Is Short-Term Exposure to PM2.5 Relevant to Childhood Kawasaki Disease?
Abstract
1. Introduction
2. Materials and Methods
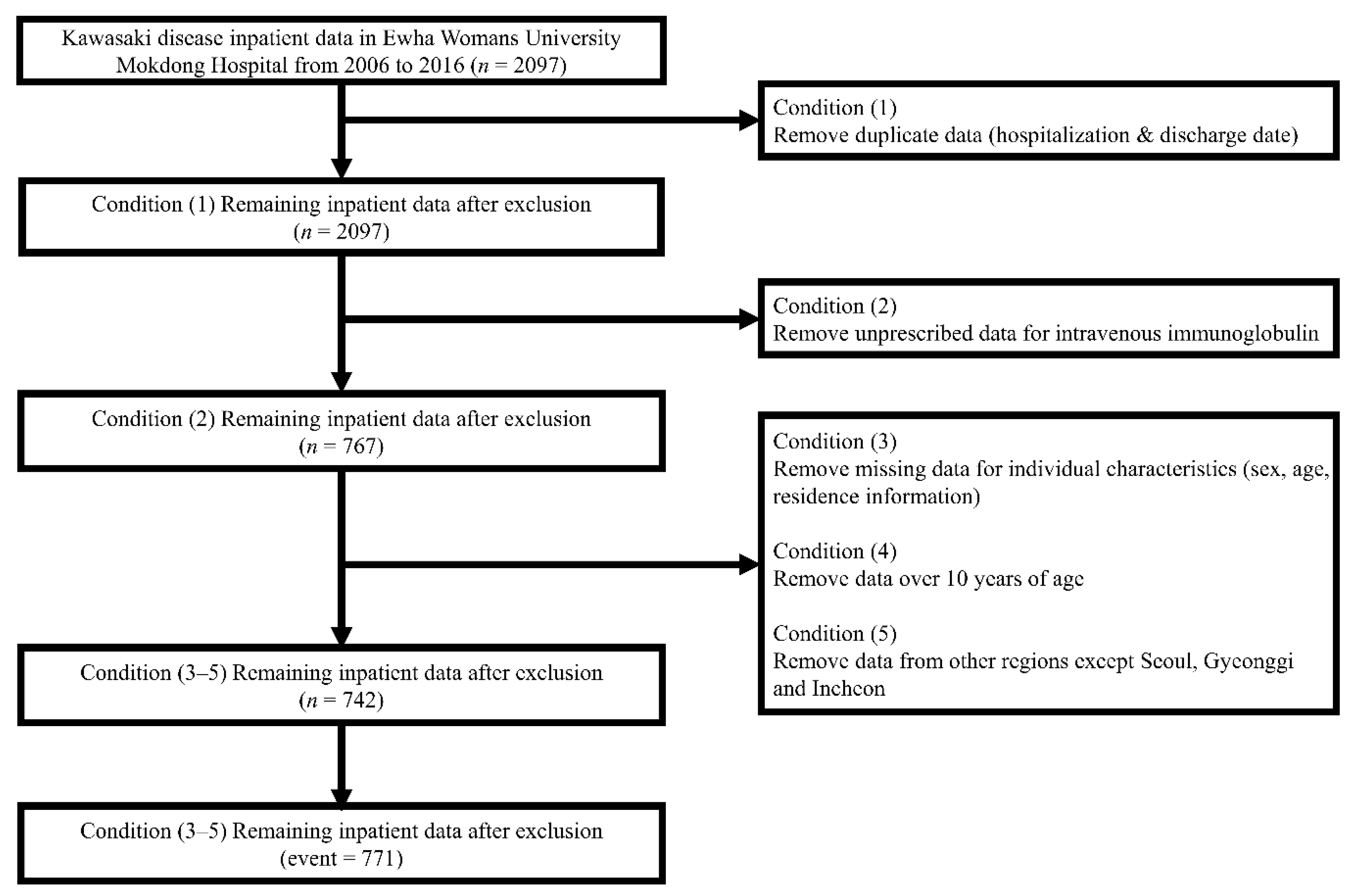
2.1. Kawasaki Disease Inpatient Definitions
2.2. Environmental Variable
2.3. Study Design
2.4. Statistical Analysis
3. Results
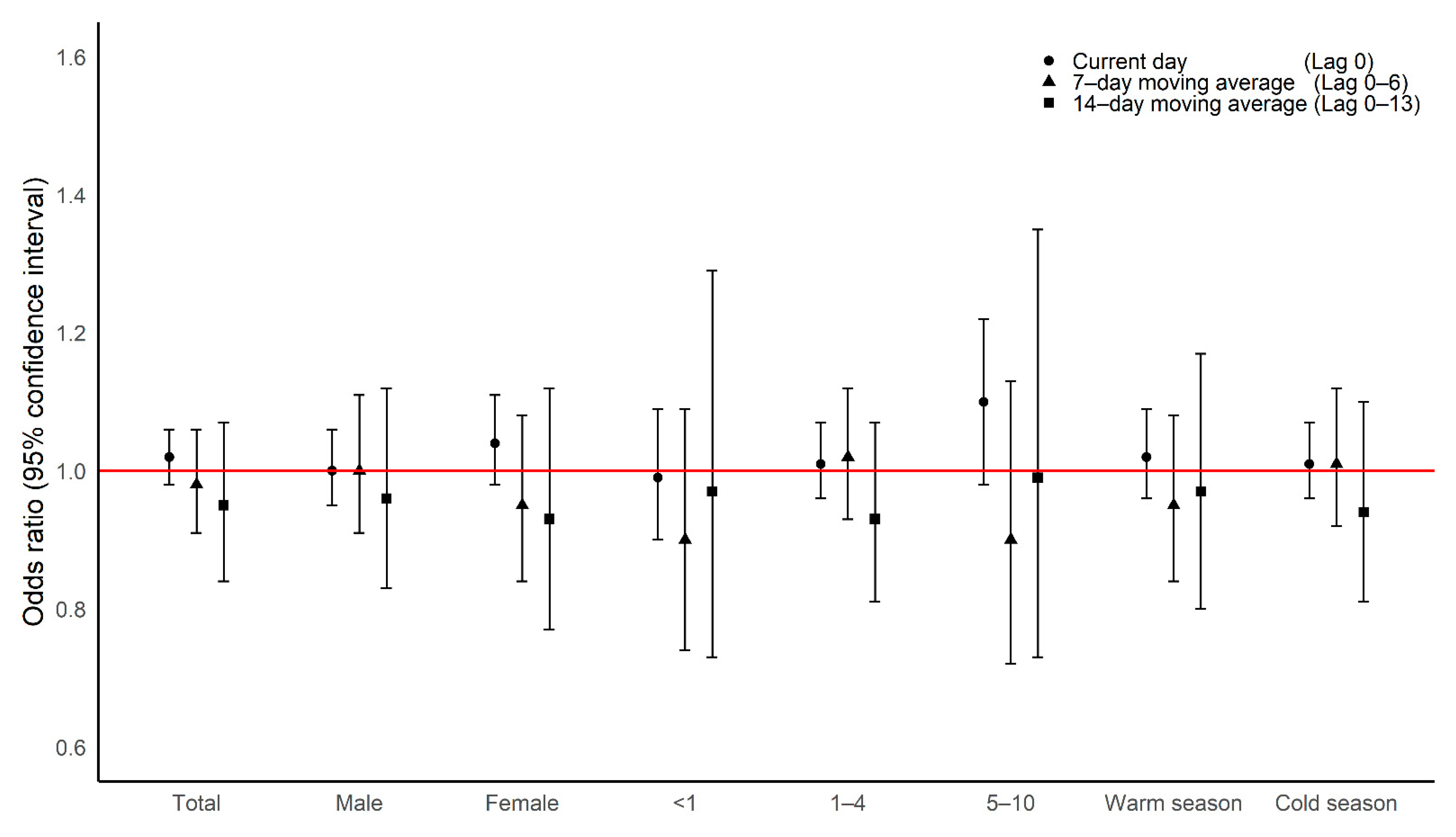
Main Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| KD | Kawasaki disease |
| PM2.5 | particulate matter with a diameter equal to or less than 2.5 μm |
| OR | odds ratio |
| CI | confidence interval |
| SD | standard deviation |
References
- Kawasaki, T.; Kosaki, F.; Okawa, S.; Shigematsu, I.; Yanagawa, H. A new infantile acute febrile mucocutaneous lymph node syndrome (MLNS) prevailing in Japan. Pediatrics 1974, 54, 271–276. [Google Scholar] [PubMed]
- Melish, M.E.; Hicks, R.M.; Larson, E.J. Mucocutaneous lymph node syndrome in the United States. Am. J. Dis. Child. 1976, 130, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Ichinose, E.; Yoshioka, F.; Takechi, T.; Matsunaga, S.; Suzuki, K.; Rikitake, N. Fate of coronary aneurysms in Kawasaki disease: Serial coronary angiography and long-term follow-up study. Am. J. Cardiol. 1982, 49, 1758–1766. [Google Scholar] [CrossRef]
- Rowley, A.H.; Shulman, S.T. Kawasaki syndrome. Pediatr. Clin. N. Am. 1999, 46, 313–329. [Google Scholar] [CrossRef]
- Fujiwara, H.; Hamashima, Y. Pathology of the heart in Kawasaki disease. Pediatrics 1978, 61, 100–107. [Google Scholar]
- Lin, M.T.; Wu, M.H. The global epidemiology of Kawasaki disease: Review and future perspectives. Glob. Cardiol. Sci. Pract. 2017, 2017, e201720. [Google Scholar] [CrossRef]
- Jung, C.R.; Chen, W.T.; Lin, Y.T.; Hwang, B.F. Ambient air pollutant exposures and hospitalization for Kawasaki disease in Taiwan: A case-crossover study (2000–2010). Environ. Health Perspect. 2017, 125, 670–676. [Google Scholar] [CrossRef]
- Zeft, A.S.; Burns, J.C.; Yeung, R.S.; McCrindle, B.W.; Newburger, J.W.; Dominguez, S.R.; Anderson, M.S.; Arrington, C.; Shulman, S.T.; Yoon, J.; et al. Kawasaki disease and exposure to fine particulate air pollution. J. Pediatr. 2016, 177, 179–183 e1. [Google Scholar] [CrossRef]
- Yorifuji, T.; Tsukahara, H.; Kashima, S.; Doi, H. Intrauterine and early postnatal exposure to particulate air pollution and Kawasaki disease: A nationwide longitudinal survey in Japan. J. Pediatr. 2018, 193, 147–154 e2. [Google Scholar] [CrossRef]
- Skamarock, W.C.; Klemp, J.B. A time-split nonhydrostatic atmospheric model for weather research and forecasting applications. J. Comput. Phys. 2008, 227, 3465–3485. [Google Scholar] [CrossRef]
- Bae, M.; Kim, B.-U.; Kim, H.C.; Kim, S. A multiscale tiered approach to quantify contributions: A case study of PM2.5 in South Korea during 2010–2017. Atmosphere 2020, 11, 141. [Google Scholar] [CrossRef]
- Kim, H.C.; Kim, E.; Bae, C.; Cho, J.H.; Kim, B.-U.; Kim, S. Regional contributions to particulate matter concentration in the Seoul metropolitan area, South Korea: Seasonal variation and sensitivity to meteorology and emissions inventory. Atmos. Chem. Phys. 2017, 17, 10315–10332. [Google Scholar] [CrossRef]
- Son, K.; You, S.; Kim, H.C.; Kim, B.-U.; Kim, S. Inter-comparisons of spatially interpolated short-term and long-term PM2.5 concentrations of local authorities in South Korea 2015~2017. J. Korean Soc. Atmos. Environ. 2020, 36, 185–197. [Google Scholar] [CrossRef]
- Oh, J.; Lee, S.; Kim, M.H.; Kwag, Y.; Kim, H.S.; Kim, S.; Ye, S.; Ha, E. The impact of PM2.5 on acute otitis media in children (aged 0–3): A time series study. Environ. Int 2020, 145, 106133. [Google Scholar] [CrossRef]
- Han, C.; Kim, S.; Lim, Y.H.; Bae, H.J.; Hong, Y.C. Spatial and temporal trends of number of deaths attributable to ambient PM2.5 in the Korea. J. Korean Med. Sci. 2018, 33, e193. [Google Scholar] [CrossRef]
- Kelly, F.J. Oxidative stress: Its role in air pollution and adverse health effects. Occup. Environ. Med. 2003, 60, 612–616. [Google Scholar] [CrossRef]
- Chen, R.; Zhao, Z.; Sun, Q.; Lin, Z.; Zhao, A.; Wang, C.; Xia, Y.; Xu, X.; Kan, H. Size-fractionated particulate air pollution and circulating biomarkers of inflammation, coagulation, and vasoconstriction in a panel of young adults. Epidemiology 2015, 26, 328–336. [Google Scholar] [CrossRef]
- Brook, R.D.; Rajagopalan, S.; Pope, C.A., 3rd; Brook, J.R.; Bhatnagar, A.; Diez-Roux, A.V.; Holguin, F.; Hong, Y.; Luepker, R.V.; Mittleman, M.A.; et al. Metabolism, particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation 2010, 121, 2331–2378. [Google Scholar] [CrossRef]
- Burns, J.C.; Mason, W.H.; Glode, M.P.; Shulman, S.T.; Melish, M.E.; Meissner, C.; Bastian, J.; Beiser, A.S.; Meyerson, H.M.; Newburger, J.W. Clinical and epidemiologic characteristics of patients referred for evaluation of possible Kawasaki disease. United States Multicenter Kawasaki Disease Study Group. J. Pediatr. 1991, 118, 680–686. [Google Scholar] [CrossRef]
- Burns, J.C.; Herzog, L.; Fabri, O.; Tremoulet, A.H.; Rodo, X.; Uehara, R.; Burgner, D.; Bainto, E.; Pierce, D.; Tyree, M.; et al. Seasonality of Kawasaki disease: A global perspective. PLoS ONE 2013, 8, e74529. [Google Scholar] [CrossRef]
- Du, Z.D.; Zhao, D.; Du, J.; Zhang, Y.L.; Lin, Y.; Liu, C.; Zhang, T.; Beijing Kawasaki Research Group. Epidemiologic study on Kawasaki disease in Beijing from 2000 through 2004. Pediatr. Infect. Dis. J. 2007, 26, 449–451. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.P.; Yan, W.L.; Huang, M.; Huang, M.R.; Chen, S.; Huang, G.Y.; Liu, F. Epidemiologic features of Kawasaki disease in Shanghai from 2013 through 2017. J. Epidemiol. 2020, 30, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Rodo, X.; Ballester, J.; Cayan, D.; Melish, M.E.; Nakamura, Y.; Uehara, R.; Burns, J.C. Association of Kawasaki disease with tropospheric wind patterns. Sci. Rep. 2011, 1, 152. [Google Scholar] [CrossRef] [PubMed]
- Rodo, X.; Curcoll, R.; Robinson, M.; Ballester, J.; Burns, J.C.; Cayan, D.R.; Lipkin, W.I.; Williams, B.L.; Couto-Rodriguez, M.; Nakamura, Y.; et al. Tropospheric winds from northeastern China carry the etiologic agent of Kawasaki disease from its source to Japan. Proc. Natl. Acad. Sci. USA 2014, 111, 7952–7957. [Google Scholar] [CrossRef] [PubMed]
- Bauer, R.N.; Diaz-Sanchez, D.; Jaspers, I. Effects of air pollutants on innate immunity: The role of Toll-like receptors and nucleotide-binding oligomerization domain-like receptors. J. Allergy Clin. Immunol. 2012, 129, 14–24. [Google Scholar] [CrossRef]
- Lin, Z.; Meng, X.; Chen, R.; Huang, G.; Ma, X.; Chen, J.; Huang, M.; Huang, M.; Gui, Y.; Chu, C.; et al. Ambient air pollution, temperature and kawasaki disease in Shanghai, China. Chemosphere 2017, 186, 817–822. [Google Scholar] [CrossRef]
- Corinaldesi, E.; Pavan, V.; Andreozzi, L.; Fabi, M.; Selvini, A.; Frabboni, I.; Lanzoni, P.; Paccagnella, T.; Lanari, M. Environmental factors and Kawasaki disease onset in Emilia-Romagna, Italy. Int. J. Environ. Res. Public Health 2020, 17, 1529. [Google Scholar] [CrossRef]
- Verdoni, L.; Mazza, A.; Gervasoni, A.; Martelli, L.; Ruggeri, M.; Ciuffreda, M.; Bonanomi, E.; D’Antiga, L. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: An observational cohort study. Lancet 2020, 395, 1771–1778. [Google Scholar] [CrossRef]
- Toubiana, J.; Poirault, C.; Corsia, A.; Bajolle, F.; Fourgeaud, J.; Angoulvant, F.; Debray, A.; Basmaci, R.; Salvador, E.; Biscardi, S.; et al. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: Prospective observational study. BMJ 2020, 369, m2094. [Google Scholar] [CrossRef]
- Belhadjer, Z.; Meot, M.; Bajolle, F.; Khraiche, D.; Legendre, A.; Abakka, S.; Auriau, J.; Grimaud, M.; Oualha, M.; Beghetti, M.; et al. Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS-CoV-2 pandemic. Circulation 2020, 142, 429–436. [Google Scholar] [CrossRef]
- Rodriguez-Gonzalez, M.; Castellano-Martinez, A.; Cascales-Poyatos, H.M.; Perez-Reviriego, A.A. Cardiovascular impact of COVID-19 with a focus on children: A systematic review. World J. Clin. Cases 2020, 8, 5250–5283. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.; Seo, G.H.; Kim, K.Y.; Kim, D.S. Epidemiologic study on Kawasaki disease in Korea, 2007–2014: Based on health insurance review & Assessment service claims. J. Korean Med. Sci 2016, 31, 1445–1449. [Google Scholar] [PubMed]
- Uehara, R.; Belay, E.D. Epidemiology of Kawasaki disease in Asia, Europe, and the United States. J. Epidemiol. 2012, 22, 79–85. [Google Scholar] [CrossRef] [PubMed]
| Category | Non-Event | Event |
|---|---|---|
| Sex | ||
| Boys | 1549 | 458 |
| Girls | 1080 | 313 |
| Season | ||
| Warm season a | 1365 | 402 |
| Cold season b | 1264 | 369 |
| Age group | ||
| <1 | 558 | 164 |
| 1–4 | 1729 | 505 |
| 5–10 | 342 | 102 |
| Regions | ||
| Seoul | 2168 | 637 |
| Gyeonggi | 389 | 113 |
| Incheon | 72 | 21 |
| Exposure Variables | Case Periods (n = 771) | Control Periods (n = 2629) | Mean Difference | 95% Confidence Interval | ||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| PM2.5 (μg/m3) | 34.13 | 22.37 | 33.33 | 20.46 | 0.80 | −0.96, 2.57 |
| Mean temperature (°C) | 12.82 | 10.93 | 12.70 | 11.19 | 0.12 | −0.76, 1.00 |
| Mean humidity (%) | 62.57 | 14.83 | 62.34 | 14.86 | 0.23 | −0.97, 1.41 |
| Sex | Age Group | Season | Regions | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lag | Total | Boys | Girls | <1 | 1–4 | 5–10 | Warm Season a | Cold Season b | Seoul | Gyeonggi | Incheon |
| 0–1 | 1.01 (0.96, 1.06) | 1.01 (0.95, 1.07) | 1.02 (0.94, 1.10) | 0.93 (0.82, 1.06) | 1.02 (0.96, 1.08) | 1.07 (0.95, 1.22) | 1.00 (0.92, 1.09) | 1.02 (0.95, 1.09) | 1.00 (0.95, 1.06) | 1.08 (0.94, 1.25) | 0.97 (0.76, 1.24) |
| 0–2 | 1.00 (0.95, 1.06) | 1.01 (0.94, 1.08) | 0.98 (0.89, 1.08) | 0.92 (0.79, 1.06) | 1.01 (0.95, 1.08) | 1.03 (0.89, 1.19) | 0.96 (0.88, 1.05) | 1.03 (0.96, 1.11) | 0.99 (0.93, 1.05) | 1.11 (0.95, 1.29) | 0.93 (0.68, 1.28) |
| 0–3 | 0.99 (0.93, 1.05) | 1.00 (0.93, 1.08) | 0.96 (0.86, 1.06) | 0.90 (0.76, 1.06) | 1.00 (0.93, 1.08) | 0.99 (0.83, 1.17) | 0.94 (0.85, 1.04) | 1.02 (0.95, 1.11) | 0.97 (0.90, 1.03) | 1.13 (0.95, 1.34) | 0.94 (0.66, 1.34) |
| 0–4 | 0.98 (0.92, 1.05) | 1.00 (0.92, 1.09) | 0.95 (0.85, 1.06) | 0.91 (0.77, 1.07) | 1.01 (0.93, 1.09) | 0.95 (0.78, 1.15) | 0.94 (0.84, 1.05) | 1.02 (0.94, 1.11) | 0.96 (0.90, 1.04) | 1.14 (0.94, 1.37) | 0.96 (0.64, 1.43) |
| 0–5 | 0.99 (0.92, 1.06) | 1.01 (0.92, 1.10) | 0.95 (0.85, 1.07) | 0.90 (0.75, 1.07) | 1.02 (0.93, 1.10) | 0.94 (0.77, 1.15) | 0.95 (0.84, 1.07) | 1.02 (0.93, 1.11) | 0.96 (0.89, 1.04) | 1.15 (0.94, 1.40) | 1.09 (0.72, 1.66) |
| 0–6 | 0.98 (0.91, 1.06) | 1.00 (0.91, 1.11) | 0.95 (0.84, 1.08) | 0.90 (0.74, 1.09) | 1.02 (0.93, 1.12) | 0.90 (0.72, 1.13) | 0.95 (0.84, 1.08) | 1.01 (0.92, 1.12) | 0.96 (0.88, 1.04) | 1.15 (0.93, 1.43) | 1.08 (0.66, 1.77) |
| 0–7 | 0.99 (0.91, 1.07) | 1.01 (0.90, 1.12) | 0.96 (0.84, 1.09) | 0.89 (0.73, 1.10) | 1.03 (0.93, 1.13) | 0.87 (0.69, 1.11) | 0.95 (0.83, 1.09) | 1.02 (0.91, 1.13) | 0.96 (0.88, 1.05) | 1.17 (0.93, 1.47) | 0.97 (0.58, 1.61) |
| 0–8 | 0.97 (0.89, 1.06) | 0.99 (0.89, 1.11) | 0.95 (0.82, 1.09) | 0.89 (0.71, 1.11) | 1.01 (0.91, 1.12) | 0.89 (0.70, 1.14) | 0.94 (0.81, 1.08) | 1.00 (0.90, 1.12) | 0.95 (0.86, 1.05) | 1.15 (0.90, 1.47) | 0.95 (0.55, 1.63) |
| 0–9 | 0.98 (0.89, 1.07) | 0.99 (0.88, 1.11) | 0.96 (0.82, 1.11) | 0.89 (0.70, 1.13) | 1.00 (0.89, 1.12) | 0.94 (0.73, 1.20) | 0.94 (0.80, 1.10) | 1.01 (0.89, 1.13) | 0.95 (0.86, 1.05) | 1.16 (0.90, 1.51) | 0.93 (0.51, 1.68) |
| 0–10 | 0.97 (0.88, 1.08) | 0.99 (0.87, 1.12) | 0.95 (0.81, 1.11) | 0.91 (0.71, 1.16) | 0.99 (0.88, 1.12) | 0.95 (0.73, 1.23) | 0.95 (0.81, 1.12) | 0.99 (0.87, 1.13) | 0.95 (0.85, 1.06) | 1.19 (0.90, 1.56) | 0.86 (0.45, 1.64) |
| 0–11 | 0.96 (0.87, 1.07) | 0.98 (0.85, 1.12) | 0.94 (0.80, 1.11) | 0.94 (0.73, 1.22) | 0.97 (0.85, 1.10) | 0.95 (0.72, 1.25) | 0.96 (0.81, 1.14) | 0.97 (0.85, 1.11) | 0.94 (0.84, 1.05) | 1.16 (0.87, 1.55) | 0.89 (0.45, 1.77) |
| 0–12 | 0.96 (0.86, 1.07) | 0.97 (0.84, 1.12) | 0.94 (0.79, 1.12) | 0.98 (0.75, 1.29) | 0.95 (0.83, 1.09) | 0.96 (0.72, 1.29) | 0.98 (0.81, 1.17) | 0.95 (0.82, 1.10) | 0.93 (0.83, 1.05) | 1.15 (0.84, 1.58) | 1.01 (0.49, 2.07) |
| 0–13 | 0.95 (0.84, 1.07) | 0.96 (0.83, 1.12) | 0.93 (0.77, 1.12) | 0.97 (0.73, 1.29) | 0.93 (0.81, 1.07) | 0.99 (0.73, 1.35) | 0.97 (0.80, 1.17) | 0.94 (0.81, 1.10) | 0.93 (0.82, 1.05) | 1.14 (0.81, 1.60) | 1.02 (0.45, 2.29) |
| 0–14 | 0.93 (0.82, 1.05) | 0.94 (0.80, 1.11) | 0.91 (0.75, 1.10) | 0.90 (0.67, 1.22) | 0.92 (0.79, 1.07) | 0.99 (0.72, 1.36) | 0.94 (0.77, 1.15) | 0.93 (0.79, 1.09) | 0.90 (0.79, 1.03) | 1.13 (0.80, 1.61) | 0.99 (0.42, 2.35) |
| Single Pollutant | Two-Pollutant Model | ||||
|---|---|---|---|---|---|
| Lag | PM2.5 | Adjusted SO2 | Adjusted NO2 | Adjusted CO | Adjusted O3 |
| 0–1 | 1.01 (0.96, 1.06) | 1.00 (0.94, 1.06) | 1.03 (0.98, 1.10) | 1.02 (0.95, 1.09) | 1.01 (0.96, 1.07) |
| 0–2 | 1.00 (0.95, 1.06) | 0.99 (0.93, 1.06) | 1.02 (0.96, 1.08) | 1.02 (0.95, 1.09) | 1.00 (0.94, 1.06) |
| 0–3 | 0.99 (0.93, 1.05) | 0.97 (0.93, 1.04) | 1.00 (0.94, 1.07) | 1.00 (0.93, 1.07) | 0.98 (0.92, 1.05) |
| 0–4 | 0.98 (0.92, 1.05) | 0.97 (0.89, 1.04) | 1.00 (0.93, 1.07) | 0.98 (0.91, 1.06) | 0.98 (0.91, 1.04) |
| 0–5 | 0.99 (0.92, 1.06) | 0.96 (0.89, 1.04) | 0.99 (0.92, 1.07) | 0.98 (0.91, 1.06) | 0.98 (0.91, 1.05) |
| 0–6 | 0.98 (0.91, 1.06) | 0.97 (0.89, 1.05) | 0.98 (0.91, 1.07) | 0.98 (0.90, 1.07) | 0.97 (0.90, 1.05) |
| 0–7 | 0.99 (0.91, 1.07) | 0.95 (0.87, 1.04) | 0.98 (0.89, 1.07) | 0.98 (0.90, 1.07) | 0.98 (0.90, 1.06) |
| 0–8 | 0.97 (0.89, 1.06) | 0.94 (0.85, 1.03) | 0.98 (0.89, 1.08) | 0.97 (0.89, 1.07) | 0.96 (0.88, 1.05) |
| 0–9 | 0.98 (0.89, 1.07) | 0.94 (0.85, 1.04) | 0.97 (0.88, 1.08) | 0.97 (0.89, 1.07) | 0.96 (0.87, 1.06) |
| 0–10 | 0.97 (0.88, 1.08) | 0.95 (0.86, 1.05) | 0.97 (0.87, 1.08) | 0.96 (0.87, 1.07) | 0.96 (0.87, 1.07) |
| 0–11 | 0.96 (0.87, 1.07) | 0.95 (0.85, 1.06) | 0.97 (0.87, 1.08) | 0.95 (0.86, 1.06) | 0.95 (0.86, 1.06) |
| 0–12 | 0.96 (0.86, 1.07) | 0.93 (0.83, 1.05) | 0.95 (0.85, 1.07) | 0.94 (0.84, 1.06) | 0.94 (0.84, 1.05) |
| 0–13 | 0.95 (0.84, 1.07) | 0.93 (0.82, 1.05) | 0.94 (0.84, 1.06) | 0.94 (0.83, 1.06) | 0.94 (0.83, 1.05) |
| 0–14 | 0.93 (0.82, 1.05) | 0.91 (0.80, 1.04) | 0.93 (0.82, 1.05) | 0.93 (0.82, 1.05) | 0.92 (0.81, 1.04) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, J.; Lee, J.H.; Kim, E.; Kim, S.; Kim, H.S.; Ha, E. Is Short-Term Exposure to PM2.5 Relevant to Childhood Kawasaki Disease? Int. J. Environ. Res. Public Health 2021, 18, 924. https://doi.org/10.3390/ijerph18030924
Oh J, Lee JH, Kim E, Kim S, Kim HS, Ha E. Is Short-Term Exposure to PM2.5 Relevant to Childhood Kawasaki Disease? International Journal of Environmental Research and Public Health. 2021; 18(3):924. https://doi.org/10.3390/ijerph18030924
Chicago/Turabian StyleOh, Jongmin, Ji Hyen Lee, Eunji Kim, Soontae Kim, Hae Soon Kim, and Eunhee Ha. 2021. "Is Short-Term Exposure to PM2.5 Relevant to Childhood Kawasaki Disease?" International Journal of Environmental Research and Public Health 18, no. 3: 924. https://doi.org/10.3390/ijerph18030924
APA StyleOh, J., Lee, J. H., Kim, E., Kim, S., Kim, H. S., & Ha, E. (2021). Is Short-Term Exposure to PM2.5 Relevant to Childhood Kawasaki Disease? International Journal of Environmental Research and Public Health, 18(3), 924. https://doi.org/10.3390/ijerph18030924






