MALDI-TOF Protein Profiling Reflects Changes in Type 1 Diabetes Patients Depending on the Increased Amount of Adipose Tissue, Poor Control of Diabetes and the Presence of Chronic Complications
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Groups
2.2. Methods
- Obesity (BMI < 30 vs. BMI > 30 kg/m2): BMI was calculated from the formula of the ratio of body weight to squared height.
- Presence of diabetic complications (retinopathy, nephropathy, and neuropathy, all complications or any of them)
- HbA1c value < 7% and > 7%: A reasonable A1C goal according to American Diabetes Association Guidelines 2020 for many nonpregnant adults [4].
2.2.1. Assessment of Diabetic Complications
Assessment of Diabetic Kidney Disease (DKD)
Assessment of Diabetic Retinopathy
Assessment of Diabetic Neuropathy
2.2.2. Laboratory Analysis
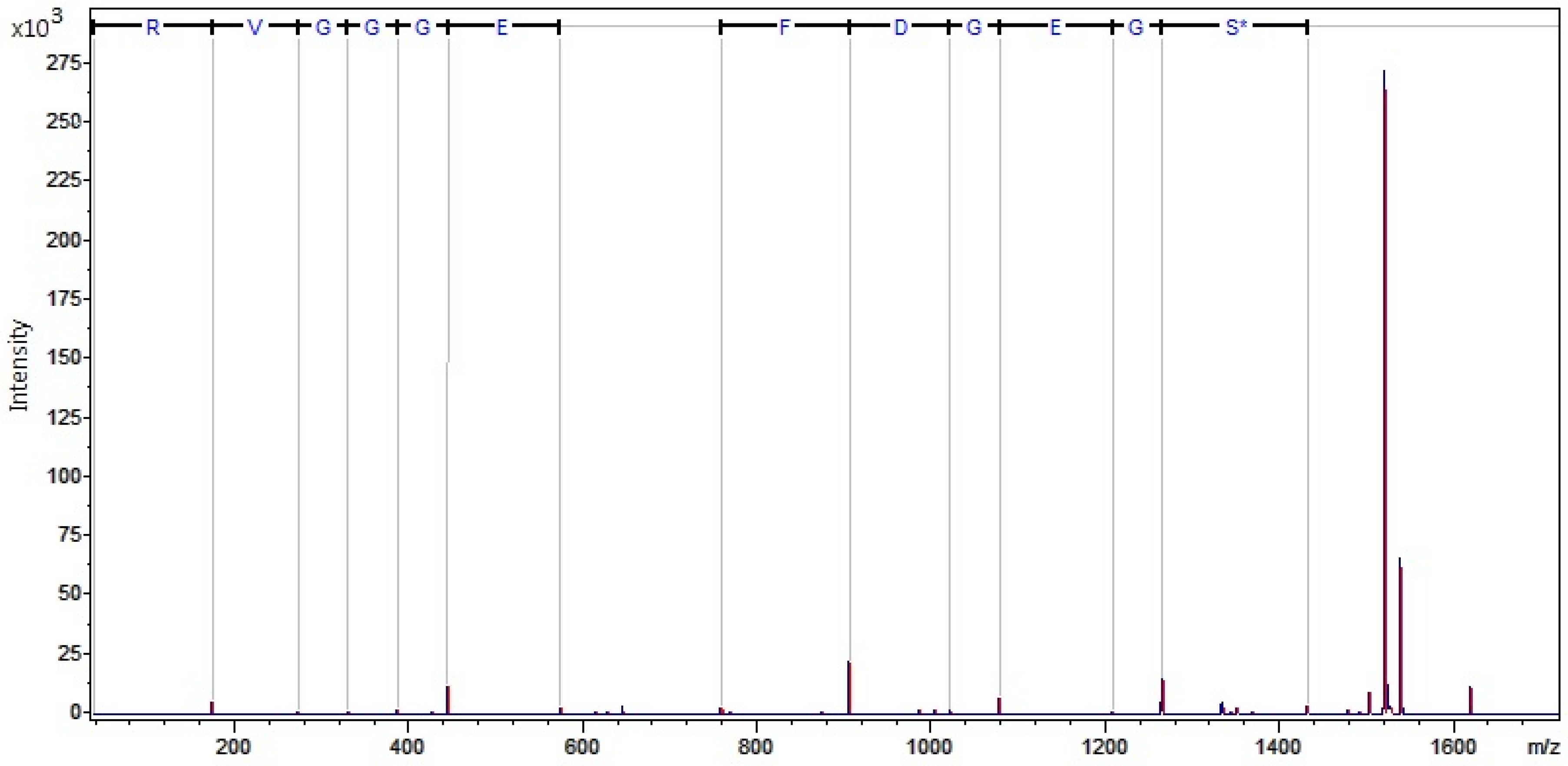
2.2.3. MALDI-TOF MS Profiling
Sample Pretreatment
MALDI-TOF MS Analysis
nanoLC MALDI-TOF/TOF MS Identification of Discriminatory Peaks
2.2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Diaz-Valencia, P.A.; Bougnères, P.; Valleron, A.J. Global epidemiology of type 1 diabetes in young adults and adults. A systematic review. BMC Public Health 2015, 15, 255. [Google Scholar] [CrossRef]
- Araszkiewicz, A.; Zozulinska-Ziolkiewicz, D.; Trepinska, M.; Wierusz-Wysocka, B. Knowledge after five-day teaching program in intensive insulin therapy performed at the onset of type 1 diabetes influence the development of late diabetic complications. Diabetes Res. Clin. Pract. 2008, 81, 61–67. [Google Scholar] [CrossRef]
- Liu, C.W.; Bramer, L.; Webb-Robertson, B.J.; Waugh, K.; Rewers, M.J.; Zhang, Q. Temporal profiles of plasma proteome during childhood development. J. Proteom. 2017, 30, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Hariri, M.A.; Elmedawar, M.; Zhu, R.; Jaffa, M.A.; Zhao, J.; Mirzaei, P.; Ahmed, A.; Kobeissy, F.; Ziyadeh, F.N.; Mechref, Y.; et al. Proteome profiling in the aorta and kidney of type 1 diabetic rats. PLoS ONE 2017, 12, e0187752. [Google Scholar] [CrossRef]
- Merchant, M.L.; Klein, J.B. Proteomics and diabetic nephropathy. Semin. Nephrol. 2007, 27, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Polish Diabetes AssociationStandards of Medical Care in Diabetesd. Diabetes Care 2020, 6, 1–106.
- Matuszewska, E.; Matysiak, J.; Brȩborowicz, A.; Olejniczak, K.; Kycler, Z.; Kokot, Z.J.; Matysiak, J. Proteomic features characterization of Hymenoptera venom allergy. Allergy Asthma Clin. Immunol. 2019, 15, 77. [Google Scholar] [CrossRef]
- Nordwall, M.; Arnqvist, H.J.; Bojestig, M.; Ludvigsson, J. Good glycemic control remains crucial in prevention of late diabetic complications – the Linköping Diabetes Complications Study. Pediatric Diabetes 2009, 10, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Krzentowski, G.; Albert, A.; Lefebvre, P.J. Risk of Developing Retinopathy in Diabetes Control and Complications Trial Type 1 Diabetic Patients With Good or Poor Metabolic Control. Diabetes Care 2001, 24, 1275–1279. [Google Scholar] [CrossRef] [PubMed]
- Hoferab, S.E.; Bennettsc, B.K.; Chana, A.; Hollowaya, B.; Karschimkusd, C.J.; Jenkinsd, A.; Silinkae, M.; CDonaghueae, K. Association between PON 1 polymorphisms, PON activity and diabetes complications. J. Diabetes Its Complicat. 2006, 20, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Løstergaard, J.A.; Ruseva, M.M.; Malik, T.H.; Hoffmann-Petersen, I.T.; Pickering, M.C.; Thiel, S.; Hansen, T.K. Increased Autoreactivity of the Complement-Activating Molecule Mannan-Binding Lectin in a Type 1 Diabetes Model. J. Diabetes Res. 2016, 2016, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Burch, T.C.; Morris, M.A.; Campbell-Thompson, M.; Pugliese, A.; Nadler, J.L.; Nyalwidhe, J.O. ProteomicAnalysisofDiseaseStratified HumanPancreasTissueIndicatesUnique SignatureofType1Diabetes. PLoS ONE 2015, 10, e0135663. [Google Scholar] [CrossRef]
- Nyalwidhe, J.O.; Grzesik, W.J.; Burch, T.C.; Semeraro, M.L.; Waseem, T.; Gerling, I.C.; Mirmira, R.G.; Morris, M.A.; Nadler, J.L. Comparative quantitative proteomic analysis of disease stratified laser captured microdissected human islets identifies proteins and pathways potentially related to type 1 diabetes. PLoS ONE 2017, 12, e0183908. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Fillmore, T.L.; Schepmoes, A.A.; Clauss, T.R.W.; Gritsenko, M.A.; Mueller, P.W.; Rewers, M.; Atkinson, M.A.; Smith, R.D.; Metz, T.O. Serum proteomics reveals systemic dysregulation of innate immunity in type 1 diabetes. J. Exp. Med. 2013, 210, 191–203. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, V.N.; Limaneto, A.B.M.; Vantilburg, M.F.; Deoliveira, A.C.; Moreira, M.; Duartepintolobo, M.; Rondina, D.; Fernandes, V.O.; Diasrangelmontenegro, A.P.; Montenegrojúnior, R.M.; et al. Proteomic analysis to identify candidate biomarkers associated with type 1 diabetes. Diabetes Metab. Syndr. Obes. 2018, 11, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Rowe, P.; Wasserfall, C.; Croker, B.; Thompson, M.C.-; Pugliese, A.; Atkinson, M.; Schatz, D. IncreasedComplementActivationin HumanType1DiabetesPancreata. Diabetes Care 2013, 36, 3815–3817. [Google Scholar] [CrossRef] [PubMed]
- Vontoerne, C.; Laimighofer, M.; Achenbach, P.; Beyerlein, A.; Lasherasgala, T.; Krumsiek, J.; Theis, F.J.; Ziegler, A.G.; Hauck, S.M. Peptide serum markers in islet autoantibody-positive children. Diabetologia 2017, 60, 287–295. [Google Scholar] [CrossRef] [PubMed]
- García-Ramírez, M.; Canals, F.; Hernández, C.; Colomé, N.; Ferrer, C.; Carrasco, E.; García-Arumí, J.; Simó, R. Proteomic analysis of human vitreous fluid by fluorescencebased difference gel electrophoresis (DIGE): A new strategy for identifying potential candidates in the pathogenesis of proliferative diabetic retinopathy. Diabetologia 2007, 50, 1294–1303. [Google Scholar]
- Moszak, M.; Klupczynska, A.; Kanikowska, A.; Kokot, Z.J.; Zawada, A.; Grzymisławska, M.; Grzymisławski, M. The influence of 3-week body mass reduction program on the metabolic parameters and free amino acid profiles in adult Polish people with obesity. Adv. Clin. Exp. Med. 2018, 27, 749–757. [Google Scholar] [CrossRef]
- Zhi, W.; Sharma, A.; Purohit, S.; Miller, E.; Bode, B.; Anderson, S.W.; Reed, J.C.; Steed, R.D.; Steed, L.; Hopkins, D.; et al. Discovery and Validation of Serum Protein Changes in Type 1 Diabetes Patients Using High Throughput Two Dimensional Liquid Chromatography-Mass Spectrometry and Immunoassays. Mol. Cell. Proteom. 2011, 10, M111.02203–M111.02210. [Google Scholar] [CrossRef] [PubMed]


| Variable | Median (IQR) |
|---|---|
| Sex [M/F], n (%) | 50 (48.5)/ 53 (51.5) |
| Age [y] | 34 (30–42) |
| DD [y] | 17 (12–23) |
| IFI [y] | 12 (8–18) |
| WHR [n] | 0.9 (0.8–0.9) |
| BMI [kg/m2] | 26 (23–29) |
| TBF [kg] | 19 (14–27) |
| VF [n] | 5 (3–8) |
| DDI [µ/kg/d] | 0.5 (0.4–0.6) |
| HbA1c [%] | 8 (7–9) |
| AST [U/L] | 19 (16–24) |
| ALT [U/L] | 19 (14–26) |
| Creatynine, [μmol/L] | 80 (71–88) |
| eGFR [mL/min/1.73 m2] | 88 (77–90) |
| hsCRP [mg/dL] | 2 (1–3) |
| T-ch [mmol/L] | 48 (42–54) |
| TAG [mmol/L] | 1 (1–2) |
| HDL-ch [mmol/L] | 2 (1–2) |
| LDL-ch [mmol/L] | 3 (2–3) |
| non-HDL-c [mmol/L] | 3 (3–4) |
| Complications | Incidence Frequency in Numbers (n = 103) | Incidence Frequency as a Ratio (103 = 100%) |
|---|---|---|
| Diabetic retinopathy | 39 | 37.9% |
| Diabetic renal disease | 9 | 8.7% |
| Autonomic neuropathy | 21 | 20.4% |
| Peripheral neuropathy | 32 | 31.1% |
| The group with any complications | 54 | 52.4 |
| Value | EBF N = 46 | NBF N = 57 | EBF/NBF P < 0.05 | HbA1c > 7% N = 80 | HbA1c < 7% N = 23 | HbA1cp < 0.05 | PofC N = 55 | AofC N = 48 | PofC/ AofC P < 0.05 |
|---|---|---|---|---|---|---|---|---|---|
| Age [y] | 35.0 (31.0–46.0) | 33.0 (29.0–40.0) | 0.09 | 34.0 (25.0–40.0) | 35.0 (31.0–42.0) | 0.64 | 38.0 (32.0–47.0) | 32.0 (24.0–37.0) | 0.00 |
| Sex [M/F] | 23/23 | 27/30 | 0.82 | 35/45 | 15/8 | 0.07 | 25/30 | 25/23 | 0.51 |
| DD [y] | 18.0 (12.0–23.5) | 16.0 (11.0–23.0) | 0.90 | 15.0 (11.0–20.0) | 19.0 (7.0–21.0) | 0.38 | 20.0 (15.0–29.0) | 13.0 (8.0–17.0) | 0.00 |
| TBF [kg] | 27.6 (23.1–33.2) | 14.1 (10.9–18.2) | 0.00 | 21.7 (14.1–29.7) | 17.3 (11.8–20.0) | 0.07 | 20.0 (14.1–27.1) | 18.2 (13.5–25.0) | 0.32 |
| BMI [kg/m2] | 29.3 (27.8–30.6) | 23.7 (21.4–25.7) | 0.00 | 27.1 (24.0–29.8) | 24.2 (22.1–28.3) | 0.06 | 26.4 (23.4–30.3) | 27.1 (23.0–28.8) | 0.66 |
| VF [n] | 8.0 (6.5–10.0) | 4.0 (2.0–5.0) | 0.00 | 5.0 (3.0–8.0) | 6.0 (3.0–7.0) | 0.93 | 6.0 (4.0–8.0) | 5.0 (2.0–7.0) | 0.12 |
| WHR [n] | 0.9 (0.8–0.9) | 0.8 (0.8–0.9) | 0.00 | 0.9 (0.8–0.9) | 0.8 (0.9–1.0) | 0.04 | 6.0 (4.0–8.0) | 5.0 (2.0–7.0) | 0.05 |
| HbA1c [%] | 8.4 (7.3–8.9) | 7.8 (6.8–8.9) | 0.18 | 8.9 (8.4–9.8) | 6.5 (6.2–6.8) | 0.00 | 7.9 (6.9–9.0) | 8.4 (7.2–8.9) | 0.75 |
| AST [IU/L] | 19.0 (15.5–27.0) | 19.0 (16.0–22.0) | 0.54 | 18.0 (16.0–27.0) | 19.0 (15.0–22.0) | 0.66 | 19.0 (15.0–24.0) | 19.0 (16.0–27.0) | 0.38 |
| ALT [IU/L] | 21.0 (14.0–28.5) | 17.0 (14.0–25.0) | 0.13 | 18.0 (13.0–27.0) | 21.0 (15.0–25.0) | 0.61 | 18.0 (14.0–24.0) | 21.0 (14.0–29.0) | 0.25 |
| Creatinine [µmol/L] | 70.7 (61.9–88.4) | 77.8 (70.7–88.4) | 0.19 | 70.7 (61.9–79.6) | 79.6 (70.7–88.4) | 0.14 | 79.6 (70.7–88.4) | 79.6 (70.7–88.4) | 0.18 |
| GFR [mL/min/1.72 m2] | 88.2 (82.2–90.0) | 86.0 (75.7–90.0) | 0.31 | 90.0 (75.5–90.0) | 84.1 (76.5–90.0) | 0.38 | 83.1 (73.4–90.0) | 90.0 (84.5–90.0) | 0.01 |
| CRP [mg/dL] | 2.2 (1.0–4.5) | 1.2 (0.6–2.1) | 0.00 | 2.1 (1.0–4.1) | 1.0 (0.4–2.0) | 0.03 | 1.4 (0.7–3.1) | 1.7 (0.8–3.1) | 0.85 |
| TCh [mmol/L] | 4.9 (4.5–5.7) | 4.6 (3.9–5.1) | 0.00 | 4.8 (4.2–5.3) | 4.7 (4.1–5.5) | 0.96 | 4.9 (4.3–5.4) | 4.6 (4.0–5.2) | 0.31 |
| TAG [mmol/L] | 1.2 (1.0–1.8) | 0.9 (0.7–1.3) | 0.00 | 1.1 (0.9–1.7) | 0.9 (0.7–1.2) | 0.00 | 1.3 (0.9–1.5) | 1.3 (0.8–1.4) | 0.45 |
| HDL-ch [mmol/L] | 1.5 (1.3–1.9) | 1.7 (1.4–2.0) | 0.11 | 1.6 (1.3–1.9) | 1.7 (1.4–2.3) | 0.13 | 1.6 (1.3–2.0) | 1.6 (1.3–2.0) | 0.96 |
| LDL-ch [mmol/L] | 2.7 (2.5–3.3) | 2.4 (1.9–2.9) | 0.01 | 2.6 (2.1–3.1) | 2.5 (1.9–3.4) | 0.84 | 2.7 (2.6–3.2) | 2.5 (1.9–3.1) | 0.18 |
| non-HDL-ch [mmol/L] | 3.2 (2.9–4.0) | 2.7 (2.2–3.3) | 0.00 | 3.1 (2.6–3.6) | 2.7 (2.3–3.9) | 0.41 | 3.2 (2.7–3.6) | 2.8 (2.4–3.8) | 0.33 |
| ACR [mg/d] | 3.5 (2.5–5.2) | 3.8 (2.7–5.4) | 0.82 | 3.8 (2.5–5.4) | 3.7 (2.9–5.3) | 0.88 | 4.3 (2.9–8.1) | 3.3 (2.3–4.3) | 0.00 |
| Division Due to Excess Fat | ||||
|---|---|---|---|---|
| Model | Cross Validation [%] | Recognition Capability [%] | External Validation—Correct Classified Part of Valid Spectra [%]—TEST | External Validation—Correct Classified Part of Valid Spectra [%]—CONTROL |
| GA | 49.5 | 93.8 | 52.9 | 82.1 |
| SNN | 59.2 | 67.1 | 60.8 | 38.5 |
| QC | 58.0 | 63.4 | 43.1 | 74.4 |
| Identified peaks (m/z) classified as discriminatory based on GA | ||||
| 1537.88 | fibrinogen alpha chain | |||
| 1519.99 | complement C3 (oxidation) | |||
| 1449.61 | complement C4A | |||
| Identified peaks (m/z) classified as discriminatory based on SNN | ||||
| 1519.99 | complement C3 (oxidation) | |||
| 1537.88 | fibrinogen alpha chain | |||
| Identified peaks (m/z) classified as discriminatory based on QC | ||||
| 1435.73 | complement C4A | |||
| 1449.61 | complement C4A | |||
| 1519.99 | complement C3 (oxidation) | |||
| 1537.88 | fibrinogen alpha chain | |||
| Division Due to Diabetes Control (HbA1c > 7%) | ||||
|---|---|---|---|---|
| Model | Cross Validation [%] | Recognition Capability [%] | External Validation—Correct Classified Part of Valid Spectra [%]—TEST | External Validation—Correct Classified Part of Valid Spectra [%]—CONTROL |
| GA | 62.2 | 85.8 | 63.6 | 30.6 |
| SNN | 64.6 | 53.7 | 0 | 88.9 |
| QC | 66.9 | 66.9 | 56.8 | 55.6 |
| Identified peaks (m/z) classified as discriminatory based on GA | ||||
| 1537.88 | fibrinogen alpha chain | |||
| 1449.61 | complement C4A | |||
| 1520.00 | complement C3 (oxidation) | |||
| Identified peaks (m/z) classified as discriminatory based on SNN | ||||
| 1519.99 | complement C3 (oxidation) | |||
| 1537.88 | fibrinogen alpha chain | |||
| Identified peaks (m/z) classified as discriminatory based on QC | ||||
| 1537.88 | fibrinogen alpha chain | |||
| Division Due to Diabetes Complications | ||||
|---|---|---|---|---|
| Model | Cross Validation [%] | Recognition Capability [%] | External Validation—Correct Classified Part of Valid Spectra [%]—TEST | External Validation—Correct Classified Part of Valid Spectra [%]—CONTROL |
| GA | 48.2 | 84.6 | 46.2 | 67.6 |
| SNN | 48.1 | 65.6 | 53.8 | 79.4 |
| QC | 38.0 | 63.1 | 46.2 | 58.8 |
| Identified peaks (m/z) classified as discriminatory based on GA | ||||
| 1537.88 | fibrinogen alpha chain | |||
| 1617.79 | fibrinogen alpha chain (peak 1537 phosphorylation) | |||
| 1435.73 | complement C4A | |||
| Identified peaks (m/z) classified as discriminatory based on SNN | ||||
| 1537.88 | fibrinogen alpha chain | |||
| 1435.73 | complement C4A | |||
| 1520.00 | complement C3 (oxidation) | |||
| 1617.79 | fibrinogen alpha chain (peak 1537 phosphorylation) | |||
| Identified peaks (m/z) classified as discriminatory based on QC | ||||
| 1537.88 | fibrinogen alpha chain | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zawada, A.; Naskręt, D.; Matuszewska, E.; Kokot, Z.; Grzymisławski, M.; Zozulińska-Ziółkiewicz, D.; Dobrowolska, A.; Matysiak, J. MALDI-TOF Protein Profiling Reflects Changes in Type 1 Diabetes Patients Depending on the Increased Amount of Adipose Tissue, Poor Control of Diabetes and the Presence of Chronic Complications. Int. J. Environ. Res. Public Health 2021, 18, 2263. https://doi.org/10.3390/ijerph18052263
Zawada A, Naskręt D, Matuszewska E, Kokot Z, Grzymisławski M, Zozulińska-Ziółkiewicz D, Dobrowolska A, Matysiak J. MALDI-TOF Protein Profiling Reflects Changes in Type 1 Diabetes Patients Depending on the Increased Amount of Adipose Tissue, Poor Control of Diabetes and the Presence of Chronic Complications. International Journal of Environmental Research and Public Health. 2021; 18(5):2263. https://doi.org/10.3390/ijerph18052263
Chicago/Turabian StyleZawada, Agnieszka, Dariusz Naskręt, Eliza Matuszewska, Zenon Kokot, Marian Grzymisławski, Dorota Zozulińska-Ziółkiewicz, Agnieszka Dobrowolska, and Jan Matysiak. 2021. "MALDI-TOF Protein Profiling Reflects Changes in Type 1 Diabetes Patients Depending on the Increased Amount of Adipose Tissue, Poor Control of Diabetes and the Presence of Chronic Complications" International Journal of Environmental Research and Public Health 18, no. 5: 2263. https://doi.org/10.3390/ijerph18052263
APA StyleZawada, A., Naskręt, D., Matuszewska, E., Kokot, Z., Grzymisławski, M., Zozulińska-Ziółkiewicz, D., Dobrowolska, A., & Matysiak, J. (2021). MALDI-TOF Protein Profiling Reflects Changes in Type 1 Diabetes Patients Depending on the Increased Amount of Adipose Tissue, Poor Control of Diabetes and the Presence of Chronic Complications. International Journal of Environmental Research and Public Health, 18(5), 2263. https://doi.org/10.3390/ijerph18052263






