Adenomyosis and Infertility—Review of Medical and Surgical Approaches
Abstract
1. Introduction
2. Definition and Symptoms
3. Pathogenesis
4. Diagnostics
4.1. Ultrasound
4.2. Hysteroscopy
4.3. MRI (Magnetic Resonance Imaging)
4.4. Histological Evaluation
5. Biological Influence of Adenomyosis on Fertility—Possible Mechanisms
6. Impact of Adenomyosis Treatment on Fertility and Implication for Clinical Practice
6.1. Surgical Methods
6.2. Pharmacological Methods
7. IVF Outcome in Adenomyosis
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Graziano, A.; Monte, G.L.; Piva, I.; Caserta, D.; Karner, M.; Engl, B.; Marci, R. Diagnostic findings in adenomyosis: A pictorial review on the major concerns. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 1146–1154. [Google Scholar] [PubMed]
- Harada, T.; Khine, Y.M.; Kaponis, A.; Nikellis, T.; Decavalas, G.; Taniguchi, F. The Impact of Adenomyosis on Women’s Fertility. Obstet. Gynecol. Surv. 2016, 71, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Chung, J.P.; Wang, S.; Li, T.C.; Duan, H. The Investigation and Management of Adenomyosis in Women Who Wish to Improve or Preserve Fertility. BioMed Res. Int. 2018, 2018, 6832685. [Google Scholar] [CrossRef] [PubMed]
- Ecker, A.M.; Chamsy, D.; Austin, R.M.; Guido, R.S.; Lee, T.T.; Mansuria, S.M.; Rindos, N.B.; Donnellan, N.M. Use of Uterine Characteristics to Improve Fertility-Sparing Diagnosis of Adenomyosis. J. Gynecol. Surg. 2018, 34, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Senturk, L.M.; Imamoglu, M. Adenomyosis: What is new? Womens Health 2015, 11, 717–724. [Google Scholar] [CrossRef]
- Eisenberg, V.H.; Arbib, N.; Schiff, E.; Goldenberg, M.; Seidman, D.S.; Soriano, D. Sonographic Signs of Adenomyosis Are Prevalent in Women Undergoing Surgery for Endometriosis and May Suggest a Higher Risk of Infertility. BioMed Res. Int. 2017, 2017, 8967803. [Google Scholar] [CrossRef]
- Van den Bosch, T.; Van Schoubroeck, D. Ultrasound diagnosis of endometriosis and adenomyosis: State of the art. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 51, 16–24. [Google Scholar] [CrossRef]
- Genc, M.; Genc, B.; Cengiz, H. Adenomyosis and accompanying gynecological pathologies. Arch. Gynecol. Obstet. 2015, 291, 877–881. [Google Scholar] [CrossRef]
- Bergeron, C.; Amant, F.; Ferenczy, A. Pathology and physiopathology of adenomyosis. Best Pract. Res. Clin. Obstet. Gynaecol. 2006, 20, 511–521. [Google Scholar] [CrossRef]
- Leyendecker, G.; Wildt, L.; Mall, G. The pathophysiology of endometriosis and adenomyosis: Tissue injury and repair. Arch. Gynecol. Obstet. 2009, 280, 529–538. [Google Scholar] [CrossRef]
- Garavaglia, E.; Audrey, S.; Annalisa, I.; Stefano, F.; Iacopo, T.; Laura, C.; Massimo, C. Adenomyosis and its impact on women fertility. Iran. J. Reprod. Med. 2015, 13, 327–336. [Google Scholar] [PubMed]
- Yen, C.F.; Huang, S.J.; Lee, C.L.; Wang, H.S.; Liao, S.K. Molecular Characteristics of the Endometrium in Uterine Adenomyosis and Its Biochemical Microenvironment. Reprod. Sci. 2017, 24, 1346–1361. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Zhang, T.; Liu, F.; Ni, X.; Huo, R.; Shi, Z. The differential expression of mRNAs and long noncoding RNAs between ectopic and eutopic endometria provides new insights into adenomyosis. Mol. Biosyst. 2016, 12, 362–370. [Google Scholar] [CrossRef] [PubMed]
- De Bruijn, A.M.; van den Bosch, T.; de Leeuw, R.A.; Dueholm, M.; Exacoustos, C.; Valentin, L.; Bourne, T.; Timmerman, D.; Huirne, J.A. A sonographic classification and reporting system for diagnosing adenomyosis. Ultrasound Obstet. Gynecol. 2019, 53, 151. [Google Scholar]
- Di Spiezio Sardo, A.; Calagna, G.; Santangelo, F.; Zizolfi, B.; Tanos, V.; Perino, A.; De Wilde, R.L. The Role of Hysteroscopy in the Diagnosis and Treatment of Adenomyosis. BioMed Res. Int. 2017, 2017, 2518396. [Google Scholar] [CrossRef]
- Khandeparkar, M.S.; Jalkote, S.; Panpalia, M.; Nellore, S.; Mehta, T.; Ganesan, K.; Parikh, F.R. High resolution magnetic resonance imaging in the detection of subtle nuances of uterine adenomyosis in infertility. Glob. Reprod. Health 2018, 3, e14. [Google Scholar] [CrossRef]
- Kunz, G.; Beil, D.; Huppert, P.; Noe, M.; Kissler, S.; Leyendecker, G. Adenomyosis in endometriosis--prevalence and impact on fertility. Evidence from magnetic resonance imaging. Hum. Reprod. 2005, 20, 2309–2316. [Google Scholar] [CrossRef]
- Vigano, P.; Corti, L.; Berlanda, N. Beyond infertility: Obstetrical and postpartum complications associated with endometriosis and adenomyosis. Fertil. Steril. 2015, 104, 802–812. [Google Scholar] [CrossRef]
- Buggio, L.; Monti, E.; Gattei, U.; Dridi, D.; Vercellini, P. Adenomyosis: Fertility and obstetric outcome. A comprehensive literature review. Minerva Ginecol. 2018, 70, 295–302. [Google Scholar]
- Vlahos, N.F.; Theodoridis, T.D.; Partsinevelos, G.A. Myomas and Adenomyosis: Impact on Reproductive Outcome. BioMed Res. Int. 2017, 2017, 5926470. [Google Scholar] [CrossRef]
- Benagiano, G.; Brosens, I.; Habiba, M. Adenomyosis: A life-cycle approach. Reprod. Biomed. Online 2015, 30, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.H.; Chang, M.Y.; Shiau, C.S.; Hou, H.C.; Soong, Y.K. Effect of a sonographically diffusely enlarged uterus without distinct uterine masses on the outcome of in vitro fertilization-embryo transfer. J. Assist. Reprod. Genet. 1999, 16, 369–372. [Google Scholar] [CrossRef] [PubMed]
- Maubon, A.; Faury, A.; Kapella, M.; Pouquet, M.; Piver, P. Uterine junctional zone at magnetic resonance imaging: A predictor of in vitro fertilization implantation failure. J. Obstet. Gynaecol. Res. 2010, 36, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Benagiano, G.; Brosens, I. The endometrium in adenomyosis. Womens Health 2012, 8, 301–312. [Google Scholar] [CrossRef]
- Vannuccini, S.; Tosti, C.; Carmona, F.; Huang, S.J.; Chapron, C.; Guo, S.W.; Petraglia, F. Pathogenesis of adenomyosis: An update on molecular mechanisms. Reprod. Biomed. Online 2017, 35, 592–601. [Google Scholar] [CrossRef]
- Fischer, C.P.; Kayisili, U.; Taylor, H.S. HOXA10 expression is decreased in endometrium of women with adenomyosis. Fertil. Steril. 2011, 95, 1133–1136. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, T.; Xia, E.; Yang, X.; Sun, X.; Zhou, Y. Expression of integrin β3 and osteopontin in the eutopic endometrium of adenomyosis during the implantation window. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 170, 419–422. [Google Scholar] [CrossRef]
- Jiang, Y.; Jiang, R.; Cheng, X.; Zhang, Q.; Hu, Y.; Zhang, H.; Cao, Y.; Zhang, M.; Wang, J.; Ding, L.; et al. Decreased expression of NR4A nuclear receptors in adenomyosis impairs endometrial decidualization. Mol. Hum. Reprod. 2016, 22, 655–668. [Google Scholar] [CrossRef]
- Wu, D.; Li, G.; Lin, R.; Li, C.; Zhao, S. Progesterone Promotes HOXa-10 Expression in Mouse during Embryo Implantation Period. J. Anim. Vet. Adv. 2012, 11, 3076–3080. [Google Scholar]
- Yao, M.W.; Lim, H.; Schust, D.J.; Choe, S.E.; Farago, A.; Ding, Y.; Michaud, S.; Church, G.M.; Maas, R.L. Gene expression profiling reveals progesterone-mediated cell cycle and immunoregulatory roles of Hoxa-10 in the preimplantation uterus. Mol. Endocrinol. 2003, 17, 610–627. [Google Scholar] [CrossRef]
- Surrey, E.S.; Minjarez, D.A.; Schoolcraft, W.B. The incidence of aberrant endometrial alphavbeta(3) vitronectin expression in a high risk infertility population: Could prolonged GnRH agonist therapy play a role? J. Assist. Reprod. Genet. 2007, 24, 553–556. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Garcıa, P.; Nieto, A.; Sanchez, M.A.; Pizarro, M.; Flores, J.M. Expression of alphav, alpha4, alpha5 and beta3 integrin subunits, fibronectin and vitronectin in goat peri-implantation. Anim. Reprod. Sci. 2004, 80, 91–100. [Google Scholar] [CrossRef]
- Fayazi, M.; Boroujeni, M.B.; Salehnia, M.; Khansarinejad, B. Ovarian stimulation by exogenous gonadotropin decreases the implantation rate and expression of mouse blastocysts integrins. Iran. Biomed. J. 2014, 18, 8–15. [Google Scholar] [PubMed]
- Carrarelli, P.; Yen, C.F.; Funghi, L.; Arcuri, F.; Tosti, C.; Bifulco, G.; Luddi, A.; Lee, C.L.; Petraglia, F. Expression of Inflammatory and Neurogenic Mediators in Adenomyosis. Reprod. Sci. 2017, 24, 369–375. [Google Scholar] [CrossRef]
- Benagiano, G.; Brosens, I.; Habiba, M. Structural and molecular features of the endomyometrium in endometriosis and adenomyosis. Hum. Reprod. Update 2014, 20, 386–402. [Google Scholar] [CrossRef]
- Ishikawa, M.; Nakata, T.; Yaginuma, Y.; Nishiwaki, K.; Goishi, K.; Saitoh, S. Expression of superoxide dismutase (SOD) in adenomyosis. Am. J. Obstet. Gynecol. 1993, 169, 730–734. [Google Scholar] [CrossRef]
- Orazov, M.R.; Radzinsky, V.E.; Nosenko, E.N.; Khamoshina, M.B.; Dukhin, A.O.; Lebedeva, M.G. Immune-inflammatory predictors of the pelvic pain syndrome associated with adenomyosis. Gynecol Endocrinol. 2017, 33 (Suppl. S1), 44–46. [Google Scholar] [CrossRef]
- Ota, H.; Igarashi, S.; Sato, N.; Tanaka, H.; Tanaka, T. Involvement of catalase in the endometrium of patients with endometriosis and adenomyosis. Fertil. Steril. 2002, 78, 804–809. [Google Scholar] [CrossRef]
- Busca, A.; Parra-Herran, C. The role of pathologic evaluation of endometrial ablation resections in predicting ablation failure and adenomyosis in hysterectomy. Pathol. Res. Pract. 2016, 212, 778–782. [Google Scholar] [CrossRef]
- Wood, C. Adenomyosis: Difficult to diagnose, and difficult to treat. Diagn. Ther. Endosc. 2001, 7, 89–95. [Google Scholar] [CrossRef]
- Kohn, J.R.; Shamshirsaz, A.A.; Popek, E.; Guan, X.; Belfort, M.A.; Fox, K.A. Pregnancy after endometrial ablation: A systematic review. BJOG 2018, 125, 43–53. [Google Scholar] [CrossRef] [PubMed]
- De Bruijn, A.M.; Smink, M.; Lohle, P.N.; Huirne, J.A.; Twisk, J.W.; Wong, C.; Schoonmade, L.; Hehenkamp, W.J. Uterine Artery Embolization for the Treatment of Adenomyosis: A Systematic Review and Meta-Analysis. J. Vasc. Interv. Radiol. 2017, 28, 1629–1642. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Rao, F.; Setzen, R. High intensity focused ultrasound for the treatment of adenomyosis: Selection criteria, efficacy, safety and fertility. Acta Obstet. Gynecol. Scand. 2017, 96, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.Y.; Xu, X.J.; He, J. [Pregnancy outcomes and symptom improvement of patients with adenomyosis treated with high intensity focused ultrasound ablation]. Zhonghua Fu Chan Ke Za Zhi 2016, 51, 845–849. (In Chinese) [Google Scholar] [PubMed]
- Otsubo, Y.; Nishida, M.; Arai, Y.; Ichikawa, R.; Taneichi, A.; Sakanaka, M. Association of uterine wall thickness with pregnancy outcome following uterine-sparing surgery for diffuse uterine adenomyosis. Aust. N. Z. J. Obstet. Gynaecol. 2016, 56, 88–91. [Google Scholar] [CrossRef]
- Younes, G.; Tulandi, T. Conservative Surgery for Adenomyosis and Results: A Systematic Review. J. Minim. Invasive Gynecol. 2018, 25, 265–276. [Google Scholar] [CrossRef]
- Allen, C.; Hopewell, S.; Prentice, A.; Gregory, D. Nonsteroidal anti-inflammatory drugs for pain in women with endometriosis. Cochrane Database of Syst. Rev. 2017, 1, CD004753. [Google Scholar] [CrossRef]
- Nyachieo, A.; Siristatidis, C.S.; Vaidakis, D. Nonsteroidal anti-inflammatory drugs for assisted reproductive technology. Cochrane Database Syst. Rev. 2019, 10, CD007618. [Google Scholar] [CrossRef]
- Vercellini, P.; Viganò, P.; Somigliana, E.; Fedele, L. Endometriosis: Pathogenesis and treatment. Nat. Rev. Endocrinol. 2014, 10, 261–275. [Google Scholar] [CrossRef]
- Huang, B.S.; Seow, K.M.; Tsui, K.H.; Huang, C.Y.; Lu, Y.F.; Wang, P.H. Fertility outcome of infertile women with adenomyosis treated with the combination of a conservative microsurgical technique and GnRH agonist: Long-term follow-up in a series of nine patients. Taiwan. J. Obstet. Gynecol. 2012, 51, 212–216. [Google Scholar] [CrossRef]
- Vannuccini, S.; Luisi, S.; Tosti, C.; Sorbi, F.; Petraglia, F. Role of medical therapy in the management of uterine adenomyosis. Fertil. Steril. 2018, 109, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, T.; Akira, S.; Fukami, T.; Yoneyama, K.; Takeshita, T. Efficacy of Hormonal Therapies for Decreasing Uterine Volume in Patients with Adenomyosis. Gynecol. Minim. Invasive Ther. 2018, 7, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Osuga, Y.; Fujimoto-Okabe, H.; Hagino, A. Evaluation of the efficacy and safety of dienogest in the treatment of painful symptoms in patients with adenomyosis: A randomized, double-blind, multicenter, placebo-controlled study. Fertil. Steril. 2017, 108, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Dasrilsyah, R.A.; Shan, L.P.; Kwang, N.B.; Shafiee, M.N.; Omar, M.H. Spontaneous conception following GnRHa and progestogen therapy in adenomyosis. Horm. Mol. Biol. Clin. Investig. 2016, 27, 77–79. [Google Scholar] [CrossRef] [PubMed]
- Mijatovic, V.; Florijn, E.; Halim, N.; Schats, R.; Hompes, P. Adenomyosis has no adverse effects on IVF/ICSI outcomes in women with endometriosis treated with long-term pituitary down-regulation before IVF/ICSI. Eur. J. Obstet. Gynecol. Reprod. Biol. 2010, 151, 62–65. [Google Scholar] [CrossRef]
- Thalluri, V.; Tremellen, K.P. Ultrasound diagnosed adenomyosis has a negative impact on successful implantation following GnRH antagonist IVF treatment. Hum. Reprod. 2012, 27, 3487–3492. [Google Scholar] [CrossRef] [PubMed]
- Costello, M.F.; Lindsay, K.; McNally, G. The effect of adenomyosis on in vitro fertilisation and intra-cytoplasmic sperm injection treatment outcome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 158, 229–234. [Google Scholar] [CrossRef]
- Vercellini, P.; Consonni, D.; Dridi, D.; Bracco, B.; Frattaruolo, M.P.; Somigliana, E. Uterine adenomyosis and in vitro fertilization outcome: A systematic review and meta-analysis. Hum. Reprod. 2014, 29, 964–977. [Google Scholar] [CrossRef]
- Kwack, J.Y.; Kwon, Y.S. Conservative surgery of diffuse adenomyosis with TOUA: Single surgeon experience of one hundred sixteen cases and report of fertility outcomes. Kaohsiung J. Med. Sci. 2018, 34, 290–294. [Google Scholar] [CrossRef]
- Al Jama, F.E. Management of adenomyosis in subfertile women and pregnancy outcome. Oman Med. J. 2011, 26, 178–181. [Google Scholar] [CrossRef]
- Saremi, A.; Bahrami, H.; Salehian, P.; Hakak, N.; Pooladi, A. Treatment of adenomyomectomy in women with severe uterine adenomyosis using a novel technique. Reprod. Biomed. Online 2014, 28, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Kishi, Y.; Yabuta, M.; Taniguchi, F. Who will benefit from uterus-sparing surgery in adenomyosis-associated subfertility? Fertil. Steril. 2014, 102, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.H.; Wang, K.C.; Lee, N.R.; Huang, N.; Su, W.H.; Chao, H.T.; Yen, M.S.; Fuh, J.L.; Wang, P.H. Reproductive performance of severely symptomatic women with uterine adenomyoma who wanted preservation of the uterus and underwent combined surgical-medical treatment. Taiwan. J. Obstet. Gynecol. 2013, 52, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Feng, X.; Gao, L.; Huang, M. Local excision of uterine adenomyomas: A report of 86 cases with follow-up analyses. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012, 161, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Osada, H.; Silber, S.; Kakinuma, T.; Nagaishi, M.; Kato, K.; Kato, O. Surgical procedure to conserve the uterus for future pregnancy in patients suffering from massive adenomyosis. Reprod. Biomed. Online 2011, 22, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, H.; Kitade, M.; Kikuchi, I.; Kumakiri, J.; Kuroda, K.; Jinushi, M. Diagnosis, laparoscopic management, and histopathologic findings of juvenile cystic adenomyoma: A review of nine cases. Fertil. Steril. 2010, 94, 862–868. [Google Scholar] [CrossRef]
- Nishida, M.; Takano, K.; Arai, Y.; Ozone, H.; Ichikawa, R. Conservative surgical management for diffuse uterine adenomyosis. Fertil. Steril. 2010, 94, 715–719. [Google Scholar] [CrossRef]
- Hadisaputra, W.; Anggraeni, T.D. Laparoscopic resection versus myolysis in the management of symptomatic uterine adenomyosis: Alternatives to conventional treatment. Med. J. Indones 2006, 15, 9–17. [Google Scholar] [CrossRef]
- Rajuddin, R.; Jacoeb, T.Z. Management of adenomyosis in infertile women: Comparison between laparotomic resection and administration of aromatase inhibitor (Experience in 55 cases). Med. J. Indones 2006, 15, 18–23. [Google Scholar] [CrossRef][Green Version]
- Takeuchi, H.; Kitade, M.; Kikuchi, I.; Shimanuki, H.; Kumakiri, J.; Kitano, T.; Kinoshita, K. Laparoscopic adenomyomectomy and hysteroplasty: A novel method. J. Minim. Invasive Gynecol. 2006, 13, 150–154. [Google Scholar] [CrossRef]
- Fujishita, A.; Masuzaki, H.; Khan, K.N.; Kitajima, M.; Ishimaru, T. Modified reduction surgery for adenomyosis. A preliminary report of the transverse H incision technique. Gynecol. Obstet. Investig. 2004, 57, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Vannuccini, S.; Petraglia, F. Recent advances in understanding and managing adenomyosis. F1000Research 2019, 8. F1000 Faculty Rev-283. [Google Scholar] [CrossRef] [PubMed]
- Sõritsa, D.; Saare, M.; Laisk-Podar, T.; Peters, M.; Sõritsa, A.; Matt, K.; Karro, H.; Salumets, A. Pregnancy rate in endometriosis patients according to the severity of the disease after using a combined approach of laparoscopy, GnRH agonist treatment and in vitro fertilization. Gynecol. Obstet. Investig. 2015, 79, 34–39. [Google Scholar] [CrossRef] [PubMed]
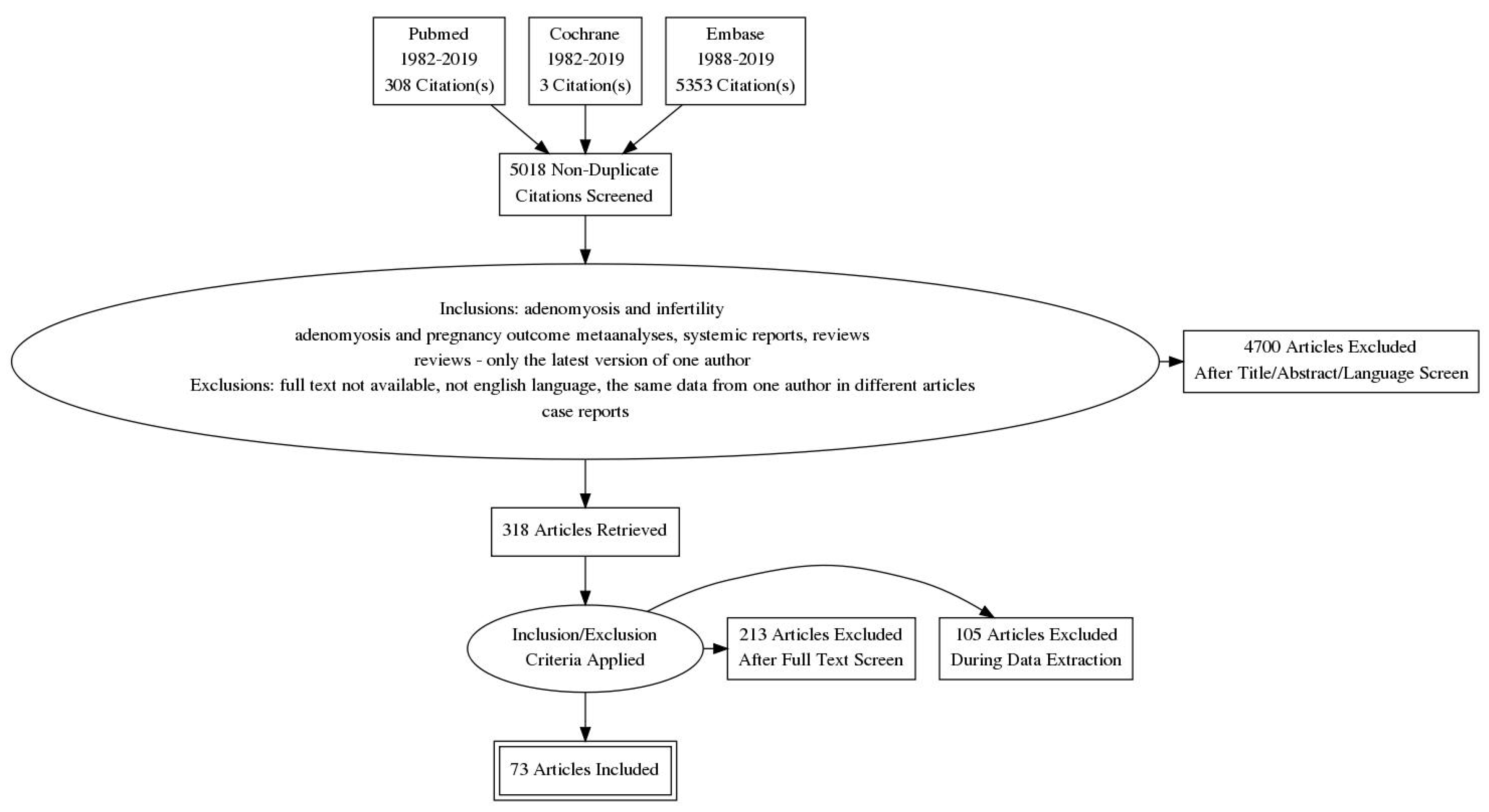
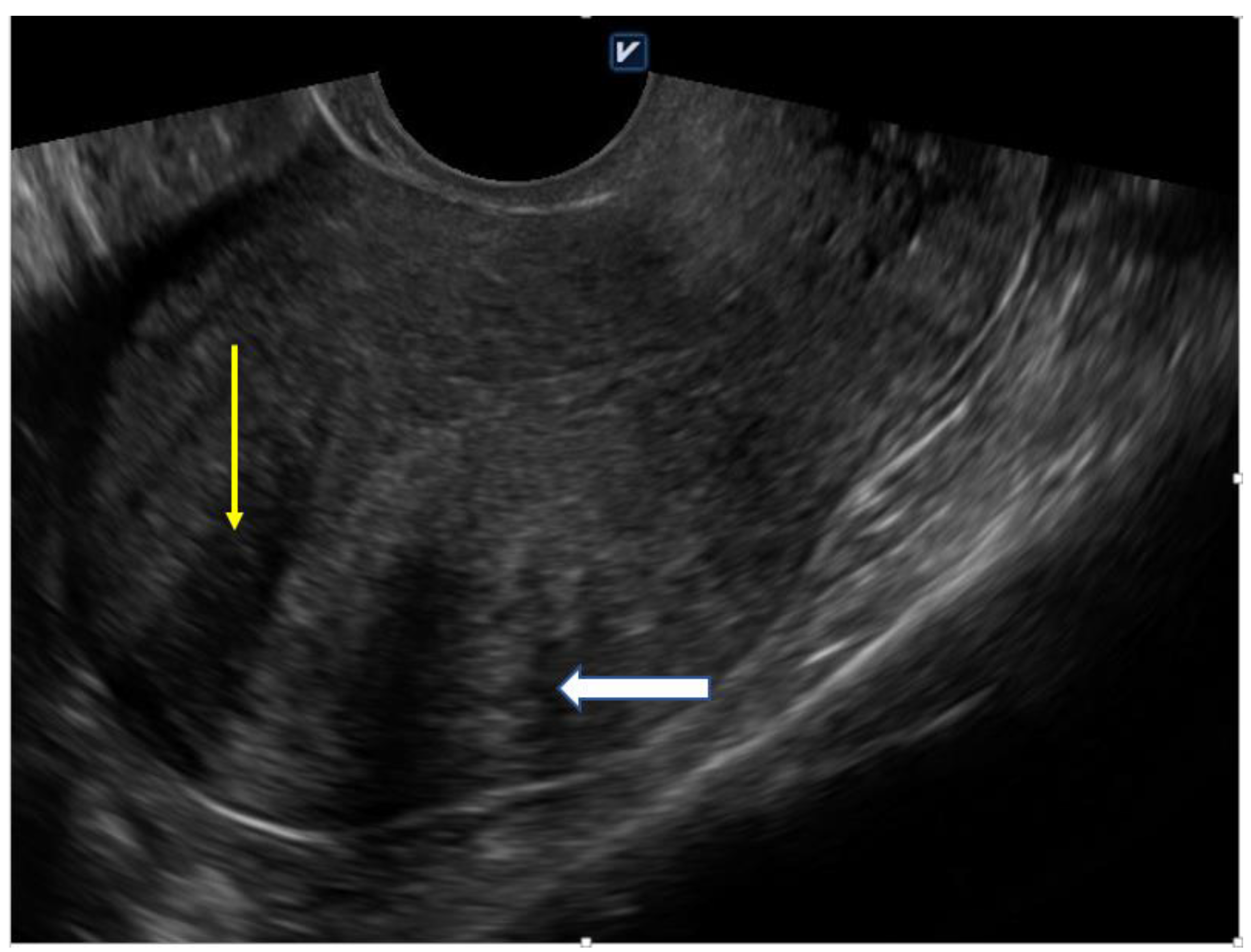
| Diffuse Adenomyosis | Focal Adenomyosis |
|---|---|
| globally enlarged uterus | focal disturbances in myometrium layer |
| asymmetric thickness anterior and posterior wall = pseudo-widening sign | sometimes focal form diagnosed as intramural myoma |
| cystic myometrium (cystic anechoic spaces) | anechoic cysts |
| junctional zone not clearly visible, thickening of the JZ | |
| heterogeneous echogenicity of the myometrium |
| Pharmacological | Surgical |
|---|---|
| Anti-inflammatory drugs | Endo-myometrial ablation |
| Oral contraceptives | High-intensity focused ultrasound |
| GnRH | Ablation |
| progestins | Electrocoagulation of adenomyosis foci |
| Resection of adenomyosis foci | |
| Hysterectomy |
| Author | Treatment | Patients | Results | ||
|---|---|---|---|---|---|
| N | Fertility | Bleeding | Pain | ||
| Kwack et al. 2018 [59] | conservative adenomyomectomy with TOUA (transient occlusion of uterine arteries) | 116 | 5/116 conception by natura l5/11 conception by ART 7 live births | menorrhagia: 52/116 complete remission 53/116 partial remission | dysmenorrhea: 98/116 complete remission 18/116 partial remission |
| Al Jama et al. 2016 [60] | treatment with Gn-RH agonist | 22 | 3/22 pregnancies 1/22 live birth | improvement in dysmenorrhea and menorrhagia was noted at the 6- and 12-month follow-up visits in both groups | |
| combined conservative surgery and Gn-RHa therapy | 18 | 8/18 pregnancies 6/18 live births | |||
| Saremi et al. 2014 [61] | resection of adenomatosis lesions with a thin margin after sagittal incision in the uterine body | 103 | 14/70 conception by ART 7/70 conception by natural 16/70 live births | decrease of 65% in the number of patients with a heavy bleeding pattern; | decrease of 41% in the number of patients with dysmenorrhoea symptoms; |
| Kishi et al. 2014 [62] | laparoscopic adenomyomectomy with laser | 102 | conception by natural: 16/75 (<40 y) and 0/27 (40 or more y) conception by ART: 15/75 (<40 y) and 1/27 (40 or more y) delivery: 26/75 (<40 y) | no data | no data |
| Chang et al. 2013 [63] | ultramini- or mini-laparotomy conservative surgery and Gn-RHa therapy | 56 | 23/56 pregnancies 15/56 live births | no precise data | VNRS-6 (six-point verbal numeric rating scale) baseline of 3.96 ± 0.41 to 0.32 ± 0.46 1st year, 0.68 ± 0.78 2nd year 1.27 ± 1.22 3rd year, |
| Dai et al. 2012 [64] | local excision of adenomyoma at laparotomy | 86 | 2/86 pregnancies | no data | alleviation of dysmenorrhea -12 months after treatment: >80% reduction in 77/79 (97.5%); 50–80% reduction in 2/79 (2.5%); -24 months after treatment: >80% reduction in 45/48 (93.8%); 50–80% reduction in 3/48 (6.2%); |
| Huang et al. 2012 [50] | excision of the adenomyosis tissue using a microsurgical technique and a six-month course of GnRHa therapy | 9 | 6/18 conception by ART 3/18 conception by natural 2/18 live births | no data | pain score 4.7 ± 0.5 before treatment 0.33 ± 0.5 after 3 months 1.0 ± 0.9 after 12 months |
| Osada et al. 2011 [65] | adenomyomectomy with a triple-flap method, without overlapping suture lines | 104 | 4/26 conception by natura l12/26 conception by ART 14 live births | VAS (visual analogue scale) hypermenorrhoea, 10 pre-surgically 3.27 ± 2.17 at 3 months, 2.89 ± 1.77 at 6 months, 2.63 ± 1.3 at 1 year, 2.87 ± 1.77 at 2 years post-surgery | The VAS findings (dysmenorrhoea, 10 pre-surgically) 1.61 ± 1.43 at 3 months, 1.54 ± 1.62 at 6 months, 1.44 ± 1.65 at 1 year, 1.67 ± 1.79 at 2 years post-surgery. |
| Takeuchi et al. 2010 [66] | laparoscopic enucleation of juvenile cystic adenomyoma | 9 | 2/3 pregnancies | no data | dysmenorrhea 8–10 on the VAS before the surgery, decreased to 2 by 6 months after |
| Nishida et al. 2010 [67] | adenomyomectomy with unilateral salpingectomy | 44 | 1/16 live births | reducing menstrual blood loss, no quantitative data | dramatic relief from dysmenorrhea, no quantitative data |
| Hadisaputra et al. 2006 [68] | laparoscopic resection +GnRH analogue after surgery | 10 | 3/10 pregnancies | no change in the symptom of menorrhagia | 75% reduction in dysmenorrhea after treatment |
| myolysis +GnRH analogue after surgery | 10 | 2/10 pregnancies | no change in the symptom of menorrhagia | 58.31% reduction in dysmenorrhea after treatment | |
| Rajuddin et al. 2006 [69] | laparotomic resection | 32 | 3/32 pregnancies 2/32 live births | after intervention, 28/32 experienced disappearance of symptoms (dysmenorrhea, pelvic pain, menorrhagia, dyspareunia), while 4/32 had remaining symptoms; | |
| treatment with aromatase inhibitor of anastrozole | 23 | 2/23 pregnancies 1/23 live births | after therapy 14/23 experienced a disappearance of symptoms, while 9/23 had remaining symptoms; | ||
| Takeuchi et al. 2006 [70] | laparoscopic adenomyomectomy and hysteroplasty | 14 | 2/14 pregnancies | all 8 cases of polyhypermenorrhea improved, no precise data available | dysmenorrhea—VAS during menstruation decreased from 10 before operation to 2.5 after operation. |
| Fujishita et al. 2004 [71] | classical reduction surgery | 5 | 0/5 pregnancies | 2/5 relief of menorrhagia and dysmenorrhea | |
| transverse H incision method and the reduction surgery | 6 | 1/6 pregnancies | 3/6 relief of symptoms | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szubert, M.; Koziróg, E.; Olszak, O.; Krygier-Kurz, K.; Kazmierczak, J.; Wilczynski, J. Adenomyosis and Infertility—Review of Medical and Surgical Approaches. Int. J. Environ. Res. Public Health 2021, 18, 1235. https://doi.org/10.3390/ijerph18031235
Szubert M, Koziróg E, Olszak O, Krygier-Kurz K, Kazmierczak J, Wilczynski J. Adenomyosis and Infertility—Review of Medical and Surgical Approaches. International Journal of Environmental Research and Public Health. 2021; 18(3):1235. https://doi.org/10.3390/ijerph18031235
Chicago/Turabian StyleSzubert, Maria, Edward Koziróg, Olga Olszak, Klaudia Krygier-Kurz, Jakub Kazmierczak, and Jacek Wilczynski. 2021. "Adenomyosis and Infertility—Review of Medical and Surgical Approaches" International Journal of Environmental Research and Public Health 18, no. 3: 1235. https://doi.org/10.3390/ijerph18031235
APA StyleSzubert, M., Koziróg, E., Olszak, O., Krygier-Kurz, K., Kazmierczak, J., & Wilczynski, J. (2021). Adenomyosis and Infertility—Review of Medical and Surgical Approaches. International Journal of Environmental Research and Public Health, 18(3), 1235. https://doi.org/10.3390/ijerph18031235






