1226. [Google Scholar] [CrossRef] [PubMed]
- Jacob, P.; Benowitz, N.L.; Destaillats, H.; Gundel, L.; Hang, B.; Martins-Green, M.; Matt, G.E.; Quintana, P.J.E.; Samet, J.M.; Schick, S.F.; et al. Thirdhand smoke: New evidence, challenges, and future directions. Chem. Res. Toxicol. 2017, 30, 270–294. [Google Scholar] [CrossRef]
- Sleiman, M.; Destaillats, H.; Smith, J.D.; Liu, C.-L.; Ahmed, M.; Wilson, K.R.; Gundel, L.A. Secondary organic aerosol formation from ozone-initiated reactions with nicotine and secondhand tobacco smoke. Atmos. Environ. 2010, 44, 4191–4198. [Google Scholar] [CrossRef]
- Sleiman, M.; Gundel, L.A.; Pankow, J.F.; Jacob, P., III; Singer, B.C.; Destaillats, H. Formation of carcinogens indoors by surface-mediated reactions of nicotine with nitrous acid, leading to potential thirdhand smoke hazards. Proc. Natl. Acad. Sci. USA 2010, 107, 6576–6581. [Google Scholar] [CrossRef]
- Schick, S.F.; Glantz, S. Concentrations of the carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in side stream cigarette smoke increase after release into indoor air: Results from unpublished tobacco industry research. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1547–1553. [Google Scholar] [CrossRef] [PubMed]
- Bahl, V.; Jacob, P., III; Havel, C.; Schick, S.F.; Talbot, P. Thirdhand cigarette smoke: Factors affecting exposure and re-mediation. PLoS ONE 2014, 9, e108258. [Google Scholar] [CrossRef] [PubMed]
- Hang, B.; Sarker, A.H.; Havel, C.; Saha, S.; Hazra, T.K.; Schick, S.; Jacob, P., III; Rehan, V.K.; Chenna, A.; Sharan, D.; et al. Thirdhand smoke causes DNA damage in human cells. Mutagenesis 2013, 28, 381–391. [Google Scholar] [CrossRef]
- Bahl, V.; Johnson, K.; Phandthong, R.; Zahedi, A.; Schick, S.F.; Talbot, P. Thirdhand cigarette smoke causes stress-induced mitochondrial hyperfusion and alters the transcriptional profile of stem cells. Toxicol. Sci. 2016, 153, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Bahl, V.; Shim, H.J.; Jacob, P., III; Dias, K.; Schick, S.F.; Talbot, P. Thirdhand smoke: Chemical dynamics, cytotoxicity, and genotoxicity in outdoor and indoor environments. Toxicol. Vitr. 2016, 32, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Bahl, V.; Weng, N.J.-H.; Schick, S.F.; Sleiman, M.; Whitehead, J.; Ibarra, A.; Talbot, P. Cytotoxicity of Thirdhand Smoke and Identification of Acrolein as a Volatile Thirdhand Smoke Chemical That Inhibits Cell Proliferation. Toxicol. Sci. 2015, 150, 234–246. [Google Scholar] [CrossRef]
- Xu, B.; Chen, M.; Yao, M.; Ji, X.; Mao, Z.; Tang, W.; Qiao, S.; Schick, S.F.; Mao, J.H.; Hang, B.; et al. Metabolomics reveals metabolic changes inn male reproductive cells exposed to thirdhand smoke. Sci. Rep. 2015, 5, 15512. [Google Scholar] [CrossRef] [PubMed]
- Martins-Green, M.; Adhami, N.; Frankos, M.; Valdez, M.; Goodwin, B.; Lyubovitsky, J.; Dhall, S.; Garcia, M.; Egiebor, I.; Martinez, B.; et al. Cigarette Smoke Toxins Deposited on Surfaces: Implications for Human Health. PLoS ONE 2014, 9, e86391. [Google Scholar] [CrossRef] [PubMed]
- Adhami, N.; Starck, S.R.; Flores, C.; Martins Green, M. A health threat to bystanders living in the homes of smokers: How smoke toxins deposited on surfaces can cause insulin resistance. PLoS ONE 2016, 11, e0149510. [Google Scholar]
- Hang, B.; Snijders, A.M.; Huang, Y.; Schick, S.F.; Wang, P.; Xia, Y.; Havel, C.; Jacob, P.; Benowitz, N.; Destaillats, H.; et al. Early exposure to thirdhand cigarette smoke affects body mass and the development of immunity in mice. Sci. Rep. 2017, 7, 41915. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, G.; Simoni, M.; Cibella, F.; Ferrara, F.; Liotta Malizia, V.; Corsello, G.; Viegi, G.; La Crutta, S. Third-hand smoke exposure and health hazards in children. Monaldi Arch. Chest Dis. 2013, 79, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Northrup, T.; Khan, A.M.; Jacob, P., III; Benowitz, N.L.; Hoh, E.; Hovell, M.F.; Matt, G.E.; Stotts, A.L. Thirdhand smoke contamination in hospital settings: Assessing exposure risk for vulnerable pediatric patients. Tob. Control 2016, 25, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.W.; Ju, Y.S.; Kang, H.R. Association between paternal smoking behavior and children’s respiratory morbidity: 5 year study inn an urban city of South Korea. Pediatric Pulmonol. 2012, 47, 338–345. [Google Scholar] [CrossRef]
- Pozuelos, G.L.; Kagda, M.S.; Schick, S.; Girke, T.; Volz, D.C.; Talbot, P. Experimental Acute Exposure to Thirdhand Smoke and Changes in the Human Nasal Epithelial Transcriptome: A Randomized Clinical Trial. JAMA Netw. Open 2019, 2, e196362. [Google Scholar] [CrossRef]
- Matt, G.E.; Quintana, P.J.; Zakarian, J.M.; Fortmann, A.L.; Chatfield, D.A.; Hoh, E.; Uribe, A.M.; Hovell, M.F. When smokers move out and nonsmokers move in: Residential thirdhand smoke pollution and exposure. Tobacco Control 2011, 20, e1. [Google Scholar] [CrossRef]
- Matt, G.E.; Quintana, P.J.E.; Hovell, M.F.; Chatfield, D.; Ma, D.S.; Romero, R.; Uribe, A. Residual tobacco smoke pollution in used cars for sale: Air, dust, and surfaces. Nicotine Tob. Res. 2008, 10, 1467–1475. [Google Scholar] [CrossRef]
- Matt, G.E.; E Quintana, P.J.; Hovell, M.F.; Bernert, J.T.; Song, S.; Novianti, N.; Juarez, T.; Floro, J.; Gehrman, C.; Garcia, M.; et al. Households contaminated by environmental tobacco smoke: Sources of infant exposures. Tob. Control. 2004, 13, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Sheu, R.; Stönner, C.; Ditto, J.C.; Klüpfel, T.; Williams, J.; Gentner, D.R. Human transport of thirdhand tobacco smoke: A prominent source of hazardous air pollutants into indoor nonsmoking environments. Sci. Adv. 2020, 6, eaay4109. [Google Scholar] [CrossRef]
- Schick, S.F.; Farraro, K.F.; Perrino, C.; Sleiman, M.; Van De Vossenberg, G.; Trinh, M.P.; Hammond, S.K.; Jenkins, B.M.; Balmes, J. Thirdhand cigarette smoke in an experimental chamber: Evidence of surface deposition of nicotine, nitrosamines and polycyclic aromatic hydrocarbons and de novo formation of NNK. Tob. Control. 2014, 23, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Schick, S.F.; Farraro, K.F.; Fang, J.; Nasari, S.; Kim, J.; Lucas, D.; Wong, J.; Giles, D.K.; Jenkins, B. An Apparatus for generating aged cigarette smoke for controlled human exposure studies. Aerosol Sci. Technol. 2012, 46, 1246–1255. [Google Scholar] [CrossRef]
- Whitehead, T.P.; Havel, C.; Metayer, C.; Benowitz, N.L.; Jacob, P., III. Tobacco alkaloids and tobacco-specific nitrosamines in dust from homes of smokeless tobacco users, active smokers, and nontobacco users. Chem. Res. Toxicol. 2015, 28, 1007–1014. [Google Scholar] [CrossRef]
- Davis, B.; Dang, M.; Kim, J.; Talbot, P. Nicotine concentrations in electronic cigarette refill and Do-It-Yourself fluids. Nicotine Tob. Res. 2014, 17, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Paszkiewick, G.M.; Pauly, J.L. Spectrofluorometric method for measuring tobacco smoke particulate matter on cigarette filters and Cambridge pads. Tob. Control 2008, 17 (Suppl. I), i53–i58. [Google Scholar] [CrossRef]
- Pauly, J.L.; Allaart, H.A.; Rodriguez, M.I.; Streck, R.J. Fibers released from cigarette filters: An additional health risk to the smoker? Cancer Res. 1995, 55, 253–258. [Google Scholar]
- Petrick, L.; Destaillats, H.; Zouev, I.; Sabach, S.; Dubowski, Y. Sorption, desorption, and surface oxidative fate of nicotine. Phys. Chem. Chem. Phys. 2010, 12, 10356–10364. [Google Scholar] [CrossRef]
- Noble, R.E. Environmental tobacco smoke uptake by clothing fabrics. Sci. Total Environ. 2000, 262, 1–3. [Google Scholar] [CrossRef]
- Cheng, C.-Y.; Huang, S.-S.; Yang, C.-M.; Tang, K.-T.; Yao, D.-J. Detection of thirdhand smoke on clothing fibers with a surface acoustic wave gas sensor. Biomicrofluidics 2016, 10, 11907. [Google Scholar] [CrossRef]
- Saini, A.; Rauert, C.; Simpson, M.J.; Harrad, S.; Diamond, M.L. Characterizing the sorption of polybrominated diphenyl ethers (PBDEs) to cotton and polyester fabrics under controlled conditions. Sci. Total Environ. 2016, 563–564, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Chien, Y.-C.; Chang, C.-P.; Liu, Z.-Z. Volatile organics off-gassed among tobacco-exposed clothing fabrics. J. Hazard. Mater. 2011, 193, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Destaillats, H.; Singer, B.C.; Lee, S.K.; Gundel, L.A. Effect of Ozone on Nicotine Desorption from Model Surfaces: Evidence for Heterogeneous Chemistry. Environ. Sci. Technol. 2006, 40, 1799–1805. [Google Scholar] [CrossRef] [PubMed]
- Barlow, S.; Renwick, A.G.; Kleiner, J.; Bridges, J.W.; Busk, L.; Dybing, E.; Edler, L.; Eisenbrand, G.; Fink-Gremmels, J.; Knaap, A.; et al. Risk assessment of substances that are both genotoxic and carcinogenic report of an International Conference organized by EFSA and WHO with support of ILSI Europe. Food Chem. Toxicol. 2006, 44, 1636–1650. [Google Scholar] [CrossRef]
- Cunningham, F.; Fiebelkorn, S.; Johnson, M.; Meredith, C. A novel application of the Margin of Exposure approach: Segregation of tobacco smoke toxicants. Food Chem. Toxicol. 2011, 49, 2921–2933. [Google Scholar] [CrossRef] [PubMed]
- Naufal, Z.; Kathman, S.; Wilson, C. Bayesian derivation of an oral cancer slope factor distribution for 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK). Regul. Toxicol. Pharm. 2009, 55, 69–75. [Google Scholar] [CrossRef] [PubMed]
- EFSA. EFSA Meeting Summary Report: EFSA/WHO International Conference with Support of ILSI Europe on Risk Assessment of Compounds that Are Both Genotoxic and Carcinogenic; EFSA: Brussels, Belgium, 2006; p. 290. [Google Scholar]
- CDC (Centers for Disease Control and Prevention). Smoking is Down, but almost 38 Million American Adults Still Smoke. 2018. Available online: https://www.cdc.gov/media/releases/2018/p0118-smoking-rates-declining.html (accessed on 30 November 2020).
- Ramírez, N.; Özel, M.Z.; Lewis, A.C.; Marcé, R.M.; Borrull, F.; Hamilton, J.F. Exposure to nitrosamines in thirdhand tobacco smoke increases cancer risk in non-smokers. Environ. Int. 2014, 71, 139–147. [Google Scholar] [CrossRef]

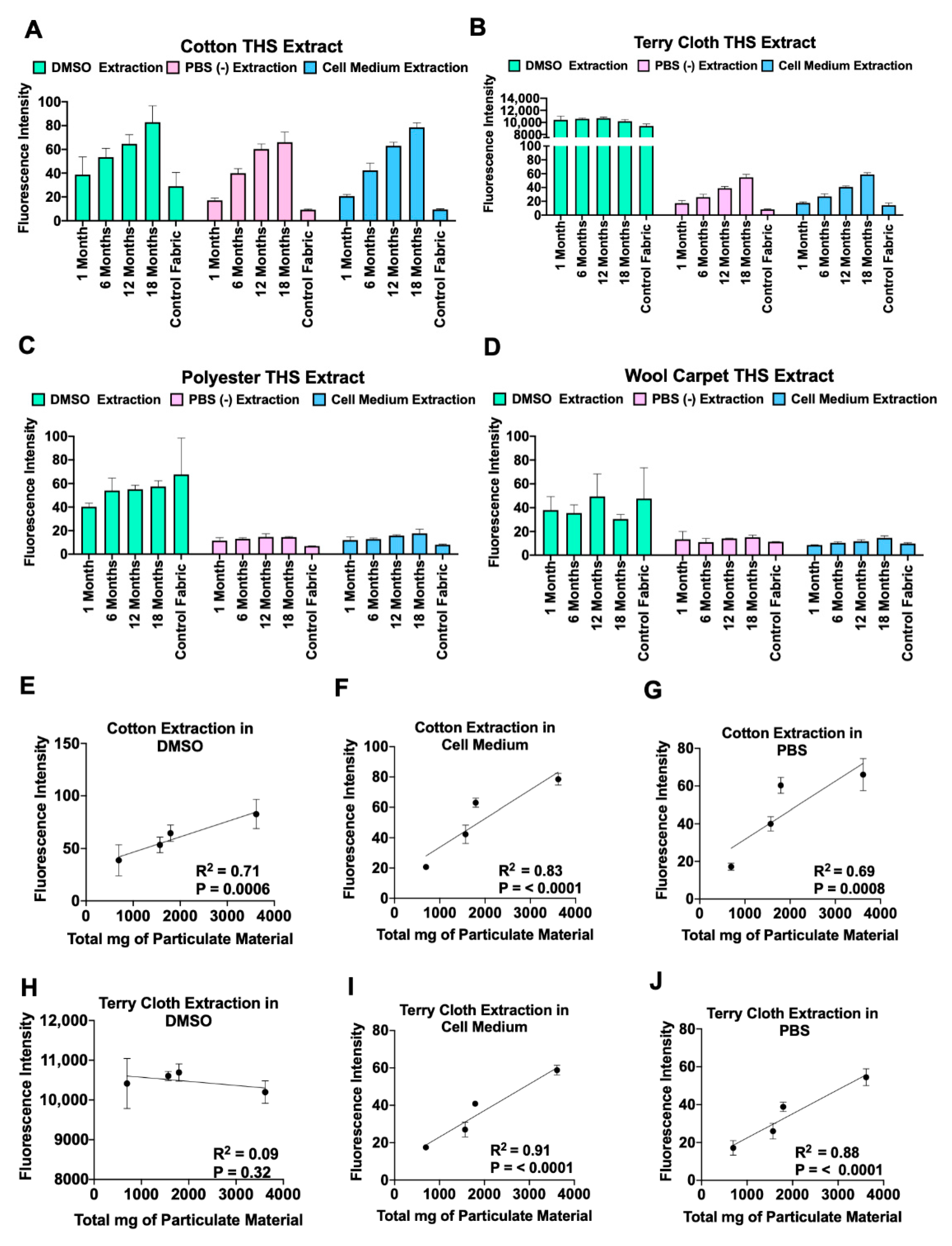

| Months | ||||
|---|---|---|---|---|
| Smoke exposure | 1 | 6 | 12 | 18 |
| Total mg of particulate material at each time interval | 696 | 873 | 226 | 1833 |
| Total mg of particulate material accumulated over time | 696 | 1569 | 1795 | 3917 |
| Total hours of smoke at each time interval | 45.5 | 74.1 | 30.3 | 140.3 |
| Total hours of smoke accumulated over time | 45.5 | 119.4 | 149.7 | 289.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pozuelos, G.L.; Jacob, P., III; Schick, S.F.; Omaiye, E.E.; Talbot, P. Adhesion and Removal of Thirdhand Smoke from Indoor Fabrics: A Method for Rapid Assessment and Identification of Chemical Repositories. Int. J. Environ. Res. Public Health 2021, 18, 3592. https://doi.org/10.3390/ijerph18073592
Pozuelos GL, Jacob P III, Schick SF, Omaiye EE, Talbot P. Adhesion and Removal of Thirdhand Smoke from Indoor Fabrics: A Method for Rapid Assessment and Identification of Chemical Repositories. International Journal of Environmental Research and Public Health. 2021; 18(7):3592. https://doi.org/10.3390/ijerph18073592
Chicago/Turabian StylePozuelos, Giovanna L., Peyton Jacob, III, Suzaynn F. Schick, Esther E. Omaiye, and Prue Talbot. 2021. "Adhesion and Removal of Thirdhand Smoke from Indoor Fabrics: A Method for Rapid Assessment and Identification of Chemical Repositories" International Journal of Environmental Research and Public Health 18, no. 7: 3592. https://doi.org/10.3390/ijerph18073592
APA StylePozuelos, G. L., Jacob, P., III, Schick, S. F., Omaiye, E. E., & Talbot, P. (2021). Adhesion and Removal of Thirdhand Smoke from Indoor Fabrics: A Method for Rapid Assessment and Identification of Chemical Repositories. International Journal of Environmental Research and Public Health, 18(7), 3592. https://doi.org/10.3390/ijerph18073592





